COVID-19 Science Update released: November 15, 2021 Edition 113

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 database, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. Abu-Raddad et al. JAMA (November 1, 2021).
Key findings:
- Among mRNA-vaccinated individuals, prior SARS-CoV-2 infection was associated with a lower risk of breakthrough infection.
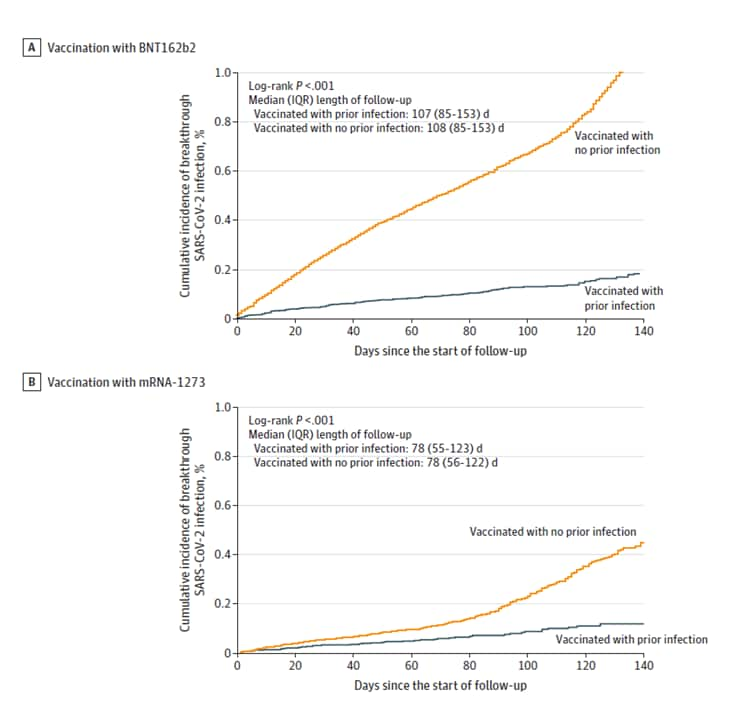
- Among individuals vaccinated with BNT162b2 (Comirnaty, Pfizer/BioNTech), cumulative incidence of breakthrough SARS-CoV-2 infection at 120 days of follow-up was 0.15% (95% CI 0.12%-0.18%) among those with and 0.83% (95% CI 0.79%-0.87%) among those without prior infection (aHR 0.18, 95% CI 0.15-0.21) (Figure).
- Among those vaccinated with mRNA-1273 (Moderna), cumulative incidence was 0.11% (95% CI 0.08%-0.15%) among those with and 0.35% (95% CI 0.32%-0.40%) among those without prior infection (aHR 0.35, 95% CI 0.25-0.48) (Figure).
Methods: Matched-cohort study (n = 617,268), Qatar, December 2020–September 2021. Each individual with prior infection matched by sex, 5-year age group, nationality, and calendar week of 1st vaccine dose to 3 individuals without prior infection. Incident infection was determined ≥14 days post-2nd vaccine dose. Limitations: Only PCR-confirmed infections included; may not be generalizable to specific age groups or to U.S. population.
Implications: Among persons fully vaccinated with mRNA vaccines, prior SARS-CoV-2 infection appears to provide additional protection against breakthrough infections.
Figure:
Note: Adapted from Abu-Raddad et al. Cumulative SARS-CoV-2 incidence among matched cohorts of A) BNT162b2-vaccinated and B) mRNA-1273–vaccinated individuals with prior SARS-CoV-2 infection and without prior SARS-CoV-2 infection. Reproduced with permission from JAMA, 2021. Published online November 1, 2021. https://doi.org/10.1001/jama.2021.19623. Copyright© 2021 American Medical Association. All rights reserved.
Association between mRNA vaccination and COVID-19 hospitalization and disease severity. Tenforde et al. JAMA (November 4, 2021).
Key findings:
- Among adults hospitalized with COVID-19, 93.9% of patients who required mechanical ventilation or who died were unvaccinated.
- mRNA vaccination was associated with decreased likelihood of mechanical ventilation or death (aOR 0.33, 95% CI 0.19-0.58).
