Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses
Updated April 26, 2024
This report provides an update to the December 29, 2023, report to include seven new human cases (5 in Cambodia, 1 in the United States and 1 in Vietnam) and recent activity in wild birds, poultry and other animals, including the multi-state veterinary outbreak in U.S. dairy cattle, and updated information on monitoring for H5N1 virus infections in the United States. Even given these updates, CDC believes the overall risk to human health associated with the ongoing outbreaks of highly pathogenic avian influenza A(H5N1) viruses has not changed and remains low to the U.S. general public at this time.
Executive summary
A small number of sporadic human cases of highly pathogenic avian influenza (HPAI) A(H5N1) have been identified worldwide since 2022, amidst a panzootic of these viruses in wild birds and poultry. Nearly all human cases reported globally since 2022 were associated with poultry exposures, and no cases of human-to-human transmission of HPAI A(H5N1) virus have been identified. One human case of HPAI A(H5N1) virus infection in a farm worker reported in April 2024 in the United States and was attributed to exposure to presumptively infected dairy cattle. One previous human case was reported in the United States in 2022. In a few cases, the source of exposure to HPAI A(H5N1) virus has been unknown. To date, HPAI A(H5N1) viruses currently circulating most commonly in birds and poultry, with spillover to mammals and humans do not have the ability to easily bind to receptors that predominate in the human upper respiratory tract. This is a major reason why the current risk to the public from HPAI A(H5N1) viruses remains low. However, because of the potential for influenza viruses to rapidly evolve and the wide global prevalence of HPAI A(H5N1) viruses in wild birds and poultry outbreaks and following the identification and spread among dairy cattle in the United States, additional sporadic human infections are anticipated. Continued comprehensive surveillance of these viruses in wild birds, poultry, mammals, and people worldwide, and frequent reassessments are critical to determine the public health risk, along with ongoing preparedness efforts.
- CDC is actively working on the domestic situation with clade 2.3.4.4b HPAI A(H5N1) viruses in wild birds, with outbreaks in poultry and backyard flocks, and infections of other animals, including dairy cattle. These activities include conducting surveillance among people with relevant exposures and preparing for the possibility that contemporary HPAI A(H5N1) viruses gain the ability for increased transmissibility to and among people.
- CDC, along with state and local public health partners, continues to monitor people in the United States who have been exposed to infected birds, poultry, or other animals for 10 days after exposure. To date, more than 8,800 people in 52 jurisdictions have been monitored since 2022, and only two human cases have been identified.
- H5 candidate vaccine viruses (CVV) produced by CDC are expected to provide good protection against current clade 2.3.4.4b HPAI A(H5N1) viruses detected in birds and mammals, including dairy cattle. These H5 CVVs are available and have been shared with vaccine manufacturers.
- Because influenza viruses are constantly changing, CDC performs ongoing analyses of HPAI A(H5N1) viruses to identify genetic changes that might allow for spread more easily to and between people, more serious illness in people, reduce susceptibility to antivirals, affect the sensitivity of diagnostic assays, or reduce neutralization of the virus by vaccine induced antibodies. To date, few genetic changes of public health concern have been identified in HPAI A(H5N1) viruses circulating in wild birds and poultry worldwide and detected in dairy cattle in the United States.
