Volume 10 — July 11, 2013
ORIGINAL RESEARCH
Adherence to Cancer Screening Guidelines and Predictors of Improvement Among Participants in the Kansas State Employee Wellness Program
Navigate This Article
Siu-kuen Azor Hui, PhD, MSPH; Kimberly K. Engelman, PhD; Theresa I. Shireman, PhD, RPh; Edward F. Ellerbeck, MD, MPH
Suggested citation for this article: Hui SA, Engelman KK, Shireman TI, Ellerbeck EF. Adherence to Cancer Screening Guidelines and Predictors of Improvement Among Participants in the Kansas State Employee Wellness Program. Prev Chronic Dis 2013;10:120212. DOI: http://dx.doi.org/10.5888/pcd10.120212.
PEER REVIEWED
Abstract
Introduction
Employee wellness programs (EWPs) have been used to implement worksite-based cancer prevention and control interventions. However, little is known about whether these programs result in improved adherence to cancer screening guidelines or how participants’ characteristics affect subsequent screening. This study was conducted to describe cancer screening behaviors among participants in a state EWP and identify factors associated with screening adherence among those who were initially nonadherent.
Methods
We identified employees and their dependents who completed health risk assessments (HRAs) as part of the Kansas state EWP in both 2008 and 2009. We examined baseline rates of adherence to cancer screening guidelines in 2008 and factors associated with adherence in 2009 among participants who were initially nonadherent.
Results
Of 53,095 eligible participants, 13,222 (25%) participated in the EWP in 2008 and 6,205 (12%) participated in both years. Among the multiyear participants, adherence was high at baseline to screening for breast (92.5%), cervical (91.8%), and colorectal cancer (72.7%). Of participants who were initially nonadherent in 2008, 52.4%, 41.3%, and 33.5%, respectively, became adherent in the following year to breast, cervical, and colorectal cancer screening. Suburban/urban residence and more frequent doctor visits predicted adherence to breast and colorectal cancer screening guidelines.
Conclusion
The effectiveness of EWPs for increasing cancer screening is limited by low HRA participation rates, high rates of adherence to screening at baseline, and failure of nonadherent participants to get screening. Improving overall adherence to cancer screening guidelines among employees will require efforts to increase HRA participation, stronger interventions for nonadherent participants, and better access to screening for rural employees.
Introduction
Employee wellness programs (EWPs) are organized, employer-sponsored health promotion and chronic disease prevention programs that offer annual free health risk assessments (HRAs) to employees and their dependents who are enrolled in employer-sponsored health plans. Based on the HRA results, personalized risk feedback and preventive care recommendations are provided to voluntary participants. During the past 40 years, EWPs have proliferated as employers try to recoup greater value from their health-related expenditures. Their goals with EWPs are to 1) improve employee health, productivity, and job satisfaction and 2) reduce health care costs associated with chronic diseases (1). EWPs are offered to employees and their dependents who purchase their health insurance plans through their employers. Several studies have shown EWPs to be effective in preventing cardiovascular diseases (CVD) among employees (2–5), but their value in cancer prevention and control is less well established.
Screening for breast, cervical, and colorectal cancer can reduce illness and death from these diseases, but adherence to cancer screening guidelines remains suboptimal (6,7). Because a large proportion of adults in the United States are employed, worksites provide an opportunity to implement interventions to improve cancer screening among employees (8). Promotion of cancer screening among employees can be highly cost-effective from the employer’s perspective because of medical and disability savings from early detection of cancer (9). Given the potential of EWP-based interventions to increase cancer screening (8), the CEO Roundtable on Cancer established standards (10) for the early detection of cancer through EWPs. These standards describe health plan benefits, education programs, and various cancer screening worksite interventions that can improve adherence to cancer screening recommendations among EWP participants. However, as of January 14, 2013, only 137 organizations were accredited with the CEO Cancer Gold Standard; most organizations provide markedly fewer cancer screening interventions for EWP participants.
