COVID-19 Science Update released: December 1, 2020 Edition 67

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Factors associated with mental health disorders among university students in France confined during the COVID-19 pandemicexternal icon. Wathelet et al. JAMA Network Open (October 23, 2020).
Key findings:
- 29,564 students (42.8%) reported at least one mental health symptom, including: anxiety (27.5%); stress (24.7%); severe distress (22.4%); severe depression (16.1%); and suicidal thoughts (11.4%).
- Risk factors associated with reporting at least one mental health symptom included:
- Female gender (OR 2.10, 95% CI 2.02-2.19, p <0.001) or non-binary gender (OR 3.57, 95% CI 2.99-4.27, p <0.001).
- Low-quality housing (OR 2.30, 95% CI 2.06-2.57, p <0.001).
- History of psychiatric follow-up (OR 3.28, 95% CI 3.09-3.48, p <0.001).
- Symptoms compatible with COVID-19 (OR 1.55, 95% CI 1.49-1.61, p <0.001).
- Social isolation (OR 3.63, 95% CI 3.35-3.92, p <0.001).
- Low quality of media information received (OR 1.56, 95% CI 1.49-1.64, p <0.001).
- Only 12.4% of students reporting severe mental health symptoms sought mental health care.
Methods: A survey of 69,054 university students in France from April 17 to May 4, 2020 who experienced quarantine during the COVID-19 pandemic collected data on the rates of self-reported suicidal thoughts, severe distress, stress, anxiety, and depression. Limitations: Small proportion (4%) of entire university population included; possible self-selection bias.
Implications: Prevention, surveillance, and access to mental healthcare services are important for university students, particularly for women, nonbinary students and those with a history of psychiatric follow up.
Incidence of COVID-19 virus transmission in three dental offices: a 6-month retrospective study.external icon Froum et al. International Journal of Periodontics & Restorative Dentistry (November 6, 2020).
Key findings:
- No occupationally-acquired infections were identified among staff involved in care of 2,810 patients that often included aerosol-generating procedures.
- No patients reported COVID-19 symptoms two weeks after having an aerosol-generating dental procedure, suggesting no transmission from office staff to patients.
Methods: Retrospective study in three different dental offices between March 15 to September 15, 2020 in New York using enhanced infection control measures. All patients were contacted two weeks post-procedure to determine whether they experienced any COVID-19 symptoms. Three staff members contracted COVID-19 during the study period while furloughed; a family member was the source of infection for all three. Limitations: Small study sample; patients may have been asymptomatically infected.
Implications: Enhanced infection control standard operating procedures appears to mitigate risk of SARS-CoV-2 transmission in dental settings even when aerosol-generating dental procedures take place.
Effectiveness of adding a mask recommendation to other public health measures to prevent SARS-CoV-2 infection in Danish mask wearers.external icon Bundgaard et al. Annals of Internal Medicine (November 18, 2020). pc
Key findings:
- 42 (1.8%) participants in the “mask recommendation” group were diagnosed with SARS-CoV-2 compared to 53 (2.1%) participants in the control group (OR = 0.82, 95% CI 0.54-1.23, p = 0.33).
Methods: Randomized controlled trial of 4,862 adults from April to June 2020 in Denmark to assess if surgical mask use reduced the wearers’ risk for SARS-CoV-2 infection. Participants in the mask group were instructed to wear a mask when outside the home during the next month. Primary outcome was SARS-CoV-2 infection diagnosed through self-collected specimens for serology and PCR or by a healthcare provider. During the study, mask use was uncommon in the community, there was no official recommendation for mask wearing, other public health prevention interventions were recommended, and community prevalence was 2%. Limitations: Self-reported primary outcome data; 46% self-reported mask use; did not assess the role of masks in source control; unblinded study could not control for disinhibition or increased risk taking from wearing a mask; study designed to detect 50% decrease in acquisition of infection and not powered to detect smaller differences in transmission between arms.
Implications: While this trial of mask-wearing suggests that masks do not protect the wearer from SARS-CoV-2 infection, Laine et alexternal icon. emphasize that it neither addresses mask-wearing as source control nor definitively shows that masks do not protect the wearer due to 46% adherence to mask-wearing. Frieden et al. external icon point out the limitations of using antibody tests with specificity of 97.5% as the primary measure of “infection” in this study.
Risk of severe COVID-19 among workers and their household members.external icon Selden et al. JAMA Internal Medicine (November 9, 2020).