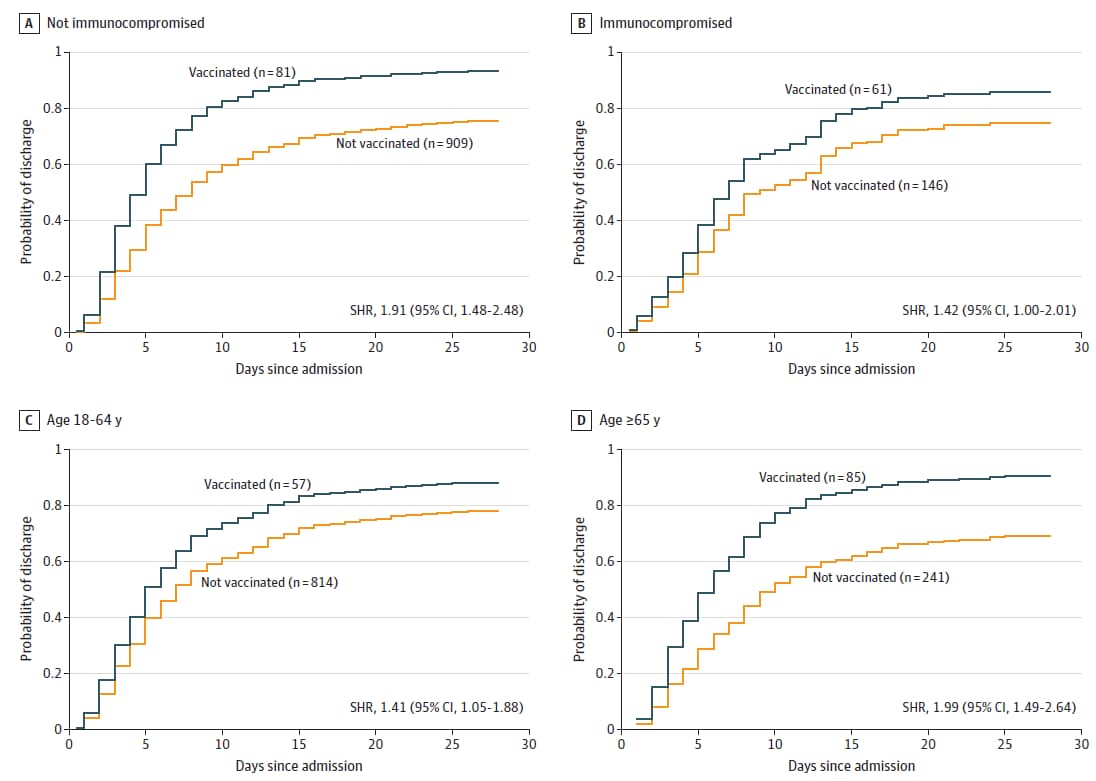
- Vaccinated patients were less likely than unvaccinated patients to require ICU-level care (24.6% vs. 40.1%, p <0.001) and more likely to have been discharged within 28 days (88.0% vs. 77.2%, p = 0.003).
- These findings remained consistent when stratified by age and immune status (Figure).
Methods: Case-control analysis of adults hospitalized with COVID-19 at 21 hospitals, United States, March–July 2021 (n = 1,187). Outcomes of fully vaccinated persons at 28 days compared to those of unvaccinated persons, adjusted for age, sex, race/ethnicity, and comorbidities. Patients considered fully vaccinated 14 days after 2nd mRNA vaccine dose. Limitations: Limited to hospitalized patients, did not assess out-of-hospital deaths; sample size limited assessment by vaccine type, SARS-CoV-2 variant, and time since vaccination; some data accrued prior to Delta period.
Implications: Breakthrough infections following mRNA COVID-19 vaccination among hospitalized patients were less severe than SARS-CoV-2 infections among unvaccinated persons hospitalized for COVID-19. These findings support prior data that COVID-19 vaccination can reduce COVID-19 severity and mortality.
Figure:
Note: Adapted from Tenforde et al. Cumulative incidence of hospital discharge by vaccination status (fully vaccinated with a 2-dose series of mRNA vaccine vs. unvaccinated) is shown for patients who are A) not immunocompromised, B) immunocompromised, C) aged 18 to 64 years, and D) aged 65 years or older. The event of interest was discharge from the hospital before day 28 in the presence of the competing event of death. Patients who remained hospitalized more than 28 days were censored at 28 days. Competing risk models were adjusted for age group (18–49, 50–64, and ≥65 years), sex, self-reported race and ethnicity, and number of medical comorbidities. Models by age group were adjusted for continuous age in years. SHR = subdistribution hazard ratio. Reproduced with permission from JAMA, 2021. Published online November 4, 2021. https://doi.org/10.1001/jama.2021.19499. Copyright© 2021 American Medical Association. All rights reserved.
Two Israeli studies evaluated the effectiveness of a 3rd dose of the BNT162b2 (Comirnaty, Pfizer/BioNTech) vaccine.
A. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Barda et al. The Lancet (October 29, 2021).
Key findings:
- Among 728,321 healthy adults who received BNT162b2, 3rd dose vaccine effectiveness (VE), compared to 2 doses, was 93% (95% CI 88%-97%) for hospital admission, 92% (95% CI 82%-97%) for severe disease, and 81% (95% CI 59%-97%) for COVID-19 related death.
- VE against hospital admission and severe disease was 93% (95% CI 87%-97%) among persons aged ≥70 years and 92% (95% 83%-97%) among those 40–69 years; few events among those aged 16–39 years limited ability to estimate VE in this group.