- Currently, HPAI A(H5N1) viruses circulating in birds and U.S. dairy cattle are believed to pose a low risk to the general public in the United States; however, people who have job-related or recreational exposures to infected birds or infected mammals are at higher risk of infection and should take appropriate precautions outlined in CDC guidance.
- Comprehensive surveillance and readiness efforts are ongoing, and CDC continually takes preparedness measures to be ready in case the risk to people from HPAI A(H5N1) virus or from other novel influenza A viruses changes.
- HPAI A(H5N1) viruses in wild birds and poultry
- HPAI A(H5N1) virus infections among mammals
- Human cases of A(H5N1)
- Table 1. Global reported A(H5N1) human cases, January 2022 through April 25, 2024
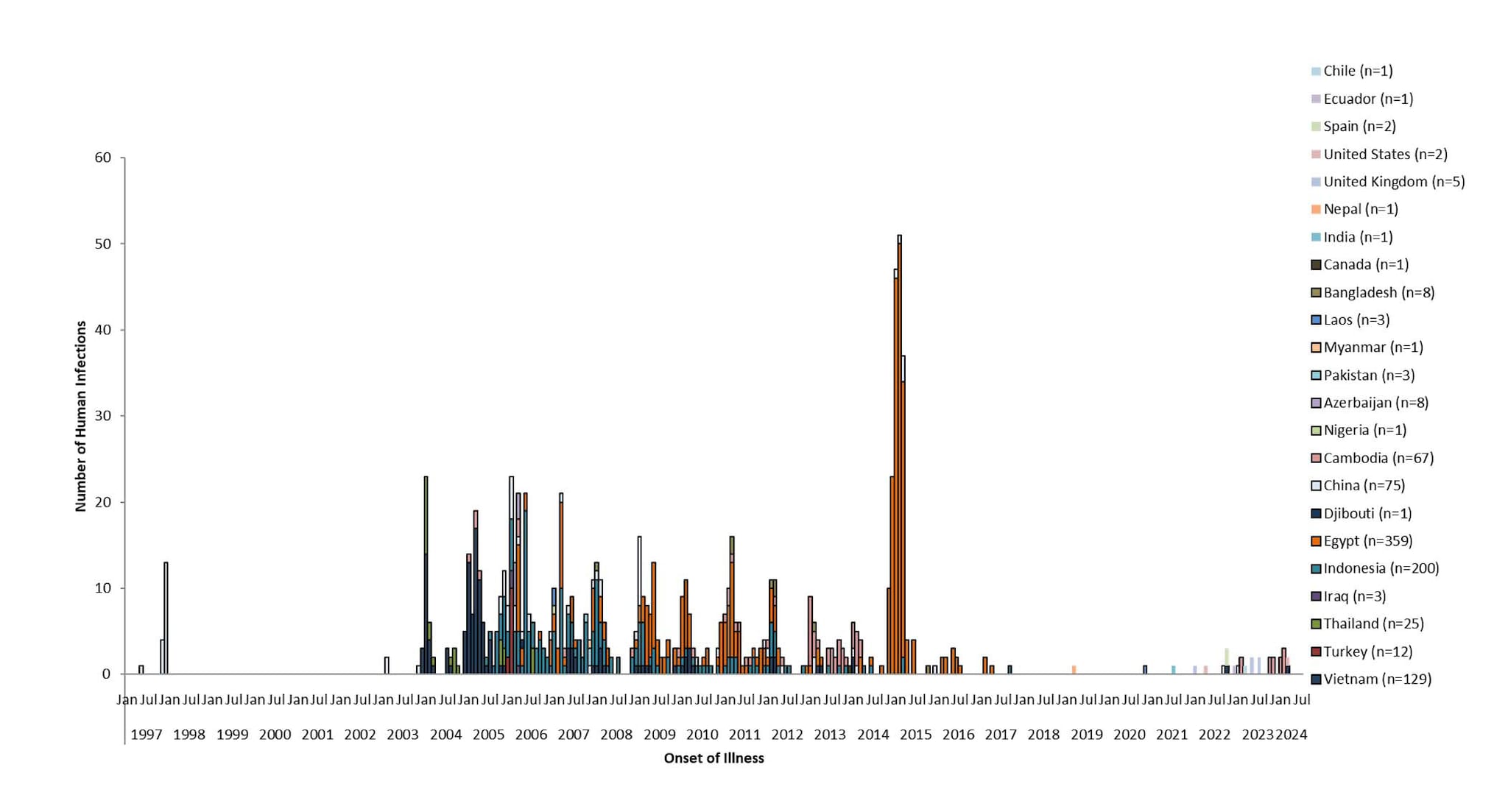
- Figure 1. Epidemic Curve of Human Cases of A(H5N1) by Illness Onset Date, 1997-2024 by Country (N=909)
- Monitoring of persons exposed to HPAI A(H5N1) viruses in the United States
HPAI A(H5N1) viruses in wild birds and poultry
Since 2005, HPAI A(H5N1) viruses have undergone extensive genetic diversification including the formation of hundreds of genotypes following reassortment with other avian influenza A viruses. Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in 2020 and were introduced into North America in late 2021 [1,2] and spread to Central and South America, resulting in wild bird infections (in terrestrial, seabird, shorebird, and migratory species) and poultry outbreaks in many countries [3-8]. In Fall 2023, the first detections of HPAI A(H5N1) viruses in birds in the Antarctica region were reported [9]. Globally, this 2.3.4.4b clade of HPAI A(H5N1) viruses has become widespread causing record numbers of bird outbreaks in wild, backyard, village, and farm birds.
In the United States, USDA APHIS monitors for avian influenza viruses in wild, commercial, and backyard birds. From January 2022 through April 25, 2024, APHIS reported HPAI A(H5)/A(H5N1) virus detections in more than 9,200 wild birds in 50 states or territories and more than 1,100 commercial and backyard flocks affecting more than 90 million birds in 48 states.
HPAI A(H5N1) virus infections among mammals
Sporadic HPAI A(H5N1) virus infections of mammals have been reported since 2003-2004 during HPAI A(H5N1) virus outbreaks in poultry or wild birds [10-12]. HPAI A(H5) viruses are known to occasionally infect mammals that eat (presumably infected) birds or poultry and mammals that are exposed to environments with a high concentration of virus.
Globally, sporadic HPAI A(H5N1) virus infections and outbreaks in a wide range of mammal species were reported by countries in different regions of the world to the World Organisation for Animal Health since January 2022. HPAI A(H5N1) virus infections of mammals have included a polar bear in the United States, farmed mink in Spain and farmed foxes and other mammals in Finland, harbor and gray seals in the United States, sea lions in Peru, Argentina, and Chile, elephant seals in Argentina, baby goats in the United States, and domesticated pets such as cats in Poland, France, South Korea, and the United States, and dogs in Italy. In March and April 2024, the United States reported HPAI A(H5N1) virus infections of dairy cattle at farms in multiple states. Spread from dairy farm-to-dairy farm was reported [292KB, 6 pages], and routes of transmission are under investigation. In the United States, from May 2022 through April 25, 2024, USDA APHIS reported HPAI A(H5N1) virus detections in wild mammals comprising a wide range of different species in 28 states.
Human cases of A(H5N1)
While HPAI A(H5N1) viruses are currently circulating widely in wild birds and poultry in many geographic regions, relatively few human cases of HPAI A(H5N1) have been reported in recent years [Figure 1]. From January 2022 through April 25, 2024, 26 sporadic human cases of A(H5N1) were reported from eight countries, including 14 cases of severe or critical illness, and seven deaths, four cases of mild illness, and eight asymptomatic cases [Table 1].