Key findings:
- 49.7% (123.2 million) of US adults met the main CDC increased risk guidelines for severe COVID-19; 61% (151.3 million) met the broader CDC guidelines for increased risk.
- 27.7% (34.1 million) who met the main CDC guidelines were essential workers who could not work at home (WAH).
- 46.1% (56.7 million) either lived with or were themselves essential workers who could not WAH; using broader CDC guidelines this increases to 49.1% (74.3 million).
Methods: Data from a 2014-2017 in-person household survey of US civilians that provided information on health and employment was used to assess the prevalence of CDC-defined risks for severe COVID-19 among adults who held essential jobs and could not WAH or who lived in households with such workers. Broader guidelines added smoking, asthma, and hypertension to these risks. Limitations: Pre-pandemic data may not reflect current employment and WAH status; self-reported risk factors.
Implications: Policies related to reopening and vaccine distribution should take into consideration not only the health risk of essential workers, but also of those with whom they live.
The COVID-19 pandemic in the US has interrupted outpatient medical services. The following articles report on the uptake of telemedicine for various health services and barriers to doing so.
PEER-REVIEWED
A. Disparities in the uptake of telemedicine during the COVID-19 surge in a multidisciplinary head and neck cancer population by patient demographic characteristics and socioeconomic statusexternal icon. Tam et al. JAMA Otolaryngology Head Neck Surgery (November 5, 2020).
Key findings:
- Of 401 visits from March 17 to April 24, 2020, 87 (25.1%) were in-person, 170 (49.1%) were telemedicine, and 89 (23.6%) were by telephone.
- There were no telemedicine visits for this health system in 2019.
- Patients with Medicaid/none/other public insurance were less likely to complete telemedicine visits compared with patients with private insurance (OR 0.26, 95% CI 0.10-0.66).
- Patients in the lowest two median household income quartiles had lower completion of telemedicine visits compared with highest income quartile (OR 0.33, 95% CI 0.14-0.82 for the second income quartile; OR 0.22, 95% CI 0.07-0.74 for lowest income quartile).
- Female patients (OR 0.27, 95% CI 0.09-0.79) and patients with Medicaid/none/other public insurance (OR 0.09, 95% CI 0.01-0.51) were less likely to complete any type of visit during COVID-19 pandemic than males or patients with private insurance.
Methods: Data on 364 encounters from Henry Ford Health System were collected from 234 patients between March 17 and April 24, 2020. Data were compared with patients being treated in same clinic during the identical timeframe in 2019. Analysis included 4 types of clinic visits, telemedicine (live audio and video), telephone, in-person and no-show. Limitations: Use of census data for socioeconomic status rather than individual data.
B. Changes in health services use among commercially insured US populations during the COVID-19 pandemicexternal icon. Whaley et al. JAMA Network Open (November 5, 2020).
Key findings:
- In-person office visits decreased by 25% in March 2020 and 68% in April 2020 compared with the previous two years.
- Regression-adjusted use of telemedicine services increased by 227.9 (95% CI 221.7-234.1) per 10,000 persons and 641.6 (95% CI 635.5-647.8) per 10,000 persons in March 2020 and April 2020, respectively.
- Telemedicine services offset 40% of in-person office visits in March 2020, and 42% of in-person office visits in April 2020.
