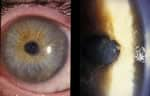
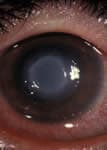
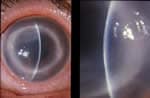
Acanthamoeba Keratitis Fact Sheet for Healthcare Professionals
The following information is provided as a resource on Acanthamoeba keratitis for physicians and ophthalmologists.
This information is not meant to be used for self-diagnosis or as a substitute for consultation with a health care provider. If you have any questions about the parasites described above or think that you may have a parasitic infection, consult a health care provider.
References
- Pussard, M. , and Pons, R. Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida). Protistologica. 1977;13:557-98.
- Centers for Disease Control (CDC). Acanthamoeba keratitis associated with contact lenses–United States. MMWR Morb Mortal Wkly Rep. 1986;35:405-8.
- Yu HS, Kong HH, Kim SY, Hahn YH, Hahn TW, Chung DI. Laboratory investigation of Acanthamoeba lugdunensis from patients with keratitis. Invest Ophthalmol Vis Sci. 2004;45:1418-26.
- Simitzis-Le Flohic AM, Hasle DP, Paniagua-Crespo E, Colin J, Lagoutte F, Donval A, Bellon C. Acanthamoeba keratitis. Epidemiologic and parasitologic study. J Fr Ophtalmol. 1989;12:361-6.
- Ledee DR, Hay J, Byers TJ, Seal DV, Kirkness CM. Acanthamoeba griffini. Molecular characterization of a new corneal pathogen. Invest Ophthalmol Vis Sci. 1996;37:544-50.
- Stothard, D.R. , Schroeder-Diedrich, J.M. , Awwad, M.H. , Gast, R.J. , Ledee, D.R. , Rodriguez-Zaragoza, S. , Dean, C.L. , Fuerst, P.A. , and Byers, T.J. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 1998;45:45-54.
- Walochnik, J. , Haller-Schober, E. , Kolli, H. , Picher, D. , Obwaller, A. , and Aspock, H. Discrimination between clinically relevant and nonrelevant Acanthamoeba strains isolated from contact lens-wearing keratitis patients in Austria. J. Clin. Microbiol. 2000;38:3932-6.
- Parmar DN, Awwad ST, Petroll WM, Bowman RW, McCulley JP, Cavanagh HD. Tandem scanning confocal corneal microscopy in the diagnosis of suspected Acanthamoeba keratitis. Ophthalmology. 2006;113:538-47.
- Schaumberg DA, Snow KK, Dana MR. The epidemic of Acanthamoeba keratitis: where do we stand? Cornea. 1998;17:3-10.
- Stehr-Green JK, Bailey TM, Visvesvara GS. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989;107:331-6.
- Jaison PL, Cao Z, Panjwani N. Binding of Acanthamoeba to [corrected] mannose-glycoproteins of corneal epithelium: effect of injury. Curr Eye Res. 1998;17:770-6. Erratum in: Curr Eye Res 1998;17:1036.
- Morton LD, McLaughlin GL, Whiteley HE. Effects of temperature, amebic strain, and carbohydrates on Acanthamoeba adherence to corneal epithelium in vitro.Infect Immun. 1991;59:3819-22.
- Yang Z, Cao Z, Panjwani N. Pathogenesis of Acanthamoeba keratitis: carbohydrate-mediated host-parasite interactions. Infect Immun. 1997;65:439-45.
- Hurt M, Niederkorn J, Alizadeh H. Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:3424-31.
- Leher H, Silvany R, Alizadeh H, Huang J, Niederkorn JY. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect Immun. 1998;66:5-10.
- Leher HF, Alizadeh H, Taylor WM, Shea AS, Silvany RS, Van Klink F, Jager MJ, Niederkorn JY. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1998;39:2666-73.
- Biddick CJ, Rogers LH, Brown TJ. Viability of pathogenic and nonpathogenic free-living amoebae in long-term storage at a range of temperatures. Appl Environ Microbiol. 1984;48:859-60.
- Neff RJ, Neff RH. The biochemistry of amoebic encystment. Symp Soc Exp Biol. 1969;23:51-81.
- Brandt FH, Ware DA, Visvesvara GS. Viability of Acanthamoeba cysts in ophthalmic solutions. Appl Environ Microbiol. 1989;55:1144-6.
Page last reviewed: December 11, 2017