One human case of HPAI A(H5N1) was reported in the United States in April 2022. The individual reported fatigue without other symptoms and a low level of A(H5N1) viral RNA was detected in a single upper respiratory tract specimen. It is possible that detection of A(H5N1) viral RNA resulted from deposition of non-infectious viral material in the upper respiratory tract of the individual and did not represent true infection, similar to the environmental contamination that was attributed to two asymptomatic cases in poultry workers reported in Spain [13]. Transient environmental deposition may also explain the detection of A(H5N1) viral RNA in cases of A(H5N1) reported in asymptomatic poultry workers in the U.K. that were investigated as part of a surveillance study [14-16].
One human case of A(H5N1) was reported in the United States in April 2024 in an adult dairy farm worker. The individual worked at a farm with sick cows presumed to be infected with HPAI A(H5N1) virus in an area in which cows at other dairy farms were confirmed with HPAI A(H5N1) virus infection. The worker only experienced conjunctivitis without any other signs or symptoms of illness. HPAI A(H5N1) virus was detected in conjunctival and nasopharyngeal swab specimens, and sequence data confirmed clade 2.3.4.4b, genotype B3.13, and close genetic relatedness to viruses detected in other dairy cattle farms in Texas. Oseltamivir was provided for treatment of the individual and for post-exposure prophylaxis of household contacts. Conjunctivitis resolved without other symptoms and household contacts remained well.
Nearly all human cases of HPAI A(H5N1) reported since January 2022 had recent exposure to sick or dead poultry, and no cases of human-to-human HPAI A(H5N1) virus transmission were identified. Fourteen cases (7 children, 7 adults) had severe or critical illness, and seven (3 children, 4 adults) died. Thirteen cases were associated with clade 2.3.4.4b HPAI A(H5N1) virus in 7 countries, and eleven cases were associated or assumed to be associated with clade 2.3.2.1c HPAI A(H5N1) viruses in Cambodia and Vietnam. None of the HPAI A(H5N1) virus genetic sequences contained any known markers of reduced susceptibility to currently recommended FDA-approved influenza antiviral medications.
Genetic data have revealed that when some mammals, including humans, are infected with HPAI A(H5N1) virus, the virus may undergo intra-host evolution resulting in genetic changes that allow more efficient replication in the lower respiratory tract or extrapulmonary tissues [17-19]. Some HPAI A(H5N1) viruses that have infected humans in 2023 and 2024 have also shown the same or similar genetic changes as those identified in wild and captive mammals. For example, sequencing of viruses from specimens collected from human cases identified in Cambodia during October and November 2023, in Vietnam in 2024 and in the dairy farm worker in Texas revealed the presence of the polymerase basic protein 2 (PB2) 627K marker, which is often associated with mammalian adaptation during infection [20]. The HPAI A(H5N1) virus sequenced from the human case in Chile identified in March 2023 had different genetic changes (PB2 591K and 701N) that are also associated with mammalian adaptation [21].
Although these genetic changes may impact mammalian disease outcome, they have not been associated with enhanced transmissibility of the virus to humans. HPAI A(H5N1) viruses do not currently have the ability to easily infect and bind to α2,6-linked sialic acid receptors that are predominant in the human upper respiratory tract [2], which would be needed to increase the risk of transmission to people [22,23].
| Country of Case | Month of illness onset or case detection | Disease Severity and Outcome | Virus Clade by sequencing or associated poultry outbreaks |
|---|---|---|---|
| Cambodia | February 2023 | Critical illness, died | Clade 2.3.2.1c |
| February 2023 | Mild illness | Clade 2.3.2.1c | |
| October 2023 | Critical illness, died | Clade 2.3.2.1c | |
| October 2023 | Critical illness, died | Clade 2.3.2.1c | |
| November 2023 | Critical illness, died | Clade 2.3.2.1c | |
| November 2023 | Mild illness | Clade 2.3.2.1c | |
| January 2024 | Severe illness, survived | Clade 2.3.2.1c | |
| January 2024 | Severe illness, survived | Clade 2.3.2.1c | |
| January 2024 | Critical illness, died | Clade 2.3.2.1c | |
| February 2024 | Severe illness, survived | Not reported | |
| February 2024 | Asymptomatic | Clade 2.3.2.1c | |
| Chile | March 2023 | Critical illness, survived | Clade 2.3.4.4b |
| China | September 2022 | Critical illness, died | Clade 2.3.4.4b |
| January 2023 | Severe illness, outcome not reported | Clade 2.3.4.4b | |
| Ecuador | December 2022 | Critical illness, survived | Clade 2.3.4.4b |
| Spain | September 2022 | Asymptomatic | Clade 2.3.4.4b |
| October 2022 | Asymptomatic | Clade 2.3.4.4b | |
| United Kingdom | January 2022 | Asymptomatic | Clade 2.3.4.4b |
| May 2023 | Asymptomatic | Clade 2.3.4.4b | |
| May 2023 | Asymptomatic | Clade 2.3.4.4b | |
| July 2023 | Asymptomatic | Clade 2.3.4.4b | |
| July 2023 | Asymptomatic | Clade 2.3.4.4b | |
| United States | April 2022 | Mild illness (fatigue) | Clade 2.3.4.4b |
| March 2024 | Mild illness (conjunctivitis) | Clade 2.3.4.4b | |
| Vietnam | October 2022 | Critical illness, survived | Not reported |
| March 2024 | Critical illness, died | Clade 2.3.2.1c |
Since 1997, a total of 909 sporadic human A(H5N1) cases have been reported from 23 countries, caused by different HPAI A(H5N1) virus clades [24,25], with a cumulative case fatality proportion of greater than 50%. Human A(H5N1) cases peaked in 2006 (115 cases, 9 countries) and 2015 (145 cases, 4 countries) primarily due to a large epidemic in Egypt with 136 cases [Figure 1].
