ORIGINAL RESEARCH
A Simulation Model for Designing Effective Interventions in Early Childhood Caries
Navigate This Article
Gary B. Hirsch, SM; Burton L. Edelstein, DDS, MPH; Marcy Frosh, JD; Theresa Anselmo, MPH, BSDH, RDH
Suggested citation for this article: Hirsch GB, Edelstein BL, Frosh M, Anselmo T. A simulation model for designing effective interventions in early childhood caries. Prev Chronic Dis 2012;9:110219. DOI: http://dx.doi.org/10.5888/pcd9.110219.
PEER REVIEWED
Abstract
Introduction
Early childhood caries (ECC) — tooth decay among children younger than 6 years — is prevalent and consequential, affecting nearly half of US 5-year-olds, despite being highly preventable. Various interventions have been explored to limit caries activity leading to cavities, but little is known about the long-term effects and costs of these interventions.
We developed a system dynamics model to determine which interventions, singly and in combination, could have the
greatest effect in reducing caries experience and cost in a population of
children aged birth to 5 years.
Methods
System dynamics is a computer simulation technique useful to policy makers in choosing the most appropriate interventions for their populations. This study of Colorado preschool children models 6 categories of ECC intervention — applying fluorides, limiting cariogenic bacterial transmission from mothers to children, using xylitol directly with children, clinical treatment, motivational interviewing, and combinations of these — to compare their relative effect and cost.
Results
The model projects 10-year intervention costs ranging from $6 million to $245 million and
relative reductions in cavity prevalence ranging from none to 79.1% from the
baseline. Interventions targeting the youngest children take 2 to 4 years longer to affect the entire population of preschool-age children but ultimately exert a greater benefit in reducing ECC; interventions targeting the highest-risk children provide the greatest return on investment, and combined interventions that target ECC at several
stages of its natural history have the greatest potential for cavity reduction. Some interventions save more in dental repair than their cost; all produce substantial reductions in repair cost.
Conclusion
By using data relevant to any geographic area, this system model can provide policy makers with information to maximize the return on public health and clinical care investments.
Introduction
Early childhood caries (ECC) — tooth decay among children younger than 6 years — is highly prevalent and consequential in the United States, despite being highly preventable. Forty-four percent of 5-year-olds have cavity experience (1). Early disease predicts lifelong cavities (2) because the process that results in cavities, once established, tends to be stable and chronic. ECC manifests frequently as pain and infection and disproportionately affects low-income children (3), leading to avoidable expenditures by Medicaid and the Children’s Health Insurance Program (4). The problem facing policy makers is selecting interventions that have greatest potential for reducing both disease and costs.
System dynamics is “a methodology for better anticipating the likely effects of interventions in dynamically complex situations” (5). It maps the causes of a persistent problem and uses computer simulation to compare alternative policies and intervention strategies that might alleviate the problem. System dynamics has been applied to health care delivery and population health for chronic conditions affecting oral health and to various public health initiatives. Although it does not predict the future, system dynamics allows policy makers and program managers to consider resolution of complex, multilayered, interactive issues in an organized way.
The objective of this study was to formulate a system dynamics model to assess and compare ECC interventions for benefits and costs among young children in Colorado. Its framework can be applied to other locales.
Methods
Our basic model structure, developed by a work group of pediatric medical, dental, and public health experts, separates children by age (0-6, 7-24, and 25-72 mo) and risk of developing ECC (low, moderate, high), using household income as a surrogate for risk. Risk is considered a key element in determining allocation of public health and dental care resources (6,7). Caries experience may vary over time with changes in socioeconomic status (8) and preventive interventions.
Stages of ECC
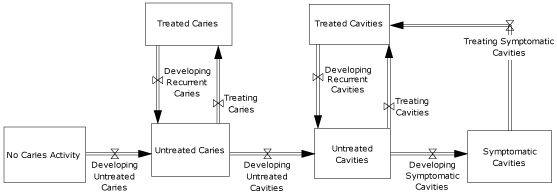
Caries is the disease process that causes cavities (Figure 1). The natural history of ECC begins with a newly erupted tooth that is not yet colonized by cariogenic bacteria. It progresses through the establishment of a biofilm that may be visible as plaque, followed by demineralization of the enamel resulting in a precavity lesion, or “white spot.” With continued progression, the enamel breaks down, resulting in a cavity. In our modeling, all phases of this dynamic chain from initial biofilm formation to cavity are termed “caries,” and the physical breakdown of the tooth structure (cavitation) is termed “cavities.” A cavity becomes symptomatic when it progresses to affect dentin and pulp. Various preventive and disease management interventions may affect this progression at any point along the way. At each point in the simulation, children move in at least 2 directions as they age and as ECC stages progress. With intervention, children can move in an additional direction if the intervention is sufficient to change risk status.
