Disease Specifics
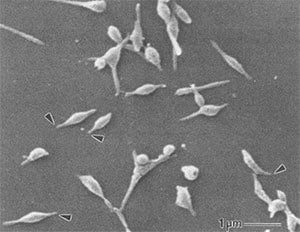
Electron micrograph of Mycoplasma pneumoniae cells. The arrows indicate the attachment organelles.
Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004
Mycoplasma pneumoniae infections have a wide spectrum of clinical symptoms and disease manifestations. Pneumonia caused by M. pneumoniae is a type of atypical bacterial pneumonia. Before tests to diagnose M. pneumoniae were available, pneumonia caused by M. pneumoniae was considered “atypical” given the differences in clinical presentation and response to treatment used for “typical” pneumonia. The varied presentation and limited diagnostic methods available present unique challenges for accurately identifying M. pneumoniae cases and appropriately treating patients.
Estimated impact
The true magnitude of this health issue in the United States is unknown. However, an estimated 2 million cases of M. pneumoniae infections occur each year. Experts estimate that M. pneumoniae infections account for between 1 and 10 in every 50 cases of community-acquired pneumonia
Mycoplasma pneumoniae
M. pneumoniae lacks a rigid cell wall, allowing it to alter its size and shape to suit its surrounding conditions. Therefore, antimicrobials that work by targeting the cell wall, like beta-lactams, do not work against these bacteria.
Pathogenesis
M. pneumoniae bacteria spread from person-to-person contact via respiratory droplets and are exclusively a human pathogen. M. pneumoniae are primarily extracellular. They evolved a specialized attachment organelle for close association with host cells. This attachment is critical to the bacteria’s survival and ability to infect. The close association between M. pneumoniae and the host cells prevents the host’s mucociliary clearance mechanisms from removing the bacterium. The bacteria attach to and damage the respiratory epithelial cells at the base of cilia. This activates the innate immune response and produces local cytotoxic effects.
Role of toxin production
M. pneumoniae produce a unique virulence factor known as Community Acquired Respiratory Distress Syndrome (CARDS) toxin. The CARDS toxin most likely aids in the colonization and pathogenic pathways of M. pneumoniae, leading to inflammation and airway dysfunction. While M. pneumoniae primarily live on the surface of the respiratory epithelial cells, they can invade tissues and replicate intracellularly. The endocytosis of M. pneumoniae by the host cells could:
- Aid in the establishment of a latent or chronic disease state
- Facilitate the bacterium in evading an immune response
- Interfere with the efficacy of certain drug therapies
- Marston BJ, Plouffe JF, File Jr TM, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. The Community-Based Pneumonia Incidence Study GroupExternal. Arch Intern Med. 1997;157:1709–18.
- Kannan TR, Baseman JB. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogensExternal. Proc Natl Acad Sci USA. 2006;103:6724–9.
- Porath A, Schlaeffer F, Lieberman D. The epidemiology of community-acquired pneumonia among hospitalized adultsExternal. J Infect. 1997;34:41–8.
- Waites KB, Atkinson TP. The role of mycoplasma in upper respiratory infectionsExternal. Curr Infect Dis Rep. 2009;11:198–206.
- Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogenExternal. Clin Microbiol Rev. 2004;17:697–728.