- 3rd dose VE against documented SARS-CoV-2 infection was 88% (95% CI 87%-90%) and against symptomatic infection was 91% (95% CI 89%-92%).
Methods: Retrospective cohort study of persons who were eligible to receive 3rd vaccine dose during July–September 2021, Israel. Cohort assembled using data from a large healthcare organization. Persons who received a 3rd dose were matched — on age, sex, place of residence, comorbidities, 2nd dose timing, and number of prior SARS-CoV-2 PCR tests — to persons who received 2 doses. Limitations: Excluded high-risk population groups (immunocompromised persons who received a 3rd dose prior to July 30, 2021 recommendation for the general public [aged >60 years] to receive a 3rd dose, healthcare workers, and those in long-term care facilities or medically confined to their homes); small number of events in some age groups limits ability to calculate age-based estimates; relatively short follow-up period (maximum of 55 days).
Figure:
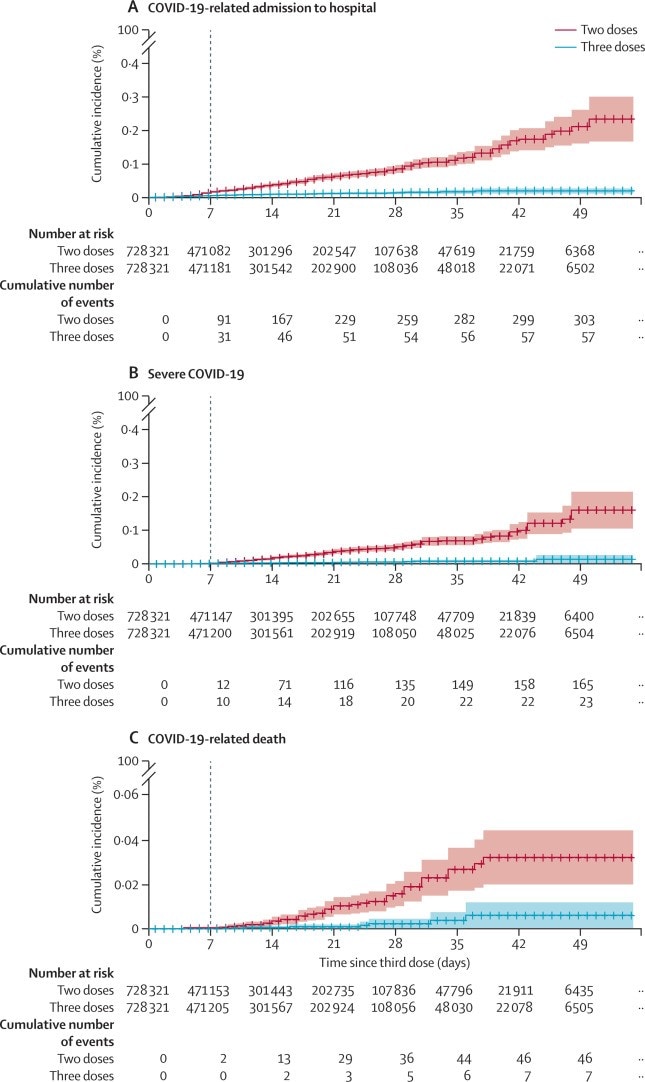
Note: Adapted from Barda et al. Cumulative incidence of A) COVID-19-related hospitalization, B) severe disease, and C) death in individuals who received 2 doses vs. 3 doses of the BNT162b2 mRNA COVID-19 vaccine. The dashed vertical line indicates day 7 after the 3rd dose was received in those vaccinated (beginning of the study period). Reprinted from The Lancet, October 29, 2021, Barda et al., Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study, online ahead of print. Copyright 2021, with permission from Elsevier.
B. Effectiveness of a third dose of BNT162b2 mRNA vaccine. Saciuk et al. The Journal of Infectious Diseases (November 2, 2021).
Key findings:
- Adjusted vaccine effectiveness (VE) of a 3rd dose of BNT162b2, compared to 2 doses, was 89.1% (95% CI 87.5%-90.5%) against PCR-confirmed SARS-CoV-2 infection.
- VE was 92.6% (95% CI 92.2%-93.0%) among persons aged <60 years and 90.7% (95% CI 89.9%-91.4%) among persons 60 years and older.
Methods: Retrospective cohort study of members of a health management organization who had no evidence of infection prior to the study period (July–October 2021), Israel (n = 947,131). VE rates were adjusted using a GLM model for age group, gender, socioeconomic status, population group, and religiosity and 10-day calendar period. Limitations: Persons >60 years were overrepresented among persons receiving the 3rd dose; asymptomatic infections may have been undetected; VE of younger age groups not reported; relatively short follow-up period (maximum of 70 days).