EWPs that incorporate cancer screening typically assess cancer screening behavior on an annual HRA questionnaire and provide full cost coverage of relevant breast, cervical, and colorectal cancer screening tests for employees. The annual HRA is commonly administered via the Internet, and it collects self-reported health-risk data by asking questions about risk factors for common health problems, including cancer. After completing the HRA, participants receive a personalized feedback report on their health-risk profile and preventive care recommendations directed toward specific identified health risks. Sometimes, participants are given referrals to programs or providers that can address their specific risks. The HRA is the critical gateway to a broader EWP, and a recent systematic review by the Task Force on Community Preventive Services found strong evidence that use of HRAs with personalized feedback combined with additional worksite interventions can improve employees’ health outcomes (4,11).
However, little is known about what happens to HRA participants who are initially nonadherent to cancer screening guidelines after they have received their personalized risk feedback report. Although previous research found that adherence to cancer screening guidelines is predictable on the basis of sociodemographic variables (eg, age, education, income) (12–14), health behavior model constructs (eg, knowledge, perceived risk, perceived barriers) (12,15,16), and engaging in other health behaviors (eg, not smoking, not being overweight) (17,18), there has been little study of predictors of adherence within the context of EWPs. This study fills in the knowledge gap of cancer screening adherence among EWP participants by examining overall participation in the EWP, changes in screening status, and factors associated with participants becoming adherent to cancer screening guidelines.
Methods
This study used 2008 and 2009 HRA data from the Kansas EWP. The data were obtained through a data use agreement between the University of Kansas Medical Center and the Kansas Health Policy Authority. Data included the basic personnel data of all eligible participants and complete responses of all HRA participants. Each person represented in these files had a unique numerical identifier. Because the coding of the numerical identifier was unknown to the authors, these data were not considered as personally identifiable, and the Human Subjects Committee at the University of Kansas Medical Center approved the study.
Participants
The participants of this study were Kansas state employees and their dependents who enrolled in the Kansas state employee health plan and completed a standard HRA in 2008 and 2009. Among the 6,205 employees and their dependents who participated in the HRA in both years, only those who were eligible for the recommended cancer screening but who were nonadherent in 2008 (N = 1,225) were included in this study (Table 1). The eligible, nonadherent populations were defined according to the clinical preventive services guide issued by the US Preventive Services Task Force (USPSTF) in 2009 (19).
Measures
The screening adherence measures used were the same on the 2008 and 2009 HRA questionnaires. Initial screening nonadherence status was derived from the 2008 HRA responses, and screening adherence status was derived from the 2009 HRA responses. Breast cancer screening nonadherence was defined as women aged 40 years or older who reported having their last mammography “more than 2 years ago” or “never”; adherence was defined as the eligible women having their last mammography “less than 1 year ago” or “1 to 2 years ago.” Cervical cancer screening nonadherence was defined as women aged 21 to 65 years who reported having their last Papanicolaou (Pap) test “more than 2 years ago” or “never”; adherence was defined as the eligible women having the last Pap test “less than 1 year ago” or “1 to 2 years ago.” Although USPSTF recommends cervical cancer screening only every 3 years for women in this age group, there was no response choice of “more than 3 years ago” on the 2008 HRA, so we could not determine the number of women who were nonadherent. Colorectal cancer screening nonadherence was defined as men or women aged 50 years or older who responded no to having had a fecal occult blood test (FOBT) in the past 12 months, sigmoidoscopy in the past 5 years, and colonoscopy in the past 10 years; screening adherence was defined as responding yes to 1 of the 3 questions.
The potential predictors of these outcomes were derived from the 2008 HRA answers. Education was dichotomized into “some college or lower” or “college graduate or higher,” income was dichotomized into $0 to $35,000 or over $35,000), living setting was dichotomized into “rural” or “suburban or urban,” and job ranking was dichotomized into “senior” (ie, professionals and executive or managers) and “junior” (all others). Perceived health risk was measured on a 5-point Likert scale by the question, “Compared to others like me, my overall risk of getting an illness or disease is . . . much higher, higher, about the same, lower, or much lower.” This variable also was dichotomized. Participants who answered “about the same, lower, or much lower” were regarded as perceiving their health to be “about the same or healthier” than that of their peers, and those who answered “higher” or “much higher” were regarded as perceiving their health to be “less healthy” than that of their peers. Number of visits to a doctor’s office or clinic in the preceding year was dichotomized into “0 or 1” and “2 or more.”