Figure 1. Stages of early childhood caries development among children aged 0 to 5 years. Symbols with 2 triangles touching at their vertices represent “valves,” indicating that various factors control the rates of flow. These factors include both biological variables such as normal rates of caries development and effects of interventions such as fluoride varnish in slowing those rates of progression. [A text description of this figure is also available.]
We set up the model to continuously reflect the initial distribution of children among age, risk, and illness groups in the absence of any interventions. Interventions change rates of flow from one status to another and, over time, yield different patterns of ECC prevalence. The model calculates a number of variables including overall fraction of children with cavities and cumulative costs of restorative care so that program costs, cost savings for restorative care, and net costs can be attributed to different interventions and combinations of interventions.
Data sources
We used multiple data sources to quantify the model and the effects of simulated interventions. To determine ECC prevalence by age and income and confirm the previously reported relationship between oral health and income, we obtained state-specific information from the Colorado Child Health Survey (9) (Rickey Tolliver, State of Colorado, written communication, November 5, 2009). We used a positive response to a question querying parents about children’s prior dental pain, cavities, broken or missing fillings, or teeth pulled because of cavities as a surrogate for a finding of ECC on clinical examination. The National Health and Nutrition Examination Survey (NHANES) stratifies pediatric cavity experience by income levels of less than 100%, 100% to 199%, and 200% or more of the federal poverty level (FPL) (10), but Colorado data supported using income breaks of up to 200% of the FPL (18.6% ECC prevalence) for “high risk,” 201% to 300% of the FPL (15.0% ECC prevalence) for “moderate risk,” and 300% or more of the FPL (8.4% ECC prevalence) for “low risk.” We categorized children for whom income data were not available (6.0% of respondents) as high-risk because they had similar ECC experience. Of the 431,070 children in Colorado’s population of 0- to 5-year-olds, 31.4% were classified as high-risk, 17.8% were moderate-risk, and 50.8% were low-risk; 8.2% were aged birth to 6 months, 24.7% were 7 to 24 months, and 67.1% were 25 to 72 months. To quantify the fraction of children with untreated and treated cavities at baseline and to compensate for lack of parental knowledge about their children’s nonsymptomatic cavity status, we adjusted Colorado findings to reflect treated and untreated cavity prevalence reported for the 1999-2002 NHANES survey after adjusting for Colorado’s income distribution. A prior secondary analysis of these NHANES data (11) was used to calculate the selected Colorado income bands.
We derived the fraction of children with symptomatic cavities from the Government Accountability Office, which conducted a secondary analysis of NHANES data to determine the number of children considered to be “in urgent need of treatment” (12). To estimate precavity caries fractions, we backward-extrapolated the rate of change in cavities between age groups (between the youngest and middle age groups from the Colorado survey and between middle and older age groups from NHANES). Results were consistent with a study that measured both precavity lesions and cavities among children (13). We used the model to more finely calibrate rates of children moving between stages of tooth decay and finally adjusted the model by using data on rates of childhood dental fillings from the Medical Panel Expenditure Survey (MEPS) (14). We revised recurrence rates (new cavities among children with prior repair) upward to maintain the model’s ability to reflect initial prevalence patterns. The model produces restorative visit rates that fall within the range suggested by MEPS data.
In addition to the prevalence of ECC, the model seeks to anticipate the extent of primary-tooth cavity experience, measured in average numbers of decayed and filled teeth. Using NHANES data (10), we adjusted for differences between national and Colorado’s income distribution and rates of cavity experience for 6- to 24-month-olds and scaled downward from the rates for children younger than 6 years.