Implications for Barda et al. and Saciuk et al.: A 3rd dose of the BNT162b2 vaccine appears to provide added protection against SARS-CoV-2 infection and severe COVID-19-related outcomes compared to 2 doses.
Association between vaccination with the BNT162b2 mRNA COVID-19 vaccine and Bell’s palsy: A population-based study. Shibli et al. The Lancet Regional Health–Europe (November 3, 2021).
Key findings:
- Among persons without a history of Bell’s palsy who received BNT162b2 (Comirnaty, Pfizer/BioNTech) vaccine, the standardized incident ratio (SIR) of observed Bell’s palsy was modestly higher than expected within 21 days following the 1st dose (1.36, 95% CI 1.14-1.61) and within 30 days following the 2nd dose (1.16, 95% CI 0.99-1.36).
- The highest attributable risk (4.46 per 100,000 vaccinees) was observed among females aged >65 years following the 1st dose (IR 2.51, 95% CI 1.65-3.68).
- Among individuals with a prior history of Bell’s palsy, SIRs were 1.15 (95% CI 0.36-2.76) and 2.15 (95% CI 1.09-3.83) following the 1st and 2nd doses, respectively.
Methods: Retrospective cohort study of >2.4 million individuals aged ≥16 years and vaccinated during December 2020–April 2021, using data from a large health system in Israel. SIRs calculated by dividing observed incidence after vaccination by expected incidence (incidence during January–May 2019) and weighted for age and sex. Limitations: No concurrent comparison group; adverse events not systematically collected; causal link not established.
Implications: BNT162b2 vaccination might be associated with a small increase in risk of Bell’s palsy, particularly among those with previous Bell’s palsy. However, the risk of Bell’s palsy following vaccination is low. More studies are needed, especially in older women and those who previously experienced Bell’s palsy.
PEER-REVIEWED
Impact of Delta variant and vaccination on SARS-CoV-2 secondary attack rate among household close contacts. Ng et al. The Lancet Regional Health–Western Pacific (November 1, 2021).
Key findings:
- The household secondary attack rate among unvaccinated contacts exposed to the SARS-CoV-2 Delta (B.1.617.2) variant was 25.8% compared with 12.9% among contacts exposed to other variants (RR 2.01, 95% bootstrap CI [BCI] 1.24-3.84).
- Among fully vaccinated Delta-exposed contacts, vaccine effectiveness against infection, symptomatic disease, and severe disease was 56.4% (95% BCI 32.6%-75.8%), 64.1% (95% BCI 37.8%-85.4%), and 100%, respectively.
- Among index cases, older age was associated with increased risk of secondary transmission (aOR 1.20 per decade, 95% robust CI [RCI] 1.03-1.39), but vaccination status was not (aOR 0.73, 95% RCI 0.38-1.40).
Methods: Retrospective cohort study in Singapore (September 2020–May 2021) determined secondary attack rates by variant type and vaccination status (n = 1,024 household contacts linked to 301 PCR-confirmed index cases). Limitations: Infection of contacts from non-household sources cannot be excluded; pre-symptomatic (when interviewed) persons may have been misclassified as asymptomatic; unable to adjust for comorbidities.
Implications: Delta variant appears more transmissible among household contacts than other variants. Vaccination might not prevent individuals with breakthrough Delta variant infection from transmitting SARS-CoV-2 to their household contacts, but prior vaccination of exposed household contacts appeared to lower their risk of becoming infected.
PREPRINTS (NOT PEER-REVIEWED)
Social vulnerability and rurality associated with higher SARS-CoV-2 infection-induced seroprevalence: A nationwide blood donor study, United States, July 2020–June 2021. Li et al. SSRN (November 2, 2021). Published in Clinical Infectious Diseases (February 7, 2022).
Key findings:
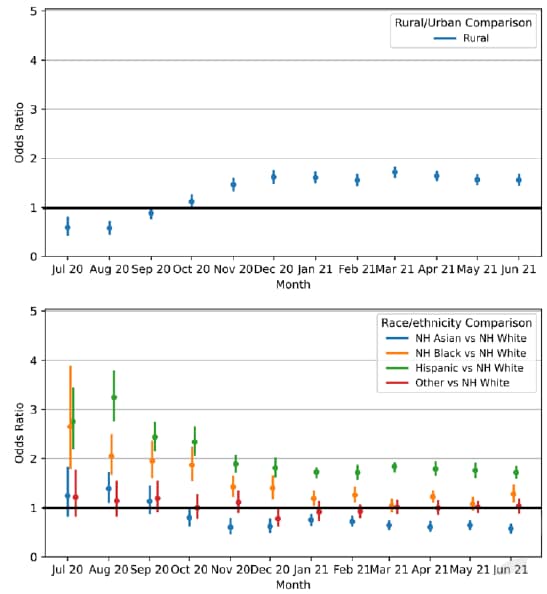
- Among U.S. blood donors in July 2020, infection-induced SARS-CoV-2 antibody seroprevalence was higher among Hispanic or Latino (aOR 2.8, 95% CI 2.2-3.4) and Black or African American persons (aOR 2.7, 95% CI 1.8-3.9) compared with White persons (Figure).
