Prophylaxis and Screening for Prevention of Viral Respiratory Infections in Neonatal Intensive Care Unit Patients: A Systematic Review
Introduction
Healthcare-associated infections, including those caused by viral respiratory pathogens, are substantial sources of morbidity and mortality in neonatal intensive care unit (NICU) patients. The frequency of these infections is unknown, but they are not uncommon.1 A review found that when infants hospitalized in the NICU were evaluated for late-onset sepsis, the incidence of respiratory virus infections was reported to be 6.6–8.0%.2
Premature infants in the NICU are at high risk for healthcare-associated infections due to their immunologic immaturity at birth.3-5 For the purposes of this document, healthcare-associated infections are infections acquired after admission to, or birth in, a healthcare facility. In addition, a child’s length of stay in the NICU is typically longer than other pediatric healthcare settings and is most strongly influenced by gestational age at birth and birth weight.6 The earliest gestational age for a preterm infant deemed “viable” was previously 26 weeks of gestation. With advances in neonatology, babies born at 22 weeks of gestation are now deemed viable,7 leading to longer NICU lengths of stay and a longer window of potential exposure to respiratory viral pathogens.
A number of factors may increase a NICU patient’s risk for healthcare-associated viral respiratory infections. The physical design of the NICU can drive increased risk of patient-to-patient transmission. Newer units may have single patient rooms; however, traditional NICU designs include close proximity of open bassinets, patient crowding in “open pod” designs, and shared patient equipment.8-10 Another challenge in NICUs, is prolonged and close exposure to visitors. Parents, siblings, and other visitors can be the source of viral respiratory infections if visiting while asymptomatic or mildly symptomatic.2,11 Notwithstanding, family members are not the only source of respiratory viral pathogens; sick healthcare personnel (HCP) also play a role in spreading diseases to premature infants. Presenteeism and ill HCP can increase the likelihood of outbreaks in the NICU setting.8,12,13 Outbreaks of influenza and pertussis in NICUs with low healthcare worker immunization rates have been reported.14,15 Thus, a robust HCP immunization program is an important prevention strategy component in this setting. The CDC provides recommendations for HCP immunizations.16
Large outbreaks of respiratory syncytial virus (RSV), varicella, and influenza have occurred in NICU settings and are challenging to detect and mitigate.8-10,17,18 The initial asymptomatic contagious period and seasonality of some respiratory viruses cause challenges in symptom screening and transmission prevention.2,11 Pertussis is also a challenge for detection since it can cause mild disease in adults, but easily transmit to children and NICU patients, causing outbreaks.19,20 Adenoviruses can also lead to outbreaks in the NICU settings.12,21,22 In one outbreak, adenovirus was introduced to a NICU by an ophthalmologist using contaminated multi-patient ophthalmologic equipment during infant eye exams for retinopathy of prematurity.12 Additional multi-patient use equipment, including shared stethoscopes, can become contaminated and are an important part of the indirect transmission chain.9,23,24
Guidance exists for prevention of viral respiratory infections in healthcare settings.25-27 The CDC Isolation Precautions Guideline provides important information about transmission-based precautions, appropriate use of personal protective equipment, cleaning and disinfection of patient care equipment, and other key elements for preventing healthcare-associated viral respiratory infections.28 The guideline Appendix also includes the type and duration of precautions recommended for selected infections and conditions. In addition, the American Academy of Pediatrics Red Book provides some pathogen-specific guidance on management of exposed patients. The Red Book also provides guidance for high-risk infants who should receive monthly palivizumab immunoprophlaxis during RSV season to reduce the risk of RSV lower respiratory tract disease. While all of these provide recommendations specific to viral respiratory infections and various populations, no evidence-based NICU-specific infection prevention and control recommendations currently exist on this topic. Clinically relevant guidelines are needed to inform the best strategies to prevent viral respiratory infections in NICU settings. This review will not cover the topic of prevention and control of SARS-CoV2 infections in the NICU setting. CDC has SARS-CoV-2 specific guidance for newborns.
B. Scope and Purpose
This systematic review aimed to evaluate available evidence related to the prevention and control of transmission of viral respiratory infections in NICUs. For the purposes of this review, a NICU was defined as level III care; however, it is important to acknowledge that many NICUs have level II units for step down care in adjoining space, sharing the same personnel.29 Additionally, healthcare-associated viral respiratory infection may be defined as a viral respiratory illness or infection acquired >72 hours after admission.30
The topics were determined by the workgroup, vetted at national infectious disease society meetings, and refined based on input received from the Healthcare Infection Control Practices Advisory Committee (HICPAC) at public meetings occurring from November 2010 to December 2022.
C. Methods
This is a targeted, systematic review of the best available evidence on the prevention and control of respiratory viral infections (excluding COVID-19), in NICUs.
C.1. Development of Key Questions
In order to inform the development of the viral respiratory infections Key Questions, electronic searches were conducted to retrieve existing relevant guidelines and to identify gaps and areas where new evidence may have been published. Gaps where the subject matter experts believed there would be sufficient evidence to answer the question were included in this effort. Additional gaps were identified where there was believed to be no relevant evidence to answer these questions, and these are further elaborated in the discussion section. The strategies used for the guideline searches and results can be found in the Appendix. The results of this initial review informed the development of a preliminary list of Key Questions. Key Questions were finalized after vetting them with HICPAC. The four questions are:
- Key Question 1: In neonatal intensive care unit patients, does prophylaxis after viral exposure (e.g., palivizumab, oseltamivir), compared to no prophylaxis, prevent the transmission of infection?