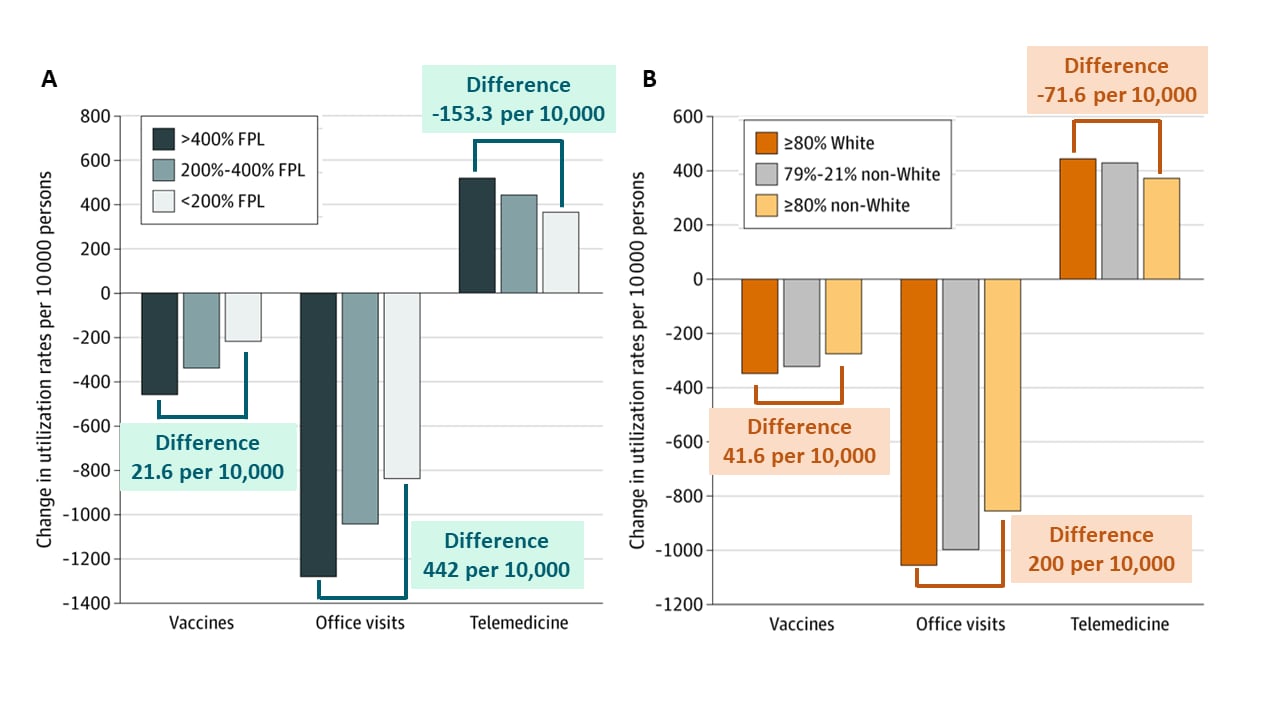
- Compared with patients living in zip codes with higher income or predominately White populations, during March and April 2020, patients living in zip codes with lower-income or majority racial/ethnic minority populations had: (Figure)
- Lower reductions in childhood vaccinations.
- Smaller reductions in in-person visits.
- Lower rates of adoption of telemedicine.
Methods: Cross-sectional study assessing trends in the use of health services (such as health care service office visits, telemedicine services, and childhood vaccination) analyzing health insurance claims data from January 1, 2018 to April 30, 2020. Limitations: Data included only individuals with employer-sponsored insurance.
Figure
Note: Adapted from Whaley et al. Regression adjusted change in vaccine receipt, office visits, and telemedicine by patient zip code-level income (A) and race (B). FPL, Federal Poverty Line, 2018 ($26,200 USD for household of 4 persons). Licensed under CC-BY.