To calculate cost savings attributable to avoided restorative care from various interventions, we obtained data for care delivered in both dental offices and ambulatory or hospital sites under general anesthesia. For office-based care, MEPS reported average restorative dental costs of $216 in 2004 for all children, adjusted for inflation at 5% per annum to $276 in 2009 dollars. We attributed an average cost of $7,204 based on Medicaid data adjusted to reflect market-level charges for the 12% of children younger than 6 years requiring care under anesthesia reported by Colorado Medicaid (M. Sajovitz, Colorado Department of Health Care Policy and Financing, written communication, September 17, 2010) and the National Survey of Ambulatory Surgery (15) (M. Hall, Centers for Disease Control and Prevention [CDC] National Center for Health Statistics, written communication, August 4, 2010).
Interventions analyzed
Among interventions of interest are those that delay transmission of cariogenic bacteria to children. To provide baseline values, the model was quantified with fractions of children by age and risk with detectable levels of cariogenic bacteria, specifically Streptococcus mutans. On the basis of studies of S. mutans prevalence among mixed-income (16) and low-income groups (17) and a putative “window of infection” of 19 to 31 months (18), we spread acquisition of S. mutans across the model’s age groups. Simulations with the model were run over 10 years to determine changes in fractions of children younger than 6 years with cavities, untreated cavities, and symptomatic cavities; numbers of decayed and filled teeth; and costs of restorative care. We weighted results against estimated program costs to estimate costs, benefits, and net savings for various interventions. Interventions were applied to entire populations or to age or risk subgroups.
Interventions considered in the analysis included 1) educational programs that reduce consumption of sugary drinks, nocturnal bottle use, and other harmful behaviors; 2) efforts to reduce S. mutans transmission from parents and other caregivers to children using xylitol gum, chlorhexidine, or behavioral interventions; and 3) use of xylitol products directly with older children; 4) aggressive screening for and treatment of caries activity to reduce progression to cavities; 5) expanded use of fluoride varnish; 6) focused preventive care and education for children who already have cavities to reduce recurrence; 7) expansion of community water fluoridation to the entire population; and 8) motivational interviewing with strong educational and behavioral components. Motivational interviewing is a brief interactive approach to counseling and educating parents that focuses on skills that move patients to action. Interventions were clustered into 6 categories: fluoride exposure, transmission reduction, xylitol administration, clinical treatment, motivational interviewing, and combinations of these.
Results
The Table reports projected disease levels in terms of the percentage of children with cavities, percentage of children with untreated cavities, and total numbers of decayed and filled teeth for the population. The Table also reports cumulative costs of restorative care, savings in restorative care compared to no intervention, and cumulative program costs. For example, simulation 1.1, community water fluoridation for all, projects a 6.6% relative reduction in cavity prevalence among Colorado children younger than 6 years (18.2% to 17.0%), a 0.8% relative reduction in their untreated cavities (71.4% to 70.8%), and 16,881 fewer affected teeth (265,923 to 249,042), at a cumulative 10-year cost reduction of $14 million ($208 million to $194 million) after expending $6 million on the intervention, for an $8 million net savings.
Fluoride interventions
Simulation 1.1 assumes that community water fluoridation is expanded to 24.6% of Colorado’s population not currently served; that fluoridation reduces cavity prevalence by 25.4%, half the average reduction reported by CDC (19) to compensate for other sources of pediatric fluoride exposure (William Maas, Pew Center on the States, written communication, September 28, 2010); and that the cost of fluoridation is $0.50 per person for the expanded population, including adults (20). The next 3 simulations consider variants of fluoride varnish application: to all children older than 6 months twice annually (1.2), to high-risk children older than 6 months 3 times annually (1.3), and to all children older than 24 months twice annually (1.4). All assume that fluoride varnish reduces decay of primary teeth by one-third (21,22) at a cost of $16 per child per application (William Maas, Pew Center on the States, written communication, September 28, 2010).
Transmission interventions
Interventions aimed at reducing transmission of cariogenic oral bacteria to children, simulations 2.1 and 2.2, are modeled on assumptions of 88% S. mutans colonization reduction among 7- to 24-month-olds and 64% reduction among 25- to 72-month-olds when mothers are provided with education and treated to suppress their oral bacteria (23,24). Associated caries reductions of 73% are anticipated (25,26), at a cost of $100 per mother.