Nearly all reported human A(H5N1) cases had poultry exposures, such as to sick or dead poultry or visiting live poultry markets. Rare, limited, and non-sustained instances of human-to-human HPAI A(H5N1) virus transmission likely occurred in a small number of family members following prolonged, close unprotected exposure with a symptomatic case-patient during 2004-2007 in multiple countries [26-29].
Figure 1. Epidemic Curve of Human Cases of A(H5N1) by Illness Onset Date, 1997-2024 by Country (N=909)
Monitoring of persons exposed to HPAI A(H5N1) viruses in the United States
Although few human cases have occurred recently, given widespread infection among poultry and wild birds, people who have job-related or recreational exposures to infected birds or sick or dead mammals are at higher risk of infection.
CDC, in collaboration with state, territorial, and local public health partners, has monitored people exposed to infected birds and poultry, cattle, or other animals beginning with their first exposure and for 10 days after their last exposure, from February 2022 through April 25, 2024:
- Total monitored: more than 8,800 people in 52 jurisdictions.
- Total illnesses reported among monitored persons: nearly 200 people.
- Number positive for influenza A(H5N1) virus: 2 people.
Of the nearly 200 people showing symptoms who were tested for novel influenza A and seasonal influenza viruses along with other respiratory viruses, HPAI A(H5N1) virus genetic material was detected in a respiratory specimen from one person in Colorado who experienced fatigue without any other illness signs or symptoms while participating in poultry culling activities, and in one person in Texas who experienced conjunctivitis without any other illness signs or symptoms while working with sick dairy cattle presumed to be infected with HPAI A(H5N1) virus. [See above section on “Human cases of A(H5N1).”]
U.S. influenza surveillance for human infections with novel influenza A viruses, including HPAI A(H5N1) virus
Human infection with a novel influenza A virus, including HPAI A(H5N1) virus, is a nationally notifiable condition (case definition: Novel Influenza A Virus Infections 2014 Case Definition | CDC)
Influenza testing is widely available in clinical laboratories and health care facilities. Assays in these settings would detect A(H5N1) virus infections as influenza A positive, and a subset of assays would be able to also determine that they are not influenza A virus subtypes H1 or H3 that commonly circulate among humans. Specimens from persons possibly exposed to HPAI A(H5N1) virus or that test positive for influenza A virus but negative for A(H1) and A(H3) subtypes should be forwarded to the appropriate state or local public health laboratory for further testing. CDC should be notified immediately in the event that any clinical specimens from suspected cases test positive for a novel influenza A virus or if the testing results of clinical specimens from suspected cases are inconclusive. Human infection with a novel influenza A virus is a nationally notifiable condition, and currently confirmatory testing is being done only at CDC. Very few specimens have been submitted to CDC for H5 testing since January 2022.
- Seasonal influenza virus detection assays that can also detect novel influenza A viruses are used in 128 public health laboratories in all 50 U.S states.
- Specific diagnostic assays to detect A(H5) viruses are available at 99 public health laboratories in all 50 states.
Per long-standing protocols, upon detection of a virus that tests positive for influenza A but is negative for human H1 or H3 genes, the public health laboratory will rapidly contact CDC and ship the specimen to CDC. Samples that are influenza A positive but negative for human H1 or H3 genes may also be tested for H5 by state public health laboratories and are rapidly sent to CDC for a diagnostic result. An investigation of the case will be initiated, and a case report form will be submitted to CDC through the novel influenza A reporting module.
Activity
Activity
Summary
Summary
Global surveillance and rapid response to human infections
Global surveillance and rapid response to human infections
CDC’s Influenza Division supports surveillance in live bird markets, backyard farms, and wild birds and/or their environments in Bangladesh, Cambodia, China, Guatemala, Kenya, Lao PDR, Peru, Thailand, and Vietnam. Surveillance data highlight the high prevalence and wide range of avian influenza A viruses in birds and help to describe the changing epidemiology of avian influenza A viruses.
In 2022, the Influenza Division tracked more than 50 human infections with avian influenza A viruses reported to the WHO from seven countries in four WHO regions. Most recently, CDC Influenza Division field staff assisted in the rapid response investigations of four human A(H5N1) cases in Cambodia during October and November 2023.
- Influenza virus and illness activity are monitored year-round through a collaborative effort between CDC and many partners, including state, local, and territorial health departments; public health and clinical laboratories; clinics; and emergency departments.
- Human cases of novel influenza A virus infection, which are human infections with non-human influenza A viruses that are different from currently spreading seasonal human influenza A viruses, are nationally notifiable. Every identified case is investigated and reported to CDC.