C. Trends in Outpatient Care Delivery and Telemedicine During the COVID-19 Pandemic in the US.external icon Patel et al. JAMA Internal Medicine (November 16, 2020).
Key findings:
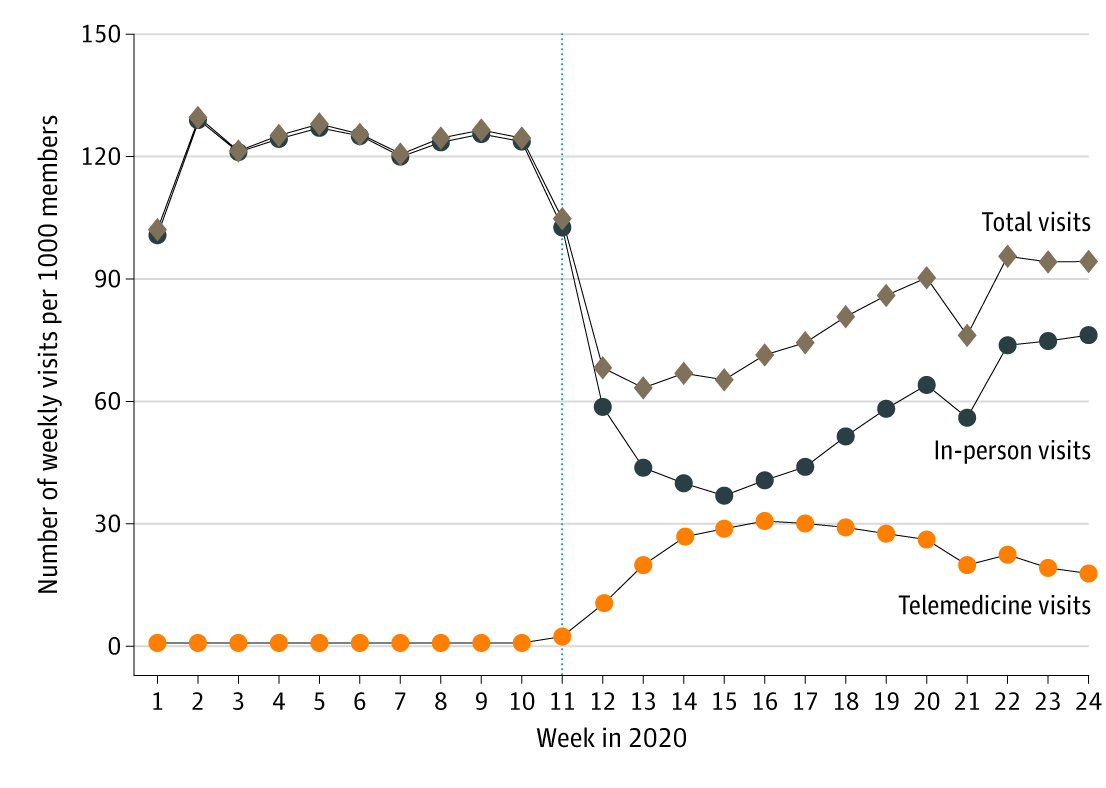
- From January 1 to June 16, 2020 (Figure 1):
- Telemedicine visits increased 17%, from 0.8 to 17.8 per 1,000 enrollees.
- In-person visits decreased 34%, from 102.7 to 76.3 per 1,000 enrollees.
- Total visits decreased 9.5%, from 103.5 to 94.1 per 1,000 enrollees.
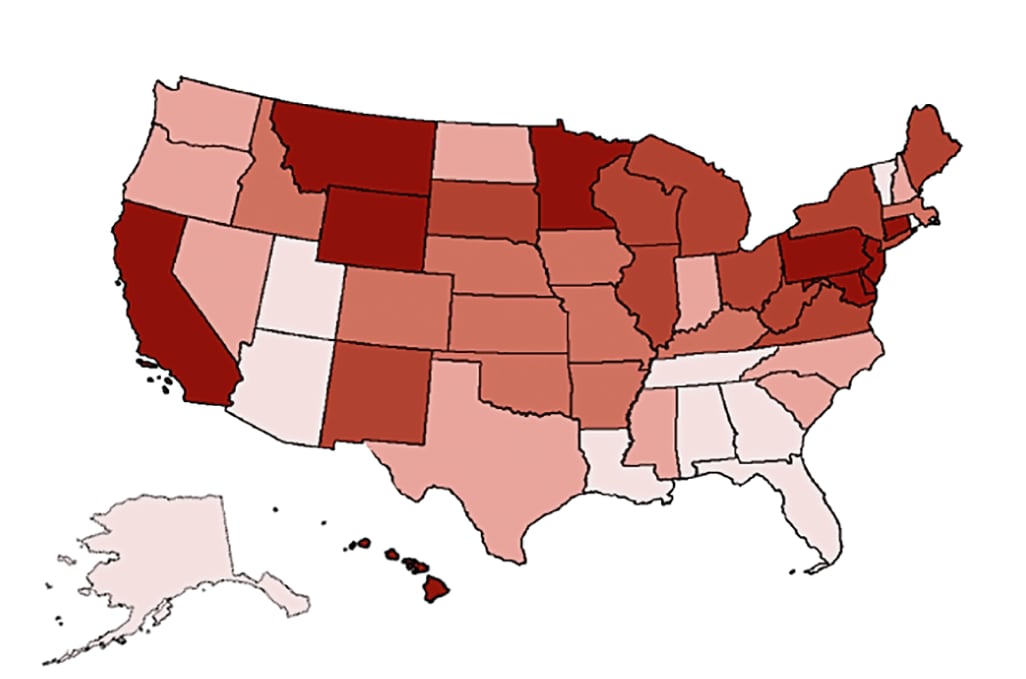
- There was a wide geographic variation in the percent of total visits delivered by telemedicine (Figure 2).
Methods: Changes in outpatient visit volume were assessed from insurance claims from 16,740,365 enrollees by capturing weekly rates per 1,000 enrollees of telemedicine, in-person, and total visits from January 1 to June 16, 2020. Limitations: Only included commercially insured persons.