Xylitol interventions with children
The effect of treating children with xylitol is reported only for children aged 24 months or older (27). Because research on xylitol interventions has reported both low-impact (44%) and high-impact (73%) reductions in cavity experience (28), simulations are modeled separately for each of these levels. Simulations 3.1 and 3.2 address all children aged 24 months or older, and simulations 3.3 and 3.4 address only those of this age group who are at high risk. Simulation 3.5 posits xylitol intervention beginning at age 7 months for children of all risk levels. The assumed cost of xylitol interventions is $100 per child.
Secondary prevention
Simulations 4.1 and 4.2 assume white-spot lesions are identified and treated before they become cavities for children older than 6 months. Simulation 4.1 assumes a fraction of untreated caries that is treated per month equal to the model’s rates for treating cavities, and 4.2 assumes a more aggressive program of screening and treatment. In simulations 4.3 and 4.4, follow-up care is intensified for children who have had prior restorative care to limit recurrence by 50% or 75%. The 2009-adjusted cost for caries management of $242 is the median between MEPS-reported costs for preventive and restorative care (14).
Motivational interviewing
Motivational interviewing, with appropriate follow-up, can reduce cavity prevalence by 63% (29), at an estimated per-child cost of $100. Simulations 5.1 and 5.2 consider motivational interviewing for all children and for high-risk children.
Combined interventions
Simulation 6.1 combines fluoride varnish for all children older than 6 months (1.2) with clinical treatment of active caries (4.1). Simulation 6.2 adds clinical care of cavities to reduce recurrence (4.3). Simulation 6.3 adds motivational interviewing (5.1). Combining interventions can have cumulative and complementary effects. Combining several interventions can produce a smaller fraction of children with cavities than can any of the single interventions.
Discussion
This study, for the first time, synthesizes information from a wide variety of sources to formulate a model that compares ECC interventions for their effect on caries and their associated costs over time.
This study of Colorado’s young children provides evidence that may be immediately applicable to policy making within and beyond the state. Expansion of community water fluoridation (1.1) was found to be cost-saving even in an environment where most of the population receives fluoridated water, and additional savings are projected from reduced treatment and retreatment costs for children older than 6 years who are not considered in the model. Fluoride varnish application for all children older than 6 months provides greater cavity reduction than does water fluoridation or other fluoride varnish interventions and saves more than half the program’s cost in reduced restorative care but is the most expensive of the fluoride interventions. Starting fluoride varnish early (age >6 mo) results in greater disease reductions than starting later (age >24 mo) and pays back a larger percentage of program costs in reduced restorative costs (55% vs 32%). Targeting the highest-risk children has a similar effect on prevalence as fluoride varnish for all children older than 24 months but at a lower program cost. Thus, when funding is limited, priority should be considered for children at highest risk. Similarly, motivational interviewing yields a greater benefit per dollar expended when targeting high-risk children.
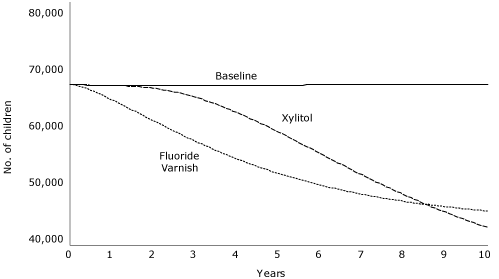
Effects of xylitol interventions with children are limited by their late application. As with fluoride varnish, reductions in restorative costs pay back a substantial portion of the program costs. Xylitol for only high-risk mothers yields a smaller overall effect than for all mothers but larger proportional reductions in restorative costs. Applying this intervention to the high-risk group was projected to achieve a net savings of $3 million. Comparing fluoride varnish for all children with xylitol for all mothers (Figure 2) reveals that over time these 2 interventions play out differently and counterintuitively. Fluoride varnish results in more immediate cavity reductions among children older than 24 months, but reducing transmission by treating mothers results in greater long-term cavity reductions for this age group. A combined xylitol intervention for both mothers and children may be useful.
Figure 2. Number of Colorado children aged 2 to 5 years projected to experience cavities during a 10-year period, given 1 of 2 interventions in a simulation model, compared to baseline. The 2 interventions were xylitol treatment of mothers to limit cariogenic bacterial transmission and fluoride varnish application to all children aged 6 months or older. [A tabular version of this figure is also available.]