- CDC is actively looking at multiple influenza indicators during the current situation to monitor for HPAI A(H5N1) viruses, including looking for spread of the virus to, or among people, in jurisdictions where the virus has been identified in people or animals.
CDC’s Influenza Division supports surveillance in live bird markets, backyard farms, and wild birds and/or their environments in Bangladesh, Cambodia, China, Guatemala, Kenya, Lao PDR, Peru, Thailand, and Vietnam. Surveillance data highlight the high prevalence and wide range of avian influenza A viruses in birds and help to describe the changing epidemiology of avian influenza A viruses.
In 2022, the Influenza Division tracked more than 50 human infections with avian influenza A viruses reported to the WHO from seven countries in four WHO regions. Most recently, CDC Influenza Division field staff assisted in the rapid response investigations of four human A(H5N1) cases in Cambodia during October and November 2023.
- Influenza virus and illness activity are monitored year-round through a collaborative effort between CDC and many partners, including state, local, and territorial health departments; public health and clinical laboratories; clinics; and emergency departments.
- Human cases of novel influenza A virus infection, which are human infections with non-human influenza A viruses that are different from currently spreading seasonal human influenza A viruses, are nationally notifiable. Every identified case is investigated and reported to CDC.
- CDC is actively looking at multiple influenza indicators during the current situation to monitor for HPAI A(H5N1) viruses, including looking for spread of the virus to, or among people, in jurisdictions where the virus has been identified in people or animals.
Virological assessments
Virological assessments
Because influenza viruses have a high error rate during replication and rapidly evolve, CDC continually conducts genetic analyses of viruses to identify changes that may impact virus phenotypes such as antigenicity, antiviral susceptibility, transmissibility, and/or pathogenesis. Genetic analysis also is performed to assess changes that may impact diagnostic test performance.
Because influenza viruses have a high error rate during replication and rapidly evolve, CDC continually conducts genetic analyses of viruses to identify changes that may impact virus phenotypes such as antigenicity, antiviral susceptibility, transmissibility, and/or pathogenesis. Genetic analysis also is performed to assess changes that may impact diagnostic test performance.
Diagnostics
Diagnostics
Various CDC influenza virus diagnostic real time RT-PCR tests detect typical human (seasonal) viruses or novel influenza A viruses (e.g., H5, H7) that may infect people through zoonotic transmission. These diagnostic tests are used in all 50 U.S states and globally. Additionally, there are CDC diagnostic tests that specifically detect A(H5) viruses, which are available in public health laboratories in all 50 U.S. states and international laboratories.
Most commercial assays used for human influenza virus testing are likely to detect HPAI A(H5N1) viruses because they target conserved proteins.
Various CDC influenza virus diagnostic real time RT-PCR tests detect typical human (seasonal) viruses or novel influenza A viruses (e.g., H5, H7) that may infect people through zoonotic transmission. These diagnostic tests are used in all 50 U.S states and globally. Additionally, there are CDC diagnostic tests that specifically detect A(H5) viruses, which are available in public health laboratories in all 50 U.S. states and international laboratories.
Most commercial assays used for human influenza virus testing are likely to detect HPAI A(H5N1) viruses because they target conserved proteins.
Candidate vaccine virus development
Candidate vaccine virus development
The development of influenza candidate vaccine viruses (CVVs), coordinated by WHO, remains an essential component of the overall global strategy for influenza pandemic preparedness. A library of H5 candidate vaccine viruses (CVV) has been produced with additional recommendations for development during bi-annual vaccine consultation meetings (See Summary of status of development and availability of A(H5N1) candidate vaccine viruses and potency testing reagents [315 KB, 6 pages] and Zoonotic influenza: candidate vaccine viruses and potency testing reagents). The CDC Influenza Risk Assessment Tool is also used to help prioritize HPAI A(H5) viruses for development of CVVs.
A/Astrakhan/3212/2020-like and A/American wigeon/South Carolina/22-000345-001/2021-like CVVs closely related HPAI A(H5N1) (clade 2.3.4.4b) viruses circulating in North America have been developed and are available for vaccine manufacturers. The two CVVs produced by the U.S. CDC (i.e., IDCDC-RG71A and IDCDC-RG78A) and one CVV produced by U.S. FDA (CBER-RG8A) encode hemagglutinin (HA) proteins that are nearly identical or identical to the HA of most recent clade 2.3.4.4b H5N1 viruses detected in birds and mammals, including dairy cattle, and could be used to produce a vaccine for people if needed. One additional clade 2.3.4.4b H5N1 CVV has been recommended for development as part of pandemic preparedness. In addition to CVVs targeting clade 2.3.4.4b viruses, CVVs have been developed for clade 2.3.2.1c viruses, such as those that have infected humans in Cambodia during 2023. Antigenic testing demonstrates that two existing clade 2.3.2.1 CVVs, NIBRG-301 (A/duck/Vietnam/NCVD-1584/2012-like) and IDCDC-RG75A (A/chicken/Ghana/20/2015-like), will offer protection against the viruses identified in Cambodia in 2023.
The development of influenza candidate vaccine viruses (CVVs), coordinated by WHO, remains an essential component of the overall global strategy for influenza pandemic preparedness. A library of H5 candidate vaccine viruses (CVV) has been produced with additional recommendations for development during bi-annual vaccine consultation meetings (See Summary of status of development and availability of A(H5N1) candidate vaccine viruses and potency testing reagents [315 KB, 6 pages] and Zoonotic influenza: candidate vaccine viruses and potency testing reagents). The CDC Influenza Risk Assessment Tool is also used to help prioritize HPAI A(H5) viruses for development of CVVs.