Figure 1
Note: Adapted from Patel et al. Trends in total visits, in-person visits, and telemedicine visits from January 1 to June 16, 2020. Reproduced with permission from JAMA Internal Medicine. 2020. doi:10.1001/jamainternmed.2020.5928. Copyright© 2020 American Medical Association. All rights reserved.
Note: Adapted from Patel et al. Change in total outpatient care visits by state January 1 to June 16, 2020, color-coded by quintile of change in total visits from lowest % change to highest % change. Permission request in process. Reproduced with permission from JAMA Internal Medicine. 2020. doi:10.1001/jamainternmed.2020.5928. Copyright© 2020 American Medical Association. All rights reserved.
Implications for three articles (Tam et al., Whaley et al., & Patel et al.): Telemedicine in the US during the early COVID-19 pandemic did not offset the drop in in-person visits and was not equally adopted across racial and socioeconomic lines. When scaling up telemedicine during and after COVID-19 pandemic it will be important to consider the special needs of socio-economically disenfranchised populations.
PEER-REVIEWED
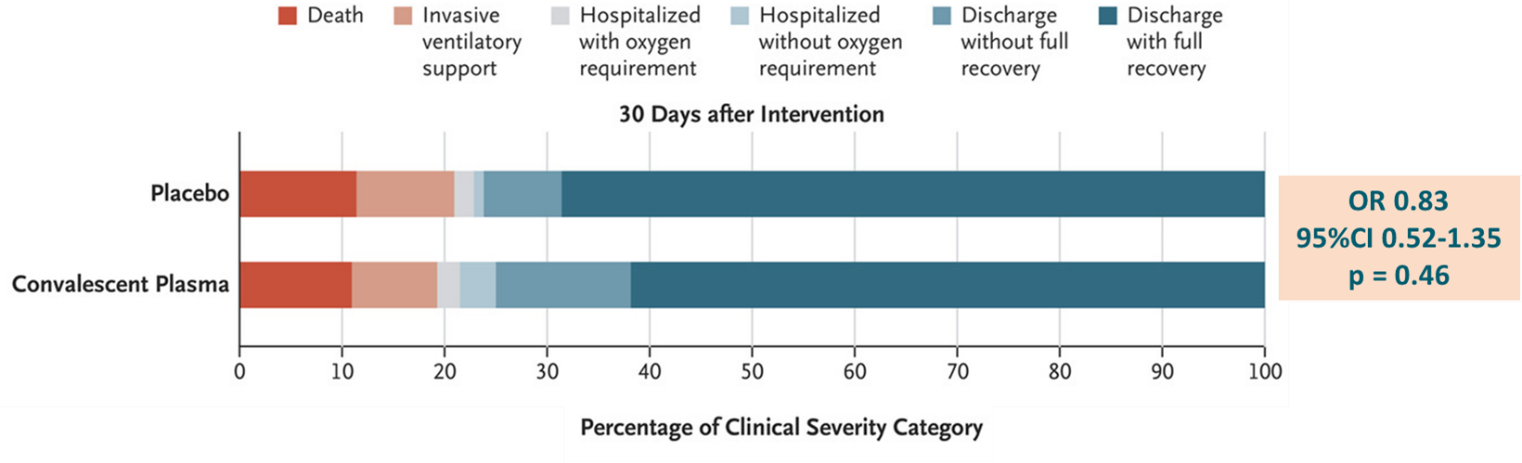
A randomized trial of convalescent plasma in COVID-19 severe pneumonia. external iconSimonovich et al. NEJM (November 24, 2020).
Key findings:
- At 30 days, there was no difference between the convalescent plasma (CP) and placebo groups in clinical outcomes (OR 0.83, 95% CI 0.52-1.35, p = 0.46) (Figure).
- No significant differences in clinical status were seen at day 7 (OR 0.88, 95% CI 0.58-1.34) or day 14 (OR 1.0, 95% CI 0.65-1.55).
- Overall mortality was 11.0% in the CP group and 11.4% in the placebo group, (risk difference 0.46%, 95% CI 7.8 -6.8).