- These disparities persisted, but had decreased by June 2021 (aOR 1.7, 95% CI 1.6-1.8 and aOR 1.3, 95% CI 1.1-1.5, respectively).
- Infection-induced seroprevalence in rural counties was lower than in urban counties (aOR 0.59, 95% 0.44-0.79) in July 2020, but was higher by June 2021 (aOR 1.6, 95% CI 1.5-1.7) (Figure).
- Counties with lower socioeconomic status had higher infection-induced seroprevalence than other counties.
Methods: National blood donor seroprevalence study, United States, July 2020–June 2020. >1.5 million specimens tested for anti-S antibodies and anti-N antibodies to distinguish infection-induced from vaccine-induced antibodies. Associations of seroprevalence with race/ethnicity, rurality, and county-level social vulnerability modeled with multivariate logistic regression, adjusting for age, gender and county. Limitations: Blood donors not representative of general population; counties approximated from ZIP codes.
Implications: Infection-induced antibody seroprevalence data can complement case-based surveillance data. Rural counties and counties with low socioeconomic status may warrant prioritization for vaccination and other public health efforts.
Figure:
Note: Adapted from Li et al. Results of multivariate regression model evaluating the association of infection-induced seropositivity with rurality (top panel; rural vs. urban counties) and race/ethnicity (lower panel; non-Hispanic Asian, non-Hispanic Black or African American, Hispanic or Latino, and Other vs. White) by month. Models adjusted for race/ethnicity, sex, age, region, rurality level, and social vulnerability (as measured by CDC’s Social Vulnerability Index). Model was run on monthly data from July 2020 to June 2021 to give a set of odds ratios with 95% confidence intervals (indicated by error bars) for all factors each month. Permission request in process.
Vaccines
- Myocarditis following COVID-19 mRNA vaccine: A case series and incidence rate determination. Perez et al. Clinical Infectious Diseases (November 3, 2021). Among 175,472 individuals aged 12–106 years in the Mayo Clinic COVID-19 Vaccine Registry (December 2020–May 2021), 7 had new onset of myocarditis following vaccination. All cases occurred within 2 weeks of an mRNA vaccine dose, and 6/7 (86%) occurred after dose 2. No cases were found after Ad26COV2.S. Compared with background incidence in a comparable population 2016–2020, overall IRR within 2 weeks following COVID vaccination was 4.18 (95% CI 1.63-8.98) and was attributable to increased IRR among males (6.69, 95% CI 2.35-15.52); IRR among females was 1.41 (95% CI 0.03-8.45).
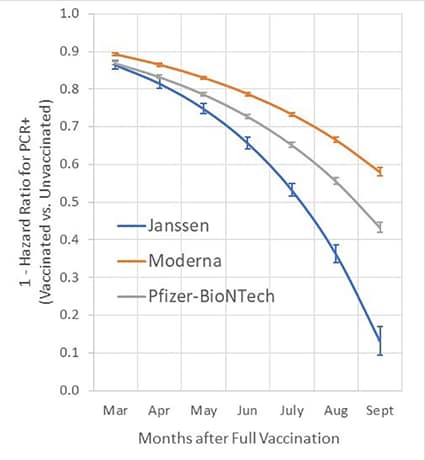
- SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Cohn et al. Science (November 4, 2021). Among 780,225 U.S. veterans, vaccine effectiveness (VE) for any vaccine against infection during February 1–October 1, 2021 declined from 87.9% to 48.1%. The VE of Ad26.COV2.S declined to 13.1%. During July–October, VE against death among persons aged <65 years was 73.0% for Ad26.COV2.S, 81.5% for mRNA-1273, and 84.3% for BNT162b2; among persons aged ≥65 years, VE against death was 52.2%, 75.5%, and 70.1%, respectively.
Note: Adapted from Cohn et al. Time dependent vaccine effectiveness against SARS-CoV-2 infection as estimated from Cox proportional hazards models, adjusted for age, race, ethnicity, sex, and comorbidity score. Vaccine effectiveness (1 – hazard ratio × 100) and 95% confidence intervals are shown. Effectiveness for each month was estimated using vaccination status and time to RT-PCR assay. Licensed under CC BY 4.0.
