Containment Strategy
Interim Guidance for a Public Health Response to Contain Novel or Targeted Multidrug-resistant Organisms (MDROs)
The Containment Strategy Guidelines address the initial response to new identifications of novel and targeted MDROs, such as Candida auris and carbapenemase-producing Enterobacterales, Pseudomonas spp., and Acinetobacter. If you are looking for the 2006 Management of Multidrug-Resistant Organisms in Healthcare Settings Guideline, see the Infection Control Website.
This document is intended for use by state, local, territorial, and tribal health departments and healthcare facilities. It was developed with input from state and local health departments and serves as general guidance for the initial response following the identification of novel or targeted multidrug-resistant organisms (MDROs) or resistance mechanisms. Response recommendations are for MDROs that are in pre-endemic stages of spread, for which a public health response to identified cases is an important strategy to limit transmission. Recommendations are not inclusive of all the actions that might be required for the control of an outbreak (e.g., sustained transmission within a facility or region).
Please refer to the complementary document, Public Health Strategies to Prevent the Spread of Novel and Targeted Multidrug-resistant Organisms (MDROs), for information about the development, implementation, and coordination of activities designed to prevent transmission of targeted MDROs at all epidemiologic stages (from pre-introduction through endemicity) across healthcare facilities within a jurisdiction. Combining prevention and response strategies has the potential to be more effective than either strategy alone.

Updates to the 2019 Interim Guidance:
- Updated definitions for organism/mechanism tiers.
- Tier 1 is now limited to novel organisms and/or resistance mechanisms that have never (or very rarely) been identified in the United States and for which experience is extremely limited, thus requiring a more extensive evaluation. Isolates that are not susceptible to any available antimicrobials, but whose transmission dynamics are well-known, are now classified as Tier 2. Note that this classification change does not change overall recommended response activities when a pan-not susceptible isolate is identified.
- Added Endemic (Tier 4) to reflect jurisdictions where organisms are endemic.
- Expanded the “Response Recommendations by Tier” to include additional details.
- Revised Tier 3 approaches to include steps to transition from response to prevention strategies.
- Added a section to describe actions that should be taken following the identification of a targeted MDRO in a region where that organism (or mechanism) is endemic.
- The section “Containment Strategies for Healthcare Facilities at High Risk for Transmission of MDROs” has been superseded by the Interim Guidance for Public Health Measures to Prevent the Spread of Novel and Targeted Multidrug-resistant Organisms (MDROs).
Goals of initial containment response include:
- Identify affected patients.
- Ensure appropriate control measures are promptly implemented to limit further spread.
- Determine if transmission within a healthcare facility and dissemination to other facilities are occurring (Tiers 1-2).
- Characterize novel organisms or mechanisms to guide further response actions, patient management, and future responses.
- Coordinate response with ongoing prevention activities (e.g., MDRO education, infection prevention and control improvement initiatives, routine colonization screening, and improved interfacility communication) in the region.
In addition to this general guidance, further pathogen-specific guidance for some MDROs can be found here:
- Vancomycin-resistant Staphylococcus aureus [PDF – 20 pages]
- Carbapenem-resistant Enterobacterales
- Candida auris
General Recommendations
Healthcare facilities and laboratories should contact state or local public health authorities promptly when targeted resistant organisms (e.g., pan-not susceptible organism or mechanisms are identified (e.g., New Delhi Metallo-β-lactamase [NDM]-producing Enterobacterales).
Health departments should use the expanded capacity for antimicrobial resistance-related laboratory testing offered through the Antimicrobial Resistance Laboratory Network (e.g., mechanism testing for carbapenemase-resistant Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii clinical isolates, Candida species identification, carbapenemase-producing organism, and Candida auris colonization screening) and should understand the availability of specific testing and processes to coordinate specimen submission to local, state, and regional public health laboratories.
All testing of clinical and surveillance specimens collected for novel and targeted MDRO prevention and response should be performed under applicable regulations for the collection, testing, and reporting of results to patients and healthcare providers.
Health Departments conducting these investigations are encouraged to consult with CDC by contacting the healthcare outbreak duty officer at haioutbreak@cdc.gov.