Methods: Double-blind, placebo-controlled trial between May 28 and August 27, 2020 at 12 sites in Argentina where 228 hospitalized adults with severe COVID-19 pneumonia received CP and 105 received placebo. The primary outcome of clinical status included death, ventilatory support, hospitalization with oxygen requirement, hospitalization without oxygen requirement, discharged without full recovery, discharge with full recovery. CP had a median titer of 1:3200 of total SARS-CoV-2 antibodies. Limitations: Conclusions cannot be drawn regarding efficacy of CP therapy earlier than the median time of entry to this trial or in patients with milder disease.
Implications: CP therapy appears non-effective in treatment of severe pneumonia in this study. These data contrast findings of nonrandomized studies showing the benefit of CP and illustrates the importance of rigorous, randomized, placebo-controlled trials in the consideration of treatment recommendations.
Figure:
Note: Adapted from Simonovich et al. Clinical outcomes among patients treated with CP compared with placebo. From NEJM, Simonovich et al., A randomized trial of convalescent plasma in COVID-19 severe pneumonia. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
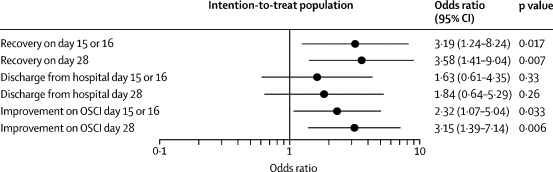
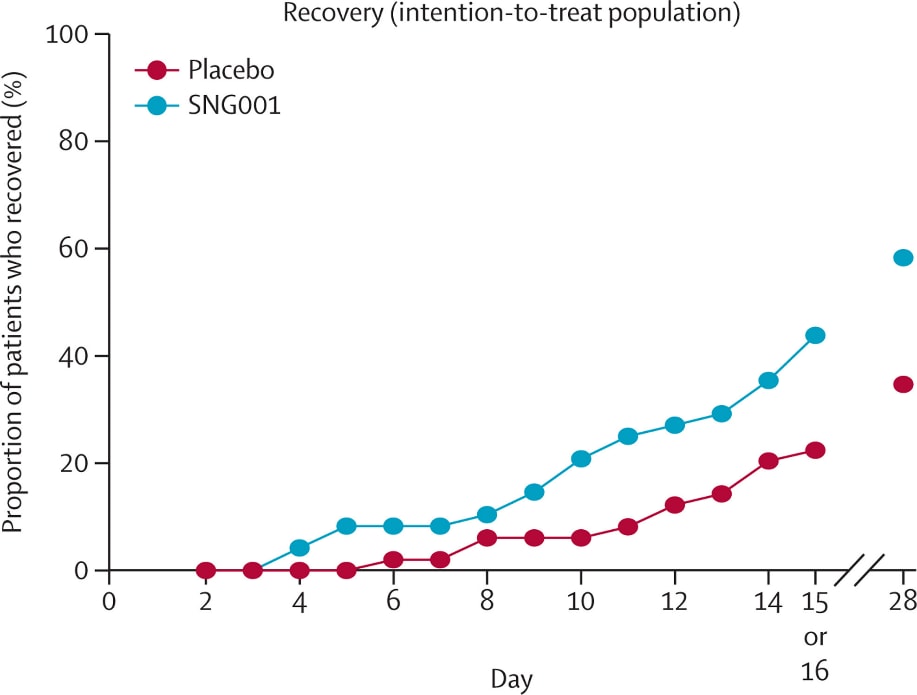
Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial.external icon Monk et al. Lancet Respiratory Medicine (November 12, 2020).
Key findings:
- Compared to placebo group, treatment group receiving inhaled interferon beta-1a had:
- Higher odds of improvement on WHO Ordinal Scale for Clinical Improvement (OSCI) on day 15 or 16 (OR 2.32, 95% CI 1.07-5.04, p = 0.033).
- Higher likelihood of achieving OSCI score of 1 (no limitation of activities) during treatment (hazard ratio [HR] 2.19, 95% CI 1.03-4.69, p = 0.043).
- Similar odds of hospital discharge by day 28 (OR 1.84, 95% CI 0.64–5.29, p = 0.26).
- Most frequently reported adverse event was headache (7 [15%] treatment group; 5 [10%] placebo group).
- Three deaths reported in placebo group, none in the treatment in group.
Methods: Hospitalized COVID-19 patients were randomized upon admission to treatment (n = 48, nebulized recombinant interferon beta-1a) vs placebo (n = 50). Intention-to-treat analysis was conducted to estimate OR of change in WHO OSCI score (0 = no infection; 8 = death). Limitations: Limited generalizability; OSCI not validated for clinical trials; imbalanced randomization on baseline comorbidities.