- Key Question 2: In neonatal intensive care unit patients, does prophylactic administration of palivizumab, compared with no palivizumab, prevent the transmission of RSV during RSV season?
- Key Question 3: In neonatal intensive care unit patients, does prophylactic administration of palivizumab, compared to no palivizumab, prevent the transmission of RSV during an RSV outbreak?
- Key Question 4: In neonatal intensive care unit patients, does screening exposed patients who are asymptomatic, compared with no screening of asymptomatic exposed patients, prevent the transmission of viral respiratory infections during an outbreak?
C.2. Systematic Literature Search
A list of search terms was developed to identify the literature most relevant to the Key Questions. The terms were incorporated into search strategies, and these searches were performed in MEDLINE, EMBASE, CINAHL, and the Cochrane Library. Subject matter experts supplemented the literature search results by recommending relevant references published prior to November 2022.
C.3. Study Selection
Titles and abstracts from references were screened by dual review (A.E., C.H., J.H., M.I., J.K.K., D.O.S., K.T.R., S.S., or E.C.S.). Full-text articles were retrieved if they were:
- Relevant to one or more Key Question;
- Primary research, systematic reviews, or meta-analyses; and
- Written in English.
The Appendix presents the full list of exclusion criteria. (Appendix Section B) The full texts of selected articles were then screened by two independent reviewers, and disagreements were resolved by discussion (A.E., C.H., J.H., M.I., J.K.K., D.O.S., K.T.R., S.S., or E.C.S.). However, no studies were retrieved that answered the key questions for NICU patients. A list of select excluded studies is included in the Appendix. The results of the study selection process are depicted in Figure 1.
Figure 1. Results of the Study Selection Process
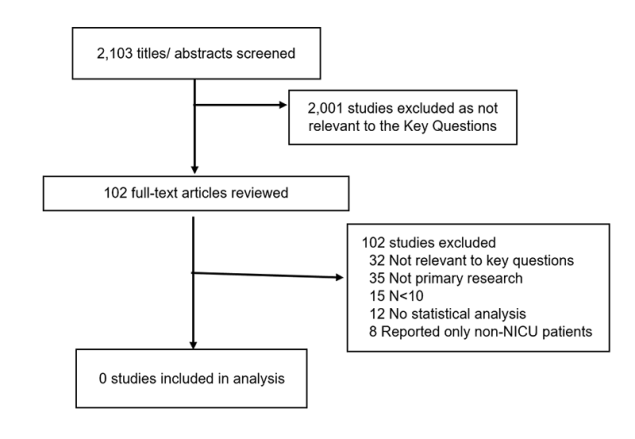
A flow chart that describes the number of studies that are included at each phase in the review. Screening began with 2,103 potentially relevant titles and abstracts. After exclusion of articles that were not relevant to the question (2,001), authors screened 102 full-text articles which resulted in 0 studies included in the final analysis.
Discussion
This systematic review identified several studies related to the key questions, but none directly addressed the questions related to preventing viral respiratory pathogen transmission or controlling outbreaks of respiratory viral infections in the NICU setting. Thus, the evidence was not sufficient to make evidence-based recommendations about the following issues:
- Administering post-exposure antiviral prophylaxis after a respiratory virus exposure to prevent hospital transmission;
- Routine use of prophylactic palivizumab during respiratory virus season to prevent RSV transmission among NICU patients;
- Use of palivizumab for post-exposure prophylaxis during an RSV outbreak in the NICU setting; and
- Screening exposed asymptomatic patients, to prevent the transmission of viral respiratory infections during an outbreak?
Additional questions considered during the scoping phase of this project include:
- How are potential respiratory pathogen exposures defined in the NICU setting?
- For infants in “open pod” NICUs, what is the optimal distance between bassinets when a patient requires contact and/or droplet isolation precautions for a respiratory viral infection?
- What is the best method to screen visitors for viral respiratory infection prior to NICU entry?
The NICU setting has unique aspects that can impact the patients’ exposure risks. As stated previously, some units have open “pod” configurations with several infants in a large, shared space, while newer NICUs have private rooms. In open pod units, shared equipment is common. In addition, different types of patient beds exist in the NICU: open warmers, enclosed isolettes for thermoregulation, bassinets, and traditional cribs. These differences can impact an infant’s level of exposure to a respiratory pathogen. Finally, admission and visitor policies may vary across NICUs. It is unclear how to measure the impact of each variable on exposure risks in the NICU, and how to define virus exposures when these factors are taken into consideration. Similarly, high-quality evidence is not available to inform detailed recommendations on patient placement (e.g., distance between bassinets) when an infant is placed in isolation precautions.
Finally, as NICUs follow the family-centered care model, family members and visitors can be screened for viral respiratory infection and educated about their role in infection prevention. Evidence suggests that screening and education are both important in infection prevention in NICUs, .31,32 However, the best approach to visitor education and screening is not established.
Acronym
Acronym
Expansion
Expansion
CDC
CDC
Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
FDA
FDA
Food and Drug Administration
Food and Drug Administration
HHS
HHS
(United States Department of) Health and Human Services
(United States Department of) Health and Human Services
HICPAC
HICPAC
Healthcare Infection Control Practices Advisory Committee
Healthcare Infection Control Practices Advisory Committee
NICU
NICU
Neonatal Intensive Care Unit
Neonatal Intensive Care Unit