A/Astrakhan/3212/2020-like and A/American wigeon/South Carolina/22-000345-001/2021-like CVVs closely related HPAI A(H5N1) (clade 2.3.4.4b) viruses circulating in North America have been developed and are available for vaccine manufacturers. The two CVVs produced by the U.S. CDC (i.e., IDCDC-RG71A and IDCDC-RG78A) and one CVV produced by U.S. FDA (CBER-RG8A) encode hemagglutinin (HA) proteins that are nearly identical or identical to the HA of most recent clade 2.3.4.4b H5N1 viruses detected in birds and mammals, including dairy cattle, and could be used to produce a vaccine for people if needed. One additional clade 2.3.4.4b H5N1 CVV has been recommended for development as part of pandemic preparedness. In addition to CVVs targeting clade 2.3.4.4b viruses, CVVs have been developed for clade 2.3.2.1c viruses, such as those that have infected humans in Cambodia during 2023. Antigenic testing demonstrates that two existing clade 2.3.2.1 CVVs, NIBRG-301 (A/duck/Vietnam/NCVD-1584/2012-like) and IDCDC-RG75A (A/chicken/Ghana/20/2015-like), will offer protection against the viruses identified in Cambodia in 2023.
Vaccines
Vaccines
Influenza A viruses of pandemic potential change over time and multiple new strains circulate in animals every year without leading to sustained human-to-human transmission. The U.S. government has a preparedness program that enables a rapid response to influenza viruses as they evolve. As part of this program, the Biomedical Advanced Research and Development Authority (BARDA) works with private industry partners to make and test small quantities of updated vaccines that match new influenza A viruses with pandemic potential as they emerge in case any of them result in sustained human-to-human transmission, while at the same time, supporting manufacturing capacity to allow for large-scale vaccine production when needed.
Influenza A viruses of pandemic potential change over time and multiple new strains circulate in animals every year without leading to sustained human-to-human transmission. The U.S. government has a preparedness program that enables a rapid response to influenza viruses as they evolve. As part of this program, the Biomedical Advanced Research and Development Authority (BARDA) works with private industry partners to make and test small quantities of updated vaccines that match new influenza A viruses with pandemic potential as they emerge in case any of them result in sustained human-to-human transmission, while at the same time, supporting manufacturing capacity to allow for large-scale vaccine production when needed.
Limitations of the Report
This report is subject to the following limitations. First, the number of reported human infections with HPAI A(H5N1) viruses is small. Conclusions regarding virus characterization analyses, transmissibility from animals to people, transmissibility among people, and clinical spectrum of illness in people should be interpreted in light of this small number. Second, detailed exposure information was not available for all exposed persons or for those being monitored for illness after exposure to HPAI A(H5N1) virus-infected wild birds, poultry, backyard flocks, and other animals, including dairy cattle in the United States. As of the date of this report, understanding of HPAI A(H5N1) virus infections of cattle is very limited. Thus, we are not able to assess the impact of exposure variables such as duration of exposure, nature of exposure (e.g., direct vs. indirect contact), and use of personal protective equipment on infection risk among persons with confirmed HPAI A(H5N1) virus infection or those being monitored after exposures to any animals confirmed or suspected with HPAI A(H5N1) virus infection.
Conclusions
- To date, CDC analyses of clade 2.3.4.4b HPAI A(H5N1) viruses detected in wild birds, poultry, and sporadically in mammals, including in dairy cattle, since late 2021 indicate that these viruses all have a high degree of genetic identity with each other and no significant mammalian adaptive substitutions, insertions, or deletions have been identified, particularly in the HA gene, which is important for zoonotic and subsequent human-to-human transmission.
- Considering the high prevalence of HPAI A(H5N1) viruses detected in wild birds and poultry worldwide, spillover into mammals (including carnivores that may feed on infected animals) and additional sporadic zoonotic infections are anticipated among people with exposures to infected sick or dead poultry, wild birds, or other infected animals.
- HA clade 2.3.4.4b A(H5N1) viruses currently circulating in wild birds and poultry worldwide lack the ability to preferentially bind to the types of sialic acid receptors that are predominant in the upper respiratory tract of humans and therefore do not currently have the ability to easily infect or transmit among people.
- Despite extensive worldwide spread of influenza A(H5N1) viruses in wild birds and poultry in recent years, only a small number of sporadic human infections with 2.3.4.4b or clade 2.3.2.1c H5N1 viruses have been reported since 2022; nearly all cases had recent exposure to poultry and no cases of human-to-human influenza A(H5N1) virus transmission have been identified.
While CDC’s assessment is that the overall threat of HA clade 2.3.4.4b A(H5N1) viruses to public health is currently low, the widespread geographic prevalence of infected birds and poultry raises the potential for exposures and infections of humans and other mammals that could result in viral evolution or reassortment events which might change the current risk assessment. Vigilance and ongoing surveillance of HPAI A(H5N1) viruses circulating in wild birds, poultry, and in sporadic infections of mammals and people worldwide is critical to monitor the public health risk and to detect genetic changes (particularly in the HA gene) that would change CDC’s risk assessment.