Implications: Nebulized recombinant interferon beta-1a treatment seems to be well-tolerated and associated with higher odds of clinical improvement and recovery among admitted patients with COVID-19.
Figure 1
Note: From Monk et al. Comparisons for recovery, hospital discharge, and improvement on the WHO OSCI on days 15 or 16 and day 28 between the treatment and placebo groups. Reprinted from The Lancet Respiratory Medicine, Monk et al., Safety and efficacy of inhaled nebulized interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomized, double-blind, placebo-controlled, phase 2 trial. Copyright 2020, with permission from Elsevier.
Figure 2
Note: From Monk et al. Proportion of treatment and placebo patients who recovered by day 15 or 16 and day 28. Reprinted from The Lancet Respiratory Medicine, Monk et al., Safety and efficacy of inhaled nebulized interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomized, double-blind, placebo-controlled, phase 2 trial. Copyright 2020, with permission from Elsevier.
Sixty-day outcomes among patients hospitalized with COVID-19.external icon Chopra et al. Annals of Internal Medicine (November 11, 2020).
Key findings:
- Nearly a quarter (24%; 398/1,648) of admitted COVID-19 patients died during hospitalization.
- 158/1,250 (12.6%) surviving patients were transferred to a skilled nursing or rehabilitation facility.
- 60-day follow-up of surviving COVID-19 patients revealed that:
- 84 died, increasing the overall mortality rate to 29% and ICU mortality rate to 65%.
- 15% (189/1,250) were readmitted to a hospital.
- Phone surveys with 42% (488/1,166) of the surviving patients revealed cardiopulmonary symptoms (33%), inability to return to work (16%), emotional impact (49%) and financial impact (37%) of COVID-19, with some reporting loss of entire savings (4%) due to COVID-19.
Methods: Observational cohort study of 1,648 patients hospitalized for COVID-19 and discharged between March 16 and July 1, 2020 from 38 Michigan hospitals. Charts were reviewed for clinical details, readmission, or death following discharge. Surviving patients not readmitted at time of follow-up participated in a phone survey on current symptoms, medical care, financial impact of medical issues, and overall mental health. Limitations: >50% lost to follow-up.
Implications: Hospitalization may be just the beginning of persistent health, emotional, and financial hardships for many persons with COVID-19. Mental health assessments and social services should be part of the inpatient care team to ensure that these resources are immediately available to patients on discharge.
Long term COVID-19
- Siegelman JN. Reflections of a COVID-19 long hauler.external icon JAMA. An emergency medicine physician shares his experiences with “mild” COVID lasting months that made it impossible for him to work, recognizing that many in the same situation cannot self-isolate.
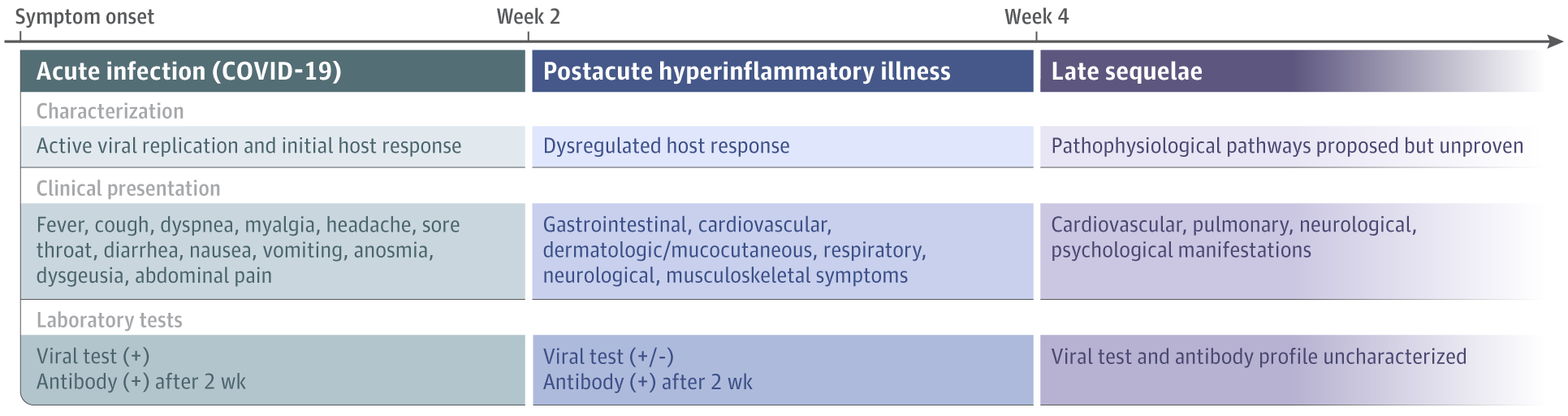
- Datta et al. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection illness beyond acute infection and public health implications.external icon JAMA. Infection with SARS-CoV-2 can lead to acute disease as well as chronic symptoms in COVID-19 “long haulers”; the authors propose a clinical and laboratory testing framework that can be refined as more is learned about long-term COVID-19-related sequelae.