Definitions
For this guidance, the term ‘healthcare facility’ refers to all acute care hospitals and post-acute care facilities that care for patients or residents who remain overnight and require medical care, nursing care or rehabilitation services. This generally excludes assisted living facilities.[1]. For the purposes of this document and the companion document Interim Guidance for Public Health Measures to Prevent the Spread of Novel and Targeted Multidrug-resistant Organisms (MDROs), these stages are:
- No cases identified.
- Limited spread: Sporadic cases or sporadic clusters of epidemiologically linked cases in single facilities or in pairs of facilities that frequently share patients (e.g., acute care hospital (ACH) and long-term acute care hospital (LTACH) or LTACH and skilled nursing facility (SNF)).
- Moderate spread: Cluster or clusters of epidemiologically linked cases identified across multiple facilities that frequently share patients (i.e., cases are primarily limited to a single patient transfer network).
- Advanced spread: Clusters of cases identified across facilities in different patient transfer networks, suggesting transmission across networks.
- Endemicity: Cases are regularly identified in healthcare facilities across the region, including those in different transfer networks. Cases primarily occur in patients admitted from facilities in the region, suggesting that transmission is sustained without new importations from outside the area.
When assessing the epidemiologic stage of an organism or mechanism, consider the most recent, available information, such as the prior 6 months. The epidemiologic stage of an organism or mechanism may change due to rapid spread, or due to additional information gained from public health response or prevention activities.
[1] Grundmann H, Livermore DM, Giske CG, et al. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill. Nov 18 2010;15(46)doi:10.2807/ese.15.46.19711-en
The following describes criteria for different categories of organisms and resistance mechanisms (Tiers 1-3) and the recommended approach to each. Definitions of each tier are accompanied by examples. Tier 1 organisms and mechanisms are novel to the United States. Tier 2 and 3 organisms and mechanisms are targeted MDROs and health departments should use local epidemiology to guide the assignment of organisms to these tiers.
Tier 1 organisms:
This category encompasses organisms or resistance mechanisms that have never (or very rarely) been identified in the United States and for which experience is extremely limited. A more extensive evaluation is needed to define the risk for transmission and the extent of spread. Examples of Tier 1 organisms and mechanisms include the initial identifications of Candida auris and mcr-1-carrying Enterobacterales in the United States. After the risk for transmission and extent of spread are well-defined, these organisms are typically moved to lower tiers.
Tier 2 organisms:
Organisms in this group include (1) MDROs that are primarily associated with healthcare settings and are not commonly identified in the region and (2) organisms for which no current treatment options exist (pan-not susceptible) and that have the potential to spread more widely within a region (e.g., have plasmid-mediated resistance mechanisms). These organisms might be more common in other areas of the United States. Information is available about how transmission of these organisms occurs and the groups primarily at risk.
Generally, these have either not been previously identified in the region or have been limited to sporadic cases or small outbreaks (i.e., correspond to “not detected” or “limited to moderate spread” epidemiologic stages). However, these MDROs might be found more commonly in other areas of the United States or even in other regions or patient sharing networks within the same jurisdiction. In most of the U.S., carbapenem-resistant Enterobacterales (CRE) with OXA-48 or metallo-β-lactamase carbapenemases (e.g., New Delhi Metallo-β-lactamase (NDM), Verona-integron-mediated carbapenemase (VIM), and imipemenemase (IMP)) and carbapenemase-producing Pseudomonas spp. meet the Tier 2 criteria. In some areas of the United States, carbapenem-resistant Enterobacterales producing Klebsiella pneumoniae carbapenemase (KPC-CRE) and C. auris also meet the Tier 2 criteria because they are not commonly identified.
Tier 3 organisms:
Organisms in this group include MDROs targeted by the facility or region for epidemiologic importance that have been identified frequently across a region, indicating advanced spread, but are not considered endemic. These organisms might be more common in other areas of the United States. Information is available about how transmission of these organisms occurs and the groups primarily at risk.