Secondary prevention is effective in reducing the fractions of children with cavities and resulting restorative costs. There is no additional programmatic cost assumed in these simulations, although such a cost might be estimated. Preventive care aimed at reducing recurrence does not, by definition, affect the fraction with cavities but does reduce the fraction of children with untreated cavities. The effect of secondary prevention is also evident in the reduced cost for restorative care that would otherwise be required for children with recurrent cavities.
Taken together, findings of simulations that project the disease reduction and cost effects of various ECC interventions suggest that 1) interventions targeting the youngest children take 2 to 4 years longer to affect the entire population of preschool-age children but lead to greater reductions in ECC, 2) interventions targeting the highest-risk children provide the highest return on investment, 3) combined interventions that target ECC at several stages of its natural history can have the most profound effect. Primary prevention provides the greatest leverage, but limiting disease progression by screening for and treating caries before cavities form is also productive.
Although the model’s framework is generalizable to any population of young children, findings will depend on the quality of the data inputs available, the geographic area considered, and the underlying rates of ECC in the target population. Needed to improve the model’s validity are more reliable and current data sources. Applying the framework more widely will allow cross-state and cross-population comparisons that can be assessed for consistency or sensitivity to changes in underlying inputs.
The strengths of this model are its flexibility, capacity for long-term projections, ability to empirically derive findings that may not be anticipated, and value in comparing alternative interventions within complex systems. Weaknesses relate primarily to the quality of data used because proxies, estimates, expert opinions, and extrapolations were employed when data were not available. As with studies of other chronic disease interventions, system dynamics is valuable for policy makers who seek the most favorable outcomes and cost efficiencies in reducing population disease burden. We conclude from this work that ECC burden among children in Colorado can be markedly reduced through a combination of interventions designed to prevent and suppress the caries process that too commonly results in dental cavities.
Acknowledgments
We gratefully acknowledge the financial support of CDC through a cooperative agreement with the Children’s Dental Health Project (CDC-RFA-DP09-908) and of the Health Resources and Services Administration (HRSA) through a Maternal and Child Health Bureau (MCHB) award to the Colorado Department of Public Health and Environment (CDPHE) (HRSA: MCHB TOHS no. H47MC08655). A 2009 expert panel at Columbia University included Pamela Koch (Columbia University Teacher’s College Center for Food & Environment), Piyumika Kularatne (Columbia University Division of Community Health), Omolara Thomas (Columbia University Medical Center), Norman Tinanoff (University of Maryland Dental School), and Kathy O’Loughlin (American Dental Association, formerly, United Healthcare and Delta Dental of Massachusetts). A 2009 expert panel in Colorado included Diane Brunson (University of Colorado Denver School of Dental Medicine), Jodi Hardin (CDPHE), Rachel Hutson (CDPHE), and Heather Dubiel (CDPHE). Additional and substantial contributions were made by Richard Manski (University of Maryland Dental School) and William Maas (University of Maryland Dental School, formerly, Pew Center on the States and CDC Division of Oral Health), Renee Calanan (CDPHE), Rickey Tolliver (CDPHE), and Anu Tate (CDHPE).
Author Information
Corresponding Author: Burton L. Edelstein, DDS, MPH, Professor, Columbia University College of Dental Medicine, 622 West 168th St, Box 20, New York, NY 10032. Telephone: 212-342-3505. E-mail: ble22@columbia.edu. Dr Edelstein is also president of the Children’s Dental Health Project.
Author Affiliations: Gary B. Hirsch, Learning Environments, Wayland, Massachusetts; Marcy Frosh, Children’s Dental Health Project, Washington, DC; Theresa Anselmo, San Luis Obispo County Health Agency, San Luis Obispo, California. At the time of this study, Ms Anselmo was affiliated with the Colorado Department of Public Health and Environment, Denver, Colorado.
References
- Iida H, Auinger P, Billings RJ, Weitzman M. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics 2007;120(4):e944-52.
- Powell LV. Caries prediction: a review of the literature. Community Dent Oral Epidemiol 1998;26(6):361-71.
- Edelstein BL, Chinn CE. Update on disparities in oral health and access to dental care for America’s children. Acad Pediatr 2009;9(6):415-9.
- Reforming States Group. Pediatric dental care in CHIP and Medicaid: paying for what kids need, getting value for state payments. Milbank Memorial Fund; 1999. http://www.milbank.org/reports/990716mrpd.html. Accessed September 26, 2011.
- Homer JB, Hirsch GB. System dynamics modeling for public health: background and opportunities. Am J Public Health 2006;96(3):452-8.
- Hale KJ. American Academy of Pediatrics Section on Pediatric Dentistry. Oral health risk assessment timing and establishment of the dental home. Pediatrics 2003;111(5 Pt 1):1113-6.
- Ramos-Gomez FJ, Crystal YO, Ng MW, Crall JJ, Featherstone JD. Pediatric dental care: prevention and management protocols based on caries risk assessment. J Calif Dent Assoc 2010;38(10):746-61.
- Pearce MS, Thomson WM, Walls AW, Steele JG. Lifecourse socio-economic mobility and oral health in middle age. J Dent Res 2009;88(10):938-41.
- Colorado Child Health Survey. Colorado Department of Public Health and Environment. http://www.cdphe.state.co.us/hs/yrbs/childhealth.html. Accessed June 6, 2011.
- Beltrán-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, Griffin SO, et al. Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis — United States, 1988-1994 and 1999-2002. MMWR Surveill Summ 2005;54(3):1-43.
- Vargas CM, Crall JJ, Schneider DA. Sociodemographic distribution of pediatric dental caries: NHANES III 1988-1994. J Am Dent Assoc 1998;129(9):1229-38.
- Medicaid: concerns remain about sufficiency of data for oversight of children’s dental services. GAO-07-826T. US Government Accountability Office; 2007. http://www.gao.gov/new.items/d07826t.pdf. Accessed December 13, 2012.
- Warren JJ, Levy SM, Kanellis MJ. Dental caries in the primary dentition: assessing prevalence of cavitated and noncavitated lesions. J Public Health Dent 2002;62(2):109-14.
- Manski RJ, Brown E. Dental use, expenses, private dental coverage, and changes, 1996 and 2004. MEPS chartbook No. 17. US Department of Health and Human Services, Agency for Healthcare Research and Quality; 2007. http://www.meps.ahrq.gov/mepsweb/data_files/publications/cb17/cb17.pdf. Accessed June 6, 2011.
- Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report 2009;(11):1-25.
- Edelstein B, Tinanoff N. Screening preschool children for dental caries using a microbial test. Pediatr Dent 1989;11(2):129-32.
- Litt MD, Reisine S, Tinanoff N. Multidimensional causal model of dental caries development in low-income preschool children. Public Health Rep 1995;110(5):607-17.
- Caulfield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res 1993;72(1):37-45.
- Truman BI, Gooch BF, Sulemana I, Gift HC, Horowitz AM, Evans CA, et al. Reviews of evidence on interventions to prevent dental caries, oral and pharyngeal cancers, and sports-related craniofacial injuries. Am J Prev Med 2002;23(1 Suppl):21-54.
- Surgeon General’s statement on community water fluoridation, 1995 [updated 2009]. US Department of Health and Human Services, Centers for Disease Control and Prevention. http://www.cdc.gov/fluoridation/fact_sheets/sg95.htm. Accessed June 6, 2011.
- Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2002;(3):CD002279.
- Kanellis MJ. Caries risk assessment and prevention: strategies for Head Start, Early Head Start, and WIC. J Public Health Dent 2000;60(3):210-7.
- Söderling E, Isokangas P, Pienihäkkinen K, Tenovuo J, Alanen P. Influence of maternal xylitol consumption on mother-child transmission of mutans streptocococci: 6-year follow up. Caries Res 2001;35(3):173-7.
- Köhler B, Bratthall D, Krasse B. Preventive measures in mothers influence the establishment of bacterium Streptococcus mutans in their infants. Arch Oral Biol 1983;28(3):225-31.
- Isokangas P, Söderling E, Pienihäkkinen K, Alanen P. Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age. J Dent Res 2000;79(11):1885-9.
- Köhler B, Andréen I. Influence of caries — preventive measures in mothers on cariogenic bacteria and caries experience in their children. Arch Oral Biol 1994;39(10):907-11.
- Ly KA, Milgrom P, Rothen M. Xylitol, sweeteners, and dental caries. Pediatr Dent 2006;28(2):154-63.
- Hayes C. The effect of non-cariogenic sweeteners on the prevention of dental caries: a review of the evidence. J Dent Educ 2001;65(10):1106-9.
- Weinstein P, Harrison R, Benton T. Motivating parents to prevent caries in their young children: one-year findings. J Am Dent Assoc 2004;135(6):731-8.
Table
 Table. Results of a Simulation Model for Designing Effective Interventions in Early Childhood Caries, Colorado
Table. Results of a Simulation Model for Designing Effective Interventions in Early Childhood Caries, Colorado
| Simulation | Children With Cavities, % | Children Whose Cavities Are Untreated, % | Decayed and Filled Teeth, n | 10-Year Cumulative Cost of Restorative Care, $ (Millions) | 10-Year Savings of Restorative Care Compared to Baseline, $ (Millions) | 10-Year Cumulative Program Cost, $ (Millions) |
|---|---|---|---|---|---|---|
| Baseline (no intervention) | 18.2 | 71.4 | 265,923 | 208 | 0 | 0 |
| Fluoride interventions | ||||||
| 1.1: Community water fluoridation for all | 17.0 | 70.8 | 249,042 | 194 | 14 | 6 |
| 1.2: Fluoride varnish for children >6 mo | 12.4 | 67.3 | 182,196 | 143 | 65 | 118 |
| 1.3: Fluoride varnish for high-risk children >6 mo | 14.7 | 68.7 | 214,281 | 174 | 34 | 56 |
| 1.4: Fluoride varnish for all children >24 mo | 16.0 | 67.8 | 233,823 | 181 | 27 | 85 |
| Transmission interventions in mothers | ||||||
| 2.1: Xylitol for all mothers | 10.8 | 68.2 | 160,609 | 152 | 56 | 79 |
| 2.2: Xylitol for mothers of high-risk children | 15.0 | 69.3 | 201,765 | 180 | 28 | 25 |
| Xyiltol interventions in children | ||||||
| 3.1: Xylitol for all children >24 mo, low-impacta | 15.2 | 66.2 | 222,741 | 172 | 36 | 93 |
| 3.2: Xylitol for all children >24 mo, high-impactb | 13.3 | 60.3 | 193,797 | 148 | 60 | 93 |
| 3.3: Xylitol for high-risk children >24 mo, low-impacta | 16.9 | 68.4 | 238,711 | 189 | 19 | 29 |
| 3.4: Xylitol for high-risk children >24 mo, high-impactb | 16.0 | 65.5 | 220,179 | 176 | 32 | 29 |
| 3.5: Xylitol for all children >6 mo, high-impactb | 5.6 | 55.3 | 82,187 | 77 | 131 | 107 |
| Clinical treatment: caries treatment and prevention of recurrence | ||||||
| 4.1: Caries treatment, children >6 mo, low treatment intensity | 14.2 | 71.4 | 209,833 | 177 | 31 | 33 |
| 4.2: Caries treatment, children >6 mo, high treatment intensity | 12.8 | 70.3 | 189,550 | 168 | 40 | 49 |
| 4.3: Prevention of recurrence, 50% reduction | 18.2 | 62.7 | 265,923 | 186 | 22 | 0 |
| 4.4: Prevention of recurrence, 75% reduction | 18.2 | 55.3 | 265,923 | 169 | 39 | 0 |
| Motivational interviewingc | ||||||
| 5.1: Motivational interviewing for all families | 6.5 | 59.4 | 95,434 | 86 | 122 | 111 |
| 5.2: Motivational interviewing for high-risk families only | 12.9 | 66.0 | 159,020 | 144 | 64 | 35 |
| Combination interventions | ||||||
| 6.1: Combination of 1.2 and 4.1 | 9.1 | 66.4 | 136,005 | 121 | 87 | 147 |
| 6.2: Combination of 1.2, 4.1, and 4.3 | 9.1 | 57.4 | 136,005 | 108 | 100 | 147 |
| 6.3: Combination of 1.2, 4.1, 4.3, and 5.1 | 3.8 | 42.4 | 56,158 | 59 | 149 | 245 |
a Low-impact assumes that xylitol interventions reduce caries at the low end (by 44%) of the reported range of impact (29).
b High-impact assumes that xylitol interventions reduce caries at the high end (by 73%) of the reported range of impact (29).
c Defined as a brief interactive approach to counseling and educating parents that focuses on skills that move patients to action.
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions.