Diagnostics for Detecting H7N9 Using rRT-PCR
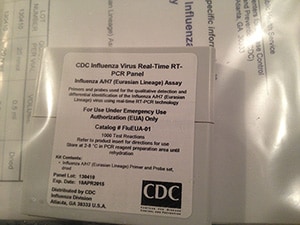
CDC has developed diagnostic test materials to specifically detect the avian influenza A(H7N9) virus that was first identified in China in 2013. These test materials include rRT-PCR reagents (primers and probes), controls and an rRT-PCR test protocol.
Reagents and Controls for A(H7N9)
These materials are available for ordering from the International Reagent Resource (IRR) website.
Note: Testing with the CDC Human Influenza Virus Real-Time RT-PCR Diagnostic Panel-Influenza A/H7 (Eurasian Lineage) Assay should not be performed unless the patient meets clinical and epidemiologic criteria for testing suspect specimens.
U.S. Domestic Ordering- (Kit name EUA)
CDC’s International Reagent Resource (IRR) provides registered users with reagents, tools, and information for studying and detecting influenza virus and other pathogens, including A(H7N9). Laboratories registered with IRR may order the CDC Human Influenza Virus Real-Time RT-PCR Diagnostic Panel, Influenza A/H7 (Eurasian Lineage) Assay (EUA) (Catalog No. FluEUA-01). This kit contains both the oligonucleotide primers and a dual-labeled hydrolysis (TaqMan®) probe used in rRT-PCR for the in vitro qualitative detection and characterization of human influenza A/H7 (Eurasian Lineage) viruses from viral RNA in respiratory specimens from patients presenting with influenza-like illness (ILI) and from virus culture and an Influenza A/H7 (Eurasian Lineage) Positive Control (EuH7PC). These reagents are intended for use in conjunction with the CDC Human Influenza Virus Real-Time RT-PCR Diagnostic Panel, Influenza A/B Typing Kit (IVD) (Catalog No. FluIVD03-9) that is currently used by U.S. public health laboratories. Non-public health entities can obtain primer sequences or testing protocols from the CLSIS website below.
International Ordering- (Kit Name RUO)
International public health laboratories registered with IRR may order the CDC Real-Time RT-PCR Influenza Virus A/H7 (Eurasian Lineage) Assay (RUO) (Catalog No. FluRUO-07) and the CDC Influenza A/H7 (Eurasian Lineage) Positive Control (EuH7PC) (RUO) (Catalog No. KK0818). To order the A(H7N9) reagents, NICs and national public health laboratories are encouraged to register with IRR by visiting the IRR’s Registration page if they have not done so already. Requests from National Influenza Centers (NICs) and national public health laboratories will be processed as soon as possible. For more information on what to expect with regard to international shipping of IRR reagents, users may refer to IRR FAQ’s page or contact IRR’s customer service team.
Emergency Use Authorization (EUA)
On April 22, 2013, the FDA issued an Emergency Use Authorization (EUA) for the CDC Human Influenza Virus Real-Time RT-PCR Diagnostic Panel-Influenza A/H7 (Eurasian Lineage) Assay. More information is available from the FDA website. Note that issuance of this EUA does NOT indicate that an actual public health emergency exists in the United States. Distribution of diagnostic test kits domestically is a preparedness measure. For information on the latest available FDA H7 molecular assays via EUA, see the FDA website. These EUAs will cease to be effective when the declaration of emergency is terminated under section 564(b)(2) of the Act or when the EUA is revoked under section 564(g) of the Act.
A(H7N9) Test Protocols
CDC’s A(H7N9) rRT-PCR test protocol is available via CDC’s Laboratory Support of Influenza Surveillance (CLSIS) Sharepoint website. The CLSIS SharePoint site provides technical support and guidance for influenza surveillance testing. As a registered user, laboratories will have access to influenza testing protocols as well as other technical support resources. All future updates and communications will be provided via this SharePoint site.
For further information or assistance, an email can be sent to clsis@cdc.gov.