Examples include KPC-CRE and Acinetobacter baumannii with plasmid-mediated oxacillinases with carbapenemase activity (e.g., OXA-23-like, OXA-24/40-like) and C. auris in certain regions of the United States where these organisms are more regularly identified but are not endemic.
Endemic (Tier 4) organisms:
These MDROs are endemic in a region and have been targeted by public health for their clinical significance and potential to spread rapidly (e.g., to other regions where they are less common or from healthcare settings into the community).
Relationship between Prevention and Response Activities
MDRO prevention strategies described in Public Health Strategies to Prevent the Spread of Novel and Targeted Multidrug-resistant Organisms (MDROs) should be considered at all epidemic stages. These encompass ongoing interventions across a group of facilities or geographic regions that are implemented based on the local epidemiology and healthcare facility characteristics. The response strategies described in the Interim Guidance for a Public Health Response to Contain Novel or Targeted Multidrug-resistant Organisms (MDROs) are intended for pre-endemic stages of spread and are implemented following the identification of a targeted MDRO. They are time-limited and focused on facilities that have recently cared for patients or residents with targeted MDROs or are epidemiologically linked to facilities that cared for these patients or residents. Ideally, response activities should be layered on existing prevention interventions. Combining these strategies has the potential to be more effective than either strategy implemented in isolation.
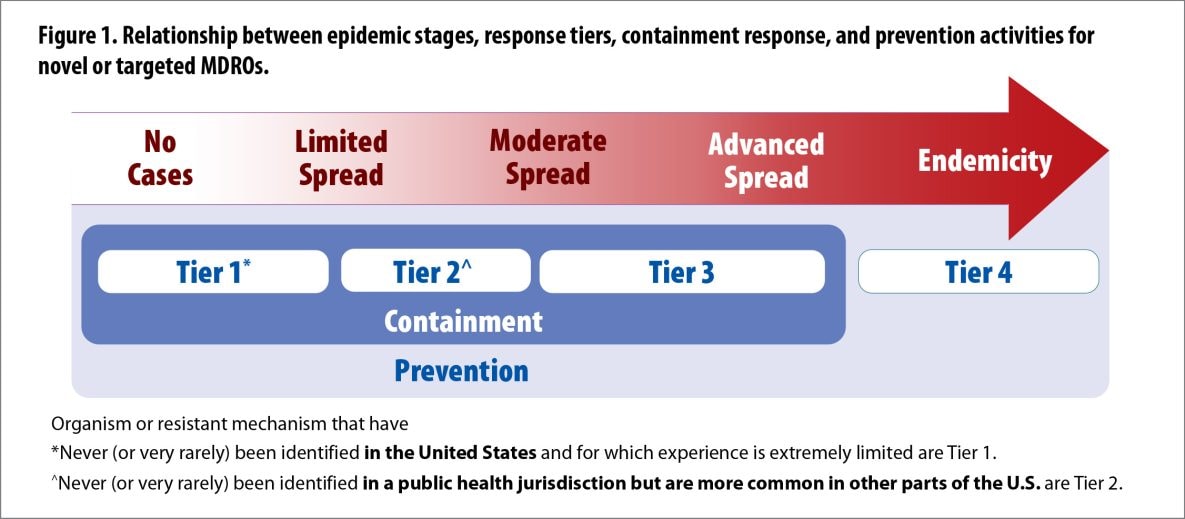
- Figure 1. Relationship between epidemic stages, response tiers, containment response, and prevention activities for novel or targeted MDROs.