Variants
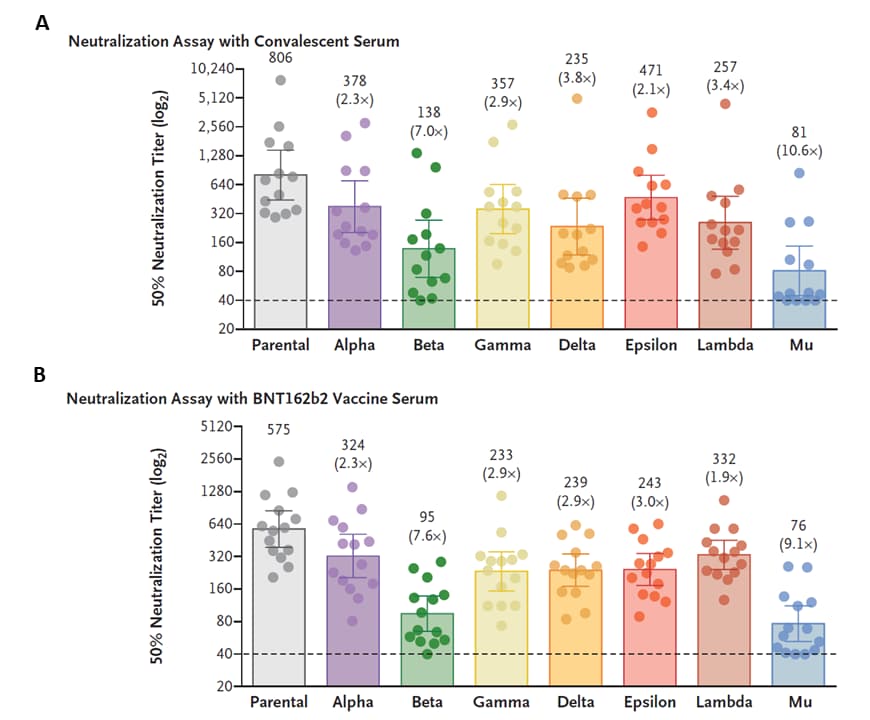
- Neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine serum. Uriu et al. NEJM (November 3, 2021). In convalescent serum samples from 13 persons in Columbia, Mu variant was 10.6 times more resistant to neutralization compared with the parental (B.1 lineage) virus. In serum samples from 14 BNT162b2 vaccinated persons, Mu variant was 9.1 times more resistant than the parental virus. Compared with the Beta variant, the Mu variant was 2.0 times more resistant to neutralization by convalescent serum and 1.5 times by vaccinated serum.
Note: Adapted from Uriu et al. SARS-CoV-2 neutralization assay results using A) convalescent serum (n = 13 patients) and B) serum from BNT162b2-vaccinated persons (n = 14) in Colombia. Neutralization assays used pseudoviruses with SARS-CoV-2 spike proteins of the Alpha, Beta, Gamma, Delta, Epsilon, Lambda, or Mu variants or the B.1 lineage with D614G mutation (parental virus). Each data point is an individual sample (circles) and indicates the 50% neutralization titer. The heights of the bars and the numbers are geometric mean titers, and I bars indicate 95% confidence intervals. Parentheses indicate average difference in neutralization resistance of the variants compared with parental virus. The horizontal dashed lines are limit of detection. From the New England Journal of Medicine, Uriu et al., Neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine serum. November 3, 2021, online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Natural History, Reinfection, and Health Impact
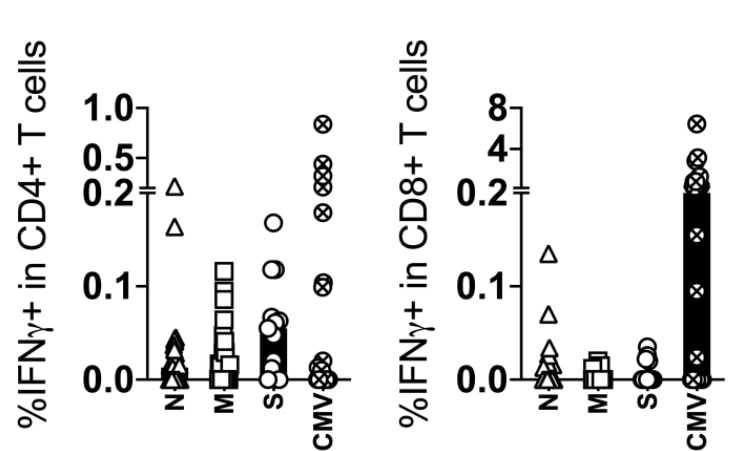
- Durability of SARS-CoV-2-specific T cell responses at 12-months post-infection. Lu et al. Journal of Infectious Diseases (October 21, 2021). Among 29 individuals who had COVID-19 and were tested for SARS-CoV-2-specific T cells 1 year after symptom onset, 22 showed at least some, typically low magnitude, T cell response (usually CD4 T cells). Patients who had been hospitalized were more likely to be positive (using a 2-fold increase over unstimulated control wells as a threshold for positivity) and had modestly higher levels of T cell responses compared to outpatients.
Note: Adapted from Lu et al. T cell responses to SARS-CoV-2 viral components at 12 months after symptom onset. SARS-CoV-2-specific CD4 T cells (left panel) were more frequently identified than CD8 T cells (right panel) following stimulation of peripheral blood mononuclear cells with SARS-CoV-2 nucleocapsid (N), membrane (M), and spike (S) proteins. Samples were considered positive for the presence of SARS-CoV-2-specific CD4 and CD8 T cells if the frequency of IFNɣ+ T cells was greater than 0.01% of T cells after subtracting the medium control value. CMV = response after stimulation with CMV peptide pool. U.S. Government work not subject to copyright.
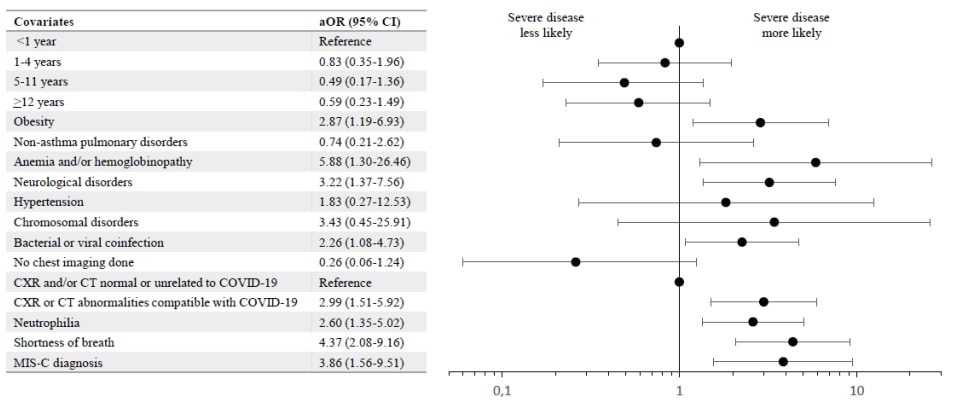
- Risk factors for severe PCR-positive SARS-CoV-2 infection in hospitalized children: A multicenter cohort study. Schober et al. medRxiv (Preprint; November 8, 2021). Among 403 children (median age 3.78 years, IQR 0.53–10.77) hospitalized with symptomatic SARS-CoV-2 infection February 2020–May 2021 in Canada, Iran, and Costa Rica, risk factors for severe disease (requirement of noninvasive ventilation, high-flow nasal cannula, mechanical ventilation, vasopressors, or death) included: anemia and/or hemoglobinopathy (aOR 5.88, 95% CI 1.30-26.46), shortness of breath (aOR 4.37, 95% CI 2.08-9.16), MIS-C (aOR 3.86, 95% CI 1.56-9.51), and neurological disorders (aOR 3.22, 95% CI 1.37-7.56).
Note: Adapted from Schober et al. Multivariable logistic regression model for factors associated with severe PCR-positive SARS-CoV-2 infection. The model evaluated individual comorbidities as risk factors for severe disease. aOR = adjusted odds ratio, CT = computed tomography, CXR = chest x-ray, MIS-C = multisystem inflammatory syndrome in children. Licensed under CC-BY-NC-ND 4.0.
Health Equity
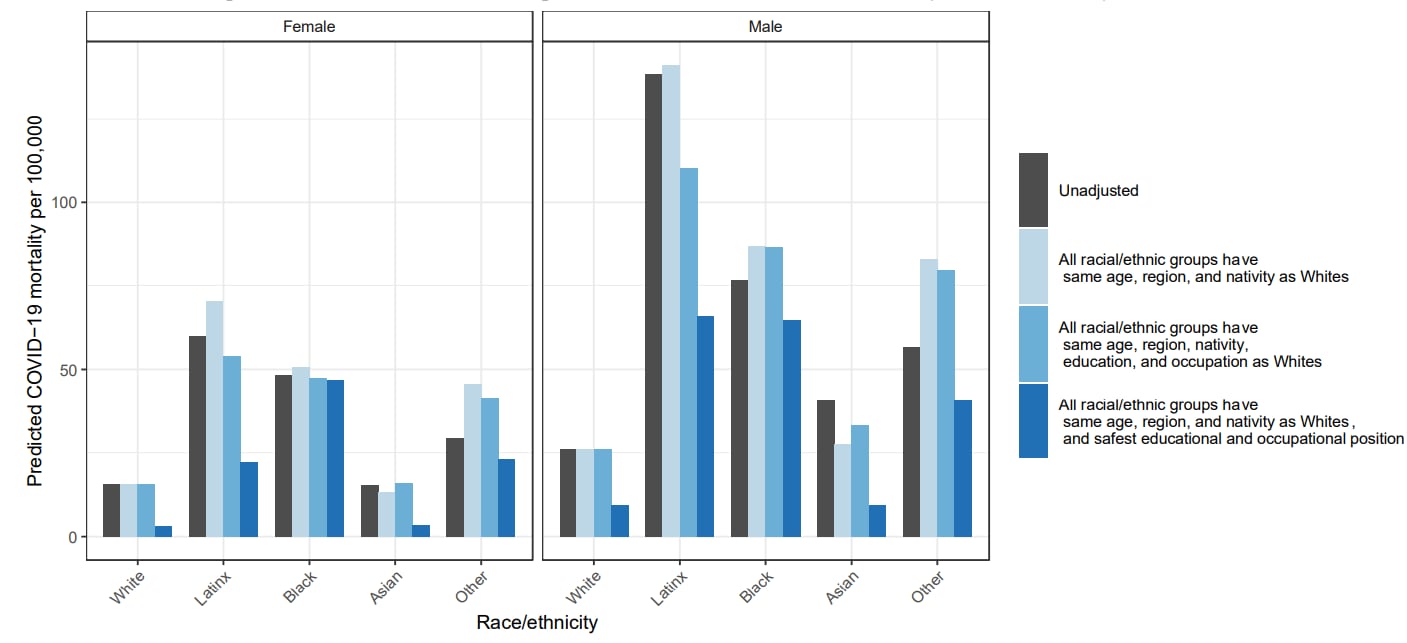
- Contributions of occupation characteristics and educational attainment to racial/ethnic inequities in COVID-19 mortality. Matthay et al. medRxiv (Preprint; October 30, 2021). Published in JAMA Network Open as Occupation and educational attainment characteristics associated with COVID-19 mortality by race and ethnicity in California (April 22, 2022). Using data from California for COVID-19-confirmed deaths and population estimates of demographics, education, and occupation, modeling estimated that COVID-19 mortality disparities may be partially (but not fully) mediated by educational attainment and occupation. Modeling estimated that, if all individuals had the COVID-19 mortality associated with the safest educational and occupational positions (Bachelor’s degree, telework access, the highest wages, and non-essential occupations), a purely theoretical state, there would have been 57% fewer COVID-19 deaths in California.
Note: Adapted from Matthay et al. Unadjusted and predicted COVID-19 mortality for individuals aged 18–65 years, per 100,000 persons, by race/ethnicity and sex, under alternative compositional, educational, and occupational distributions, California, January 1, 2020–February 12, 2021. Estimates present the predicted COVID-19 mortality risk for each racial/ethnic-sex group under the 4 scenarios shown in the legend. Licensed under CC-BY-NC-ND 4.0.
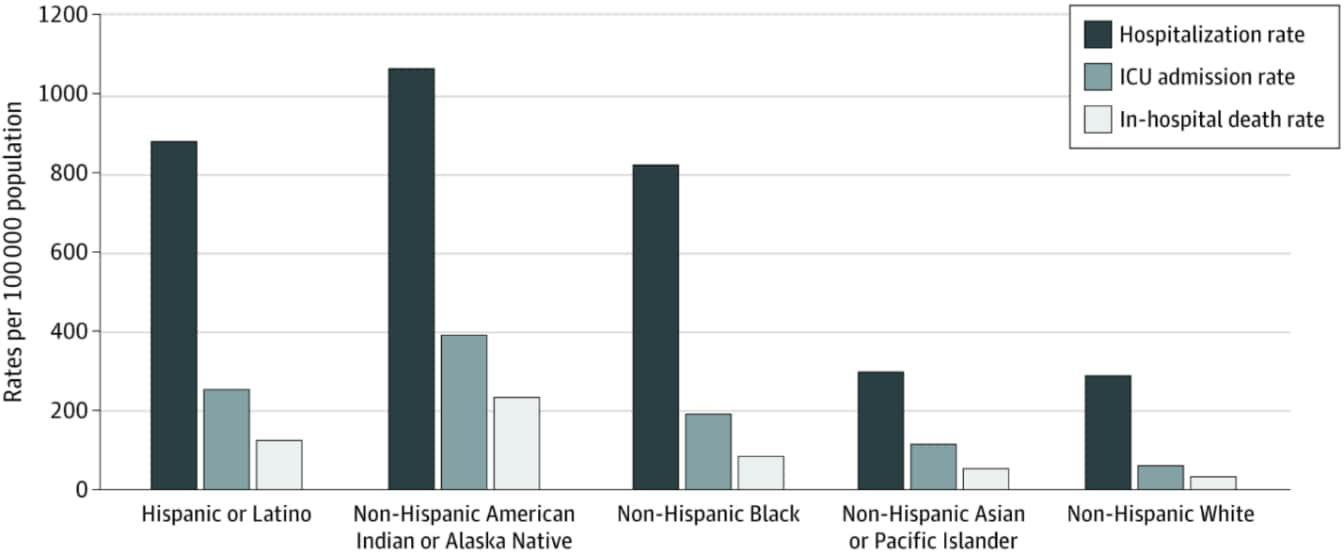
- Racial and ethnic disparities