A healthy lifestyle index was created to categorize participants into 2 groups in terms of health behaviors. The “healthy” group was coded as participants who reported not smoking, not overusing alcohol, having a body mass index below 25 kg/m2, engaging in physical activities of moderate to vigorous intensity for at least 30 minutes on 3 days per week, and eating at least 2 cups of fruit and 2.5 cups of vegetables each day. Alcohol overuse was defined as having an alcoholic drink 6 to 7 days per week and having 2 or more drinks on each day for men or 1 or more drink on each day for women (20). The definitions of health behaviors related to physical activity and fruit and vegetable intake were based on the US Department of Health and Human Services guidelines (21,22).
Procedure
An Internet-based portal for the Kansas State EWP was open during March to September in 2008 and 2009 for eligible participants to log on and complete their HRA. Because some questions on the HRA asked for clinical data, eligible participants were recommended to attend their worksite biometric screening first and then enter the data in HRA questionnaire. However, participants also could enter their clinical data obtained from their most recent doctor’s visit. As an incentive, participants received a $50 gift card if they completed both the HRA and the worksite screening.
Immediately after completing the online HRA, participants received their personalized disease risk report and preventive care recommendations electronically. Although questions were asked about participants’ last time of relevant cancer screening tests, the HRA personalized disease risk feedback report in 2008 did not include the participant’s cancer screening status and recommendations. All state employee health plans in 2008 and 2009 provided full coverage of the cost of all eligible cancer screening tests.
Statistical analyses
Chi-square tests were used to examine bivariate relationships between each cancer screening adherence outcome and each baseline predictor. Odds ratios (ORs) were calculated to identify significant associations between the hypothesized predictors and cancer screening adherence. Significant bivariate predictors were analyzed by using multivariate logistic regression to investigate whether they independently predicted the outcome.
Results
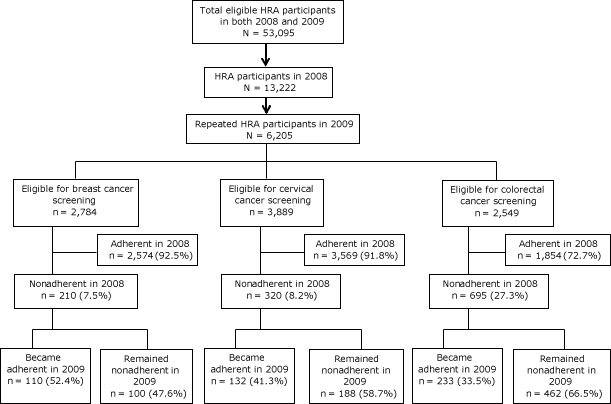
In our sample, HRA participation rates were 22% in 2008 and 15% in 2009. Among the 53,095 total health plan enrollees in 2008, 13,222 (25%) were HRA participants in 2008 and 6,205 (12%) of them also participated in 2009. Among these participants, most were adherent in their cancer screenings in 2008 (Figure). In our sample, 92.5% of eligible women were adherent to breast cancer screening, 91.8% of eligible women were adherent to cervical cancer screening, and 72.7% of eligible participants were adherent to colorectal cancer screening in 2008. Among the 210 women who were nonadherent to breast cancer screening in 2008, 110 (52.4%) became adherent in 2009. Among 320 women who were nonadherent to cervical cancer screening in 2008, 132 (41.3%) became adherent in 2009. Among 695 men and women who were nonadherent to colorectal cancer screening in 2008, 233 (33.5%) became adherent in 2009.
Figure. Flow chart depicting inclusion process of participants who completed health risk assessments (HRAs) and their cancer screening adherence behaviors, Kansas, 2008 and 2009. [A text description of this figure is also available.]
Among women who were initially nonadherent to breast cancer screening, those who lived in suburban or urban areas were more likely to become adherent the following year than those who lived in rural areas (OR = 1.99, P = .03) (Table 2). Among men and women who were initially nonadherent to colorectal cancer screening, significant bivariate predictors of improvement were suburban/urban residence (OR = 1.57, P = .01) and 2 or more doctor’s visits during the previous year (OR = 1.43, P = .04). Further multivariate logistic regression modeling showed that suburban/urban residence remained as a significant independent predictor of the improvement in colorectal cancer screening (OR = 1.54, P < .05), while number of doctor’s visits became nonsignificant (OR = 1.39, P = .056) as an independent predictor. No significant predictors were found for improvement in cervical cancer screening adherence.
Discussion
Results of this study showed that most multiple-year health risk assessment (HRA) participants were adherent to cancer screening recommendations at baseline. This high adherence rate may be due to healthier HRA participants self-selecting to participate in HRAs more often than nonparticipants, particularly when HRA participation rates are low (23–27), which is a common phenomenon in many employee wellness programs (EWPs) (28). Further self-selection bias may have occurred among these multiple-year HRA participants. Our observed cancer screening adherence rates at baseline among these multiple-year participants were slightly higher than what we observed among the single-year participants (29). The breast cancer screening adherence rate also was substantially higher than that of the general public (92.5% vs 76%), as measured by the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System (BRFSS) 2008 survey (6). Other cancer screening adherence measures used by the BRFSS survey were different and, therefore, cannot be compared directly.
Our study demonstrates multiple levels in which an EWP may fail in its efforts to enhance cancer screening adherence. First, we noted low participation rates overall. Second, multiyear HRA participants appear to be a self-selected group of healthier workers that are less likely to need help in getting cancer screening (29). Third, among multiyear HRA participants, approximately one-half to two-thirds of those initially nonadherent to cancer screening guidelines remained nonadherent the following year. This finding is disappointing considering that these were self-selected, apparently more health-conscious employees. It is reasonable to assume that non-HRA participants would have lower overall screening rates and would be less likely to increase screening from year to year.
Of the multiyear participants who were nonadherent at baseline, our results demonstrate that rural-dwelling employees and those with less frequent visits to a doctor’s office were less likely to get screened after participating in the HRA. Access to cancer screening services and physician’s recommendation are strong predictors of adherence to cancer screening (12,16,17), and our findings likely point to these mechanisms of actions. Living in suburban/urban areas makes access to screening facilities more convenient, and more frequent encounters with doctors increases the chance of receiving reminders. This finding is consistent with previous research that rural-residing individuals experience more barriers to health care access and are less likely to receive cancer screening (30–33). This finding suggests that employees who reside in rural areas may benefit the most from worksite interventions to enhance cancer screening services but that the EWP may need to address specific barriers to accessing a provider that can provide these preventive services.
Although systematic strategies to use HRAs and EWPs to enhance cancer screening have been recommended (8), these strategies may not be implemented at most worksites or not implemented effectively. For example, one of the USPSTF recommendations for maximally effective worksite health interventions is to provide cancer screening behavior feedback, recommendations, or referrals for screening resources (4,11); however, participants in this study did not receive these interventions. Although completing the HRA itself may make particularly health-conscious employees aware of their nonadherence and serve as a cue to action to initiate screening, the HRA participants did not benefit from more direct interventions to improve their cancer screening adherence. Also, it is unlikely that smaller employers in rural communities would be able to provide permanent worksite health promotion programs. Mobile solutions or partnerships with existing rural health care providers may be required.
The strengths of this study include the use of a large state employee sample and having the complete individual-level HRA response data for 2 consecutive years. This is the first study to examine predictors of improvement in adherence to cancer screening guidelines in a population of EWP participants. One limitation of this study is the lack of a non-HRA comparison group. Other limitations were the self-report nature of the HRA responses and some HRA questions that did not match standard guidelines. Furthermore, the findings from this study cannot be generalized to all employees or EWPs.