References
- Bevins SN, Shriner SA, Cumbee JC Jr, Dilione KE, Douglass KE, Ellis JW et al. Intercontinental Movement of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4 Virus to the United States, 2021. Emerg Infect Dis. 2022 May;28(5):1006-1011.
- Kandeil A, Patton C, Jones JC, Jeevan T, Harrington WN, Trifkovic S et al. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat Commun. 2023 May 29;14(1):3082.
- World Health Organization. Antigenic and genetic characteristics of zoonotic influenza A viruses and development of candidate vaccine viruses for pandemic preparedness. February 2023. https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-northern-hemisphere-recommendation-2023-2024/20230224_zoonotic_recommendations.pdf?sfvrsn=38c739fa_4 [1.17 MB, 12 pages]
- European Food Safety Authority, European Centre for Disease Prevention and Control, European Union Reference Laboratory for Avian Influenza. Avian influenza overview March – April 2023. EFSA J 2023 Jun 7;21(6):e08039.
- Ariyama N, Pardo-Roa C, Muñoz G, Aguayo C, Ávila C, Mathieu C, Almonacid LI, Medina RA, Brito B, Johow M, Neira V. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Wild Birds, Chile. Emerg Infect Dis. 2023 Sep;29(9):1842-1845. Doi: 10.3201/eid2909.230067. Epub 2023 Jul 24. PMID: 37487166; PMCID: PMC10461661
- Leguia M, Garcia-Glaessner A, Muñoz-Saavedra B, Juarez D, Barrera P, Calvo-Mac C, Jara J, Silva W, Ploog K, Amaro L, Colchao-Claux P, Johnson CK, Uhart MM, Nelson MI, Lescano J. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat Commun. 2023 Sep 7;14(1):5489. Doi: 10.1038/s41467-023-41182-0. PMID: 37679333; PMCID: PMC10484921.
- Pan American Health Organization. Epidemiological Update Outbreaks of avian influenza caused by influenza A(H5N1) in the Region of the Americas. 9 August 2023. https://www.paho.org/en/documents/epidemiological-update-outbreaks-avian-influenza-caused-influenza-ah5n1-region-americas-0
- Global Avian Influenza Viruses with Zoonotic Potential situation update, 28 March 2024. https://www.fao.org/animal-health/situation-updates/global-aiv-with-zoonotic-potential/en
- World Organisation for Animal Health. Wildlife under threat as avian influenza reaches Antarctica. 13 March 2024. https://www.woah.org/en/wildlife-under-threat-as-avian-influenza-reaches-antarctica/
- Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RAM, Amonsin A, Payungporn S et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis. 2004 Dec;10(12):2189-91.
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Pariyothorn N, Payungporn S et al. Fatal avian influenza A H5N1 in a dog. Emerg Infect Dis. 2006 Nov;12(11):1744-7.
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N et al. Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis. 2006 Apr;12(4):681-3.
- Aznar E, Casas I, González Praetorius A, Ruano Ramos MJ, Pozo F, Sierra Moros MJ et al.Influenza A(H5N1) detection in two asymptomatic poultry farm workers in Spain, September to October 2022: suspected environmental contamination. Euro Surveill. 2023 Feb;28(8):2300107. Doi: 10.2807/1560-7917.ES.2023.28.8.2300107. https://pubmed.ncbi.nlm.nih.gov/36820643/
- World Health Organization. Avian Influenza A(H5N1) – United Kingdom of Great Britain and Northern Ireland. 30 May 2023. Accessed at: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON468
- Capelastegui F, Smith J, Kumbang J, Humphreys C, Padfield S, Turner J et al. Pilot of asymptomatic swabbing of humans following exposures to confirmed avian influenza A(H5) in avian species in England, 2021/2022. Influenza Other Respir Viruses. 2023 Aug 23;17(8):e13187. Doi: 10.1111/irv.13187 https://pubmed.ncbi.nlm.nih.gov/37638093/
- UK Health Security Agency. Investigation into the risk to human health of avian influenza (influenza A H5N1) in England: technical briefing 5. Updated 14 July 2023. Accessed at: Investigation into the risk to human health of avian influenza (influenza A H5N1) in England: technical briefing 5 – GOV.UK (www.gov.uk)
- Gabriel G, Czudai-Matwich V, Klenk HD. Adaptive mutations in the H5N1 polymerase complex. Virus Res. 2013 Dec 5;178(1):53-62. Doi: 10.1016/j.virusres.2013.05.010.
- Bogs J, Kalthoff D, Veits J, Pavlova S, Schwemmle M, Mänz B et al. Reversion of PB2-627E to -627K during replication of an H5N1 Clade 2.2 virus in mammalian hosts depends on the origin of the nucleoprotein. J Virol. 2011 Oct;85(20):10691-8. Doi: 10.1128/JVI.00786-11.
- Agüero M, Monne I, Sánchez A, Zecchin B, Fusaro A, Ruano MJ et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill. 2023 Jan;28(3):2300001. Doi: 10.2807/1560-7917.ES.2023.28.3.2300001.