Tables
Summary of Response Recommendations for MDRO Containment by Tier
| Epidemic Stages | No cases identified Limited spread | Limited to moderate spread | Moderate to advanced spread | Epidemic |
|---|---|---|---|---|
| Containment Tier | Tier 1 | Tier 2 | Tier 3 | Tier 4 |
| Tier definition | Organisms or resistance mechanisms never or very rarely identified in the United States | Mechanisms and organisms not regularly found in a region. Pan-not susceptible organisms with the potential for wider spread in a region |
Mechanisms and organisms regularly (i.e., frequently) found in a region but not endemic. | Mechanisms and organisms that are endemic. |
| Response Elements | Prioritize prevention; containment principles generally do not apply. | |||
|---|---|---|---|---|
| Healthcare Investigation1 | ||||
| Review the patient’s healthcare exposures prior to and after the positive culture1 | ALWAYS
Typical review period: 30 days prior to culture collection to present |
ALWAYS
Typical review period: 30 days prior to culture collection to present |
ALWAYS
Typical review period: Current admission and sometimes immediately prior admission |
|
| Contact Investigation1 | ||||
| Screening of healthcare contacts (i.e., residents and patients) 2 | ALWAYS | ALWAYS | USUALLY | |
| Household Contact Screening | USUALLY | RARELY | RARELY | |
| Healthcare Personnel Screening | USUALLY | RARELY | RARELY | |
| Additional Actions if Transmission Identified in Healthcare | ||||
|---|---|---|---|---|
| Recurring response-driven point prevalence surveys3 | ALWAYS | ALWAYS | RARELY | |
| Evaluate potential spread to healthcare facilities that regularly share patients with the index healthcare facility4 | USUALLY | USUALLY | RARELY | |
| Clinical Laboratory Surveillance | ||||
|---|---|---|---|---|
| Retrospective lab surveillance6 | ALWAYS | ALWAYS | RARELY | |
| Prospective lab surveillance5 | ALWAYS | ALWAYS | ALWAYS | |
| Environmental Cultures | ||||
|---|---|---|---|---|
| Environmental Sampling | SOMETIMES | RARELY | RARELY | |
| Infection Control Measures | ||||
|---|---|---|---|---|
| Notify healthcare providers; promptly implement appropriate transmission-based precautions | ALWAYS | ALWAYS | ALWAYS | |
| Infection Control Assessment with observations of practice | ALWAYS | ALWAYS | SOMETIMES | |
| Clear communication of patient status with transferring facilities | ALWAYS | ALWAYS | ALWAYS | |
| Link to Prevention Activities: All Novel and Targeted MDROs | ||||
PPS: point prevalence survey
*ALWAYS: actions that should be a part of every response for a given response tier.
*USUALLY: actions that are indicated for most responses, but that might not be applicable for all novel and targeted MDRO responses for a given response tier.
*SOMETIMES: actions that that might apply, with implementation informed based on the specific scenario (including the setting and organism).
*RARELY: actions that generally are not performed for novel and targeted MDRO responses for organisms of a given response tier, but could be considered in certain situations. Decisions about implementing actions labeled “sometimes” or “rarely” should be made in consultation with public health.
1For Tier 1 and 2 organisms/mechanisms, healthcare exposures and healthcare contacts from the 30 days prior to identification of the target organism should be investigated unless information is available about the time the organism was most likely acquired. This includes any healthcare facility where the patient had an overnight stay during that time period. In some investigations; outpatient facilities and emergency departments might also be included. For Tier 3 organisms, investigation of healthcare exposures and healthcare contacts is generally limited to the current admission; however, if the admission immediately prior was within 30 days of specimen collection and occurred at a facility where the organism has never or rarely been identified, this may also be included in the investigation.
2This may include targeted screening of contacts at highest risk for acquisition and/or unit point prevalence surveys.
3Periodic (e.g., every two weeks) response-driven PPS should be conducted until transmission is controlled, defined as two consecutive PPS with no new cases identified or, in facilities with high colonization pressure, substantially decreased transmission. If high levels of transmission persist across multiple point prevalence surveys in long-term care settings, consider increasing the interval between surveys (e.g., performing every 4-6 weeks) or temporarily pausing them while reassessing infection control and implementing interventions.
4Conduct a laboratory lookback covering at least 6 months (Tier 1) and 3 months (Tier 2) prior to identification of index case.
5Prospective surveillance of clinical cultures should be conducted for 3 months after the last identified case.
6A public health investigation should also be initiated at healthcare facilities known to regularly share patients with healthcare facilities where transmission has occurred, such as post-acute care facilities. At a minimum, this should include notification of the facility and a request to retrospectively and prospectively evaluate clinical cultures for the phenotype of interest. This could also include admission screening of patients at the facility (e.g., transfers from the index facility) and/or point prevalence surveys of high-risk patients or units.