In conclusion, EWPs offer unique opportunities to improve adherence to cancer screening guidelines among employees and their dependents. However, to maximize their public health effects, EWPs need higher HRA participation rates to reduce the healthy participants effect and improved interventions to promote cancer screening among those who have not previously had screening, including cancer screening adherence status feedback, recommendations, and screening resource information. Interventions that help employees access screening services may be particularly important for rural employees. This study demonstrates the importance and utility of tracking data in EWPs to identify opportunities for improvement in cancer screening, and it suggests the need for additional worksite-based interventions to remove barriers to cancer screening for employees living in rural areas.
Acknowledgments
This work was done at University of Kansas Medical Center, and the study was not funded by any grant. The authors are grateful to Ms Cheryl Miller, Senior Manager, Health Management, Kansas Health Policy Authority, who facilitated acquisition of the data. No author of this manuscript has any conflict of interest to disclose.
Author Information
Corresponding Author: Siu-kuen Azor Hui, PhD, MSPH, Fox Chase Cancer Center, Department of Psychosocial and Behavioral Medicine, 333 Cottman Ave, Young Pavilion 4141, Philadelphia, PA 19111. Telephone: 215-728-2453. E-mail: SKAzor.Hui@fccc.edu.
Author Affiliations: Kimberly K. Engelman, Theresa I. Shireman, Edward F. Ellerbeck, Department of Preventive Medicine and Public Health, University of Kansas Medical Center, Kansas City, Kansas.
References
- Aldana SG, Merrill RM, Price K, Hardy A, Hager R. Financial impact of a comprehensive multisite workplace health promotion program. Prev Med 2005;40(2):131–7. CrossRef PubMed
- Goetzel RZ, Ozminkowski RJ. The health and cost benefits of work site health-promotion programs. Annu Rev Public Health 2008;29:303–23. CrossRef PubMed
- Goetzel RZ, Ozminkowski RJ, Bruno JA, Rutter KR, Isaac F, Wang S. The long-term impact of Johnson & Johnson’s Health & Wellness Program on employee health risks. J Occup Environ Med 2002;44(5):417–24. CrossRef PubMed
- Soler RE, Leeks KD, Razi S, Hopkins DP, Griffith M, Aten A, et al. A systematic review of selected interventions for worksite health promotion: the assessment of health risks with feedback. Am J Prev Med 2010;38(2, Suppl):S237–62. CrossRef PubMed
- Stein AD, Shakour SK, Zuidema RA. Financial incentives, participation in employer-sponsored health promotion, and changes in employee health and productivity: HealthPlus Health Quotient Program. J Occup Environ Med 2000;42(12):1148–55. CrossRef PubMed
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data, 2008. http://apps.nccd.cdc.gov/brfss/. Accessed January 6, 2013.
- Cancer trends progress report — 2009/2010 update. National Cancer Institute; 2010. http://progressreport.cancer.gov/. Accessed August 8, 2012.
- Hannon PA, Harris JR. Interventions to improve cancer screening: opportunities in the workplace. Am J Prev Med 2008;35(1 Suppl):S10–3. CrossRef PubMed
- Pyenson B, Zenner P. Cancer screening: payer cost/benefit through employee benefits programs. New York (NY): Milliman, Inc; 2005.
- What is the gold standard? CEO Roundtable on Cancer. http://www.cancergoldstandard.org/what-is-goldstandard. Accessed May 17, 2013.