- Technical Update: Summary Analysis of Genetic Sequences of Highly Pathogenic Avian Influenza A(H5N1) Viruses in Texas. April 2, 2024. Accessed at: https://www.cdc.gov/flu/avianflu/spotlights/2023-2024/h5n1-analysis-texas.htm
- Human Infection with highly pathogenic avian influenza A(H5N1) virus in Chile. Accessed at: https://www.cdc.gov/flu/avianflu/spotlights/2022-2023/chile-first-case-h5n1-addendum.htm
- Van Riel D, den Bakker MA, Leijten LM, Chutinimitkul S, Munster VJ, de Wit E et al. Seasonal and pandemic human influenza viruses attach better to human upper respiratory tract epithelium than avian influenza viruses. Am J Pathol. 2010 Apr;176(4):1614-8. Doi: 10.2353/ajpath.2010.090949.
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006 Mar 23;440(7083):435-6. Doi: 10.1038/440435a.
- Lai S, Qin Y, Cowling BJ, Ren X, Wardrop NA, Gilbert M et al. Global epidemiology of avian influenza A H5N1 virus infection in humans, 1997-2015: a systematic review of individual case data. Lancet Infect Dis. 2016 Jul;16(7):e108-e118. Doi: 10.1016/S1473-3099(16)00153-5.https://pubmed.ncbi.nlm.nih.gov/27211899/
- World Health Organization. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2024, 28 March 2024. Accessed at: https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who–2003-2024-28-march-2024
- Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med. 2005 Jan 27;352(4):333-40. Doi: 10.1056/NEJMoa044021.https://pubmed.ncbi.nlm.nih.gov/15668219/
- Wang H, Feng Z, Shu Y, Yu H, Zhou L, Zu R et al. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 2008 Apr 26;371(9622):1427-34. Doi: 10.1016/S0140-6736(08)60493-6.https://pubmed.ncbi.nlm.nih.gov/18400288/
- WHO Disease Outbreak News. 2006 – Indonesia. 31 May 2006. Accessed at: https://www.who.int/emergencies/disease-outbreak-news/item/2006_05_31-en
- World Health Organization. Human cases of avian influenza A (H5N1) in North-West Frontier Province, Pakistan, October-November 2007. Wkly Epidemiol Rec. 2008 Oct 3;83(40):359-64.
Resources
- How CDC is monitoring influenza data among people to better understand the current avian influenza A (H5N1) situation | Avian Influenza (Flu)
- Highly Pathogenic Avian Influenza A(H5N1) Virus in Animals: Interim Recommendations for Prevention, Monitoring, and Public Health Investigations | Avian Influenza (Flu) (cdc.gov)
- Case Definitions for Investigations of Human Infection with Avian Influenza A Viruses in the United States
- Recommendations for Worker Protection and Use of Personal Protective Equipment (PPE) to Reduce Exposure to Novel Influenza A Viruses Associated with Severe Disease in Humans | Avian Influenza (Flu) (cdc.gov)
- Interim Guidance on Influenza Antiviral Chemoprophylaxis of Persons Exposed to Birds with Avian Influenza A Viruses Associated with Severe Human Disease or with the Potential to Cause Severe Human Disease
- Interim Guidance on Follow-up of Close Contacts of Persons Infected with Novel Influenza A Viruses and Use of Antiviral Medications for Chemoprophylaxis
- Brief Summary for Clinicians: Evaluating and Managing Patients Exposed to Birds Infected with Avian Influenza A Viruses of Public Health Concern
- Interim Guidance on Testing and Specimen Collection for Patients with Suspected Infection with Novel Influenza A Viruses with the Potential to Cause Severe Disease in Humans
- Interim Guidance for Infection Control Within Healthcare Settings When Caring for Confirmed Cases, Probable Cases, and Cases Under Investigation for Infection with Novel Influenza A Viruses Associated with Severe Disease | Avian Influenza (Flu) (cdc.gov)
- Interim Guidance on the Use of Antiviral Medications for Treatment of Human Infections with Novel Influenza A Viruses Associated with Severe Human Disease
- Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses (cdc.gov) – December 29, 2023
- Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses (cdc.gov) – October 27, 2023
- Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses (cdc.gov) – October 5, 2023
- Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses (cdc.gov) – July 7, 2023
- Addendum: Human Infection with highly pathogenic avian influenza A(H5N1) virus in Chile (cdc.gov) – April 17, 2023
- Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses (cdc.gov) – March 17, 2023
Bird Flu Current Situation Summary | Avian Influenza (Flu) (cdc.gov)
Novel Influenza A Virus Infections (cdc.gov) An interactive dashboard of all novel influenza A virus infections in humans reported in the United States since 2010
- Reported Human Infections with Avian Influenza A Viruses
- Past Examples of Probable Limited, Non-Sustained, Person-to-Person Spread of Avian Influenza A Viruses
- Highlights in the History of Avian Influenza (Bird Flu) Timeline – 2020-2024
- Information for People Exposed to Birds Infected with Avian Influenza Viruses
- Prevention and Antiviral Treatment of Bird Flu Viruses in People
- Recommendations for Worker Protection and Use of Personal Protective Equipment (PPE) to Reduce Exposure to Novel Influenza A Viruses Associated with Severe Disease in Humans
- CDC Health Advisory, April 29, 2022 – Highly Pathogenic Avian Influenza A(H5N1) Virus: Recommendations for Human Health Investigations and Response [986 KB, 6 pages]
- Public Health Monitoring Plan for USDA/APHIS Responders to Detections of Avian Influenza Virus in Poultry [353 KB, 18 pages]
References to non-CDC sites are provided as a service and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed were current as of the date of publication.