Note: From Datta et al. Framework for spectrum of disease caused by SARS-CoV-2 from symptom onset. Reproduced with permission from JAMA. 2020. doi:10.1001/jama.2020.22717. Copyright© 2020 American Medical Association. All rights reserved.
Vaccines
- Patel et a Post licensure evaluation of COVID-19 vaccinesexternal icon. JAMA. Effectiveness of many COVID-19 vaccines may need to be evaluated using a “test-negative” design rather than a randomized trial, the challenges of which are discussed.
- Callaway E. COVID vaccine excitement builds as Moderna reports third positive result.external icon Nature. News release for the Moderna vaccine ongoing Phase III efficacy trial shown to be more than 94% efficacious.
- Callaway E. Why Oxford’s positive COVID vaccine results are puzzling scientistsexternal icon. Nature. The Oxford–AstraZeneca vaccine seems to perform better (90% efficacy) with a lower first dose and second full dose amount compared with two full doses (62% efficacy).
Other Topics
- Choi et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised hostexternal icon. NEJM. Whole-genome SARS-CoV-2 sequencing of serial specimens taken from a patient hospitalized 3 times over 154 days showed changes in spike protein at a rate suggestive of viral evolution within a persistent infection.
- Else H. Can dogs smell COVID? Here’s what the science saysexternal icon. Nature. An early pilot study suggested dogs could detect SARS-CoV-2 infection in human secretions; this article describes studies that would be needed to scale this for screening in airports or other non-medical settings.
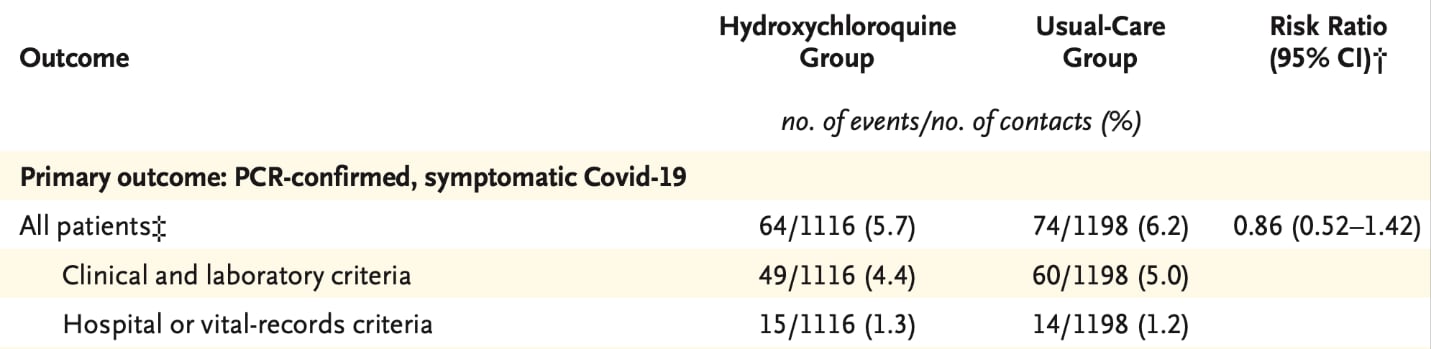
- Mitjà et al. A cluster-randomized trial of hydroxychloroquine for prevention of COVID-19external icon. NEJM. An open-label, cluster-randomized trial found that hydroxychloroquine did not prevent COVID-19.
Note: Adapted from Mitjà et al. Risk for SARS-CoV-2 infection in those receiving compared with those not receiving hydroxychloroquine. † Risk ratios were adjusted for contact-level variables. From NEJM, Mitjà et al., A cluster-randomized trial of hydroxychloroquine for prevention of COVID-19. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.