- Task Force on Community Preventive Services. Recommendations for worksite-based interventions to improve workers’ health. Am J Prev Med 2010;38(2 Suppl):S232–6. CrossRef PubMed
- Gierisch JM, Earp JA, Brewer NT, Rimer BK. Longitudinal predictors of nonadherence to maintenance of mammography. Cancer Epidemiol Biomarkers Prev 2010;19(4):1103–11. CrossRef PubMed
- Martin-Lopez R, Hernandez-Barrera V, De Andres AL, Garrido PC, De Miguel AG, Garcia RJ. Breast and cervical cancer screening in Spain and predictors of adherence. Eur J Cancer Prev 2010;19(3):239–45. CrossRef PubMed
- Paskett ED, McLaughlin JM, Reiter PL, Lehman AM, Rhoda DA, Katz ML, et al. Psychosocial predictors of adherence to risk-appropriate cervical cancer screening guidelines: a cross sectional study of women in Ohio Appalachia participating in the Community Awareness Resources and Education (CARE) project. Prev Med 2010;50(1-2):74–80. CrossRef PubMed
- McQueen A, Vernon SW, Rothman AJ, Norman GJ, Myers RE, Tilley BC. Examining the role of perceived susceptibility on colorectal cancer screening intention and behavior. Ann Behav Med 2010;40(2):205–17. CrossRef PubMed
- Price MA, Butow PN, Charles M, Bullen T, Meiser B, McKinley JM, et al. Predictors of breast cancer screening behavior in women with a strong family history of the disease. Breast Cancer Res Treat 2010;124(2):509–19. CrossRef PubMed
- Gierisch JM, O’Neill SC, Rimer BK, DeFrank JT, Bowling JM, Skinner CS. Factors associated with annual-interval mammography for women in their 40s. Cancer Epidemiol 2009;33(1):72–8. CrossRef PubMed
- Griffith KA, McGuire DB, Royak-Schaler R, Plowden KO, Steinberger EK. Influence of family history and preventive health behaviors on colorectal cancer screening in African Americans. Cancer 2008;113(2):276–85. CrossRef PubMed
- The Guide to Clinical Preventive Services. 2009 (AHRQ publication no. 09-IP006). Washington (DC): US Preventive Services Task Force, US Department of Health and Human Services, Agency for Healthcare Research and Quality; 2009.
- Food, nutrition, physical activity, and the prevention of cancer: a Global Perspective. World Cancer Research Fund, American Institute for Cancer Research; 2007. http://www.dietandcancerreport.org/cancer_resource_center/downloads/Second_Expert_Report_full.pdf. Accessed August 8, 2012.
- 2008 Physical activity guidelines for Americans. US Department of Health and Human Services; 2008. http://www.health.gov/paguidelines/pdf/paguide.pdf. Accessed May 30, 2013.
- 2005 Dietary guidelines for Americans. US Department of Health and Human Services; 2005. http://www.health.gov/dietaryguidelines/dga2005/document/pdf/DGA2005.pdf. Accessed May 30, 2013.
- Glasgow RE, McCaul KD, Fisher KJ. Participation in worksite health promotion: a critique of the literature and recommendations for future practice. Health Educ Q 1993;20(3):391–408. CrossRef PubMed
- Kizer KW, Pelletier KR, Fielding JE. Work-site health promotion programs and health care reform. West J Med 1995;162(5):467–8. PubMed
- Robroek SJ, van Lenthe FJ, van Empelen P, Burdorf A. Determinants of participation in worksite health promotion programmes: a systematic review. Int J Behav Nutr Phys Act 2009;6:26. CrossRef PubMed
- Thompson SE, Smith BA, Bybee RF. Factors influencing participation in worksite wellness programs among minority and underserved populations. Fam Community Health 2005;28(3):267–73. CrossRef PubMed
- Janer G, Sala M, Kogevinas M. Health promotion trials at worksites and risk factors for cancer. Scand J Work Environ Health 2002;28(3):141–57. CrossRef PubMed
- Cornfeld MJ, Schnoll RA, Tofani SH, Babb JS, Miller SM, Henigan-Peel T, et al. Implementation of a comprehensive cancer control program at the worksite: year one summary report. J Occup Environ Med 2002;44(5):398–406. CrossRef PubMed
- Hui SA, Engelman KK, Shireman TI, Hunt S, Ellerbeck EF. Opportunities for cancer prevention using employee wellness programs: the case of Kansas state employees. Am J Health Educ 2012;43(4):226–32. CrossRef
- Benuzillo JG, Jacobs ET, Hoffman RM, Heigh RI, Lance P, Martinez ME. Rural-urban differences in colorectal cancer screening capacity in Arizona. J Community Health 2009;34(6):523–8. CrossRef PubMed
- Jackson MC, Davis WW, Waldron W, McNeel TS, Pfeiffer R, Breen N. Impact of geography on mammography use in California. Cancer Causes Control 2009;20(8):1339–53. CrossRef PubMed
- Doescher MP, Jackson JE. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. J Public Health Manag Pract 2009;15(3):200–9. PubMed
- Holt CL, Shipp M, Eloubeidi M, Clay KS, Smith-Janas MA, Janas MJ, et al. Use of focus group data to develop recommendations for demographically segmented colorectal cancer educational strategies. Health Educ Res 2009;24(5):876–89. CrossRef PubMed
Tables
 Table 1. Demographic Characteristics of Participants (N = 1,225) of an Employee Worksite Wellness Program Who Were Nonadherent to Cancer Screening Guidelines in 2008, Kansas, 2009
Table 1. Demographic Characteristics of Participants (N = 1,225) of an Employee Worksite Wellness Program Who Were Nonadherent to Cancer Screening Guidelines in 2008, Kansas, 2009
| Demographic Variable | Breast Cancer Screening | Cervical Cancer Screening | Colorectal Cancer Screening |
|---|---|---|---|
| (n = 210) | (n = 320) | (n = 695) | |
| No. (%) Who Were Nonadherent in 2008 | |||
| Age, y | |||
| 20–29 | — | 21 (6.6) | — |
| 30–39 | — | 37 (11.6) | — |
| 40–49 | 137 (65.2) | 102 (31.9) | — |
| 50–59 | 64 (30.5) | 143 (44.7) | 588 (84.6) |
| ≥60 | 9 (4.3) | 17 (5.3) | 107 (15.4) |
| Race/ethnicitya | |||
| White | 188 (89.5) | 288 (90.0) | 651 (93.7) |
| African American | 8 (3.8) | 11 (3.4) | 19 (2.7) |
| American Indian/Native American | 11 (5.2) | 11 (3.4) | 14 (2.0) |
| Asian/Pacific Islander | 8 (3.8) | 13 (4.1) | 18 (2.5) |
| Hispanic | 5 (2.4) | 7 (2.2) | 9 (1.3) |
| Education | |||
| Some high school or less | 0 | 1 (0.3) | 7 (1.0) |
| High school graduate | 31 (14.8) | 52 (16.3) | 104 (15.0) |
| Some college | 66 (31.4) | 101 (31.6) | 245 (35.3) |
| College graduate | 80 (38.1) | 107 (33.4) | 202 (29.1) |
| Postgrad/Professional degree | 33 (15.7) | 59 (18.4) | 137 (19.7) |
| Income, $ | |||
| 0-20,000 | 9 (4.3) | 20 (6.3) | 40 (5.8) |
| 20,001–35,000 | 85 (40.5) | 134 (41.9) | 214 (30.8) |
| 35,001–55,000 | 78 (37.1) | 105 (32.8) | 256 (36.8) |
| 55,001–85,000 | 12 (5.7) | 25 (7.8) | 94 (13.5) |
| 85,001–100,000 | 1 (0.5) | 3 (0.9) | 12 (1.7) |
| ≥100,001 | 2 (1.0) | 4 (1.3) | 7 (1.0) |
| Do not wish to share income/missing | 23 (10.9) | 29 (9.0) | 72 (10.4) |
| Work type | |||
| Operator/assembly/labor/construction | 2 (1.0) | 3 (0.9) | 33 (4.7) |
| Production/craftsman/machinist/carpenter | 0 | 0 | 17 (2.4) |
| Service occupation/janitorial | 10 (4.8) | 12 (3.8) | 40 (5.8) |
| Clerical and administrative support | 76 (36.2) | 121 (37.8) | 189 (27.2) |
| Sales | 0 | 0 | 7 (1.0) |
| Technical support | 9 (4.3) | 13 (4.1) | 49 (7.1) |
| Professional | 81 (38.6) | 123 (38.4) | 221 (31.8) |
| Executive or manager | 25 (11.9) | 37 (11.6) | 110 (15.8) |
| Missing | 7 (3.3) | 11 (3.4) | 29 (4.2) |
Abbreviation: — , not applicable.
a Race/ethnicity categories are not mutually exclusive.
 Table 2. Factors Associated With Adhering to Cancer Screening Guidelines in 2009 Among Eligible Individuals Who Were Nonadherent in 2008, Employee Worksite Wellness Program, Kansas
Table 2. Factors Associated With Adhering to Cancer Screening Guidelines in 2009 Among Eligible Individuals Who Were Nonadherent in 2008, Employee Worksite Wellness Program, Kansas
| Baseline Predictors in 2008 | Breast Cancer Screening Adherent | Cervical Cancer Screening Adherent | Colorectal Cancer Screening Adherent | |||
|---|---|---|---|---|---|---|
| N (%) | OR (95% CI) | N (%) | OR (95% CI) | N (%) | OR (95% CI) | |
| Sex | ||||||
| Male | — | — | — | — | 237 (32.9) | 1 [Reference] |
| Female | — | — | — | — | 458 (33.8) | 1.04 (0.75–1.46) |
| Education | ||||||
| Some college or lower | 97 (52.6) | 1 [Reference] | 154 (40.9) | 1 [Reference] | 356 (30.3) | 1 [Reference] |
| College graduate or higher | 113 (52.2) | 0.99 (0.57–1.70) | 166 (41.6) | 1.03 (0.66–1.60) | 339 (36.9) | 1.34 (0.98–1.84) |
| Income, $ | ||||||
| 0–35,000 | 94 (54.3) | 1 [Reference] | 154 (44.8) | 1 [Reference] | 254 (32.3) | 1 [Reference] |
| ≥35,001 | 93 (55.9) | 1.07 (0.60–1.90) | 137 (40.1) | 0.83 (0.52–1.32) | 369 (35.0) | 1.13 (0.80–1.58) |
| Living setting | ||||||
| Rural | 63 (39.7) | 1 [Reference] | 214 (41.6) | 1 [Reference] | 212 (26.9) | 1 [Reference] |
| Suburban or urban | 143 (56.6) | 1.99 (1.09–3.63)a | 100 (40.0) | 1.07 (0.66–1.73) | 481 (36.6) | 1.57 (1.10–2.24)b |
| Job ranking | ||||||
| Junior | 97 (55.7) | 1 [Reference] | 149 (40.9) | 1 [Reference] | 335 (33.7) | 1 [Reference] |
| Senior | 106 (50.9) | 0.83 (0.48–1.44) | 160 (41.3) | 1.01 (0.64–1.59) | 331 (33.2) | 0.98 (0.71–1.35) |
| Perceived health risk | ||||||
| Less healthy | 68 (50.0) | 1 [Reference] | 87 (41.4) | 1 [Reference] | 290 (37.6) | 1 [Reference] |
| About the same or healthier | 118 (50.0) | 1.00 (0.55–1.82) | 200 (40.5) | 0.96 (0.58–1.61) | 318 (30.5) | 0.73 (0.52–1.02) |
| Healthy lifestyle | ||||||
| No | 180 (51.7) | 1 [Reference] | 274 (42.7) | 1 [Reference] | 596 (32.9) | 1 [Reference] |
| Yes | 30 (56.7) | 1.22 (0.56–2.67) | 46 (32.6) | 0.65 (0.34–1.26) | 99 (37.4) | 1.22 (0.78–1.89) |
| No. of doctor’s visits in the previous year | ||||||
| 0–1 | 91 (49.5) | 1 [Reference] | 114 (41.2) | 1 [Reference] | 239 (28.5) | 1 [Reference] |
| ≥2 | 119 (54.6) | 1.23 (0.71–2.13) | 206 (41.3) | 1.00 (0.63–1.59) | 456 (36.2) | 1.43 (1.02–2.00)c |
Abbreviation: — , not applicable.
a P = .03.
b P = .01.
c P = .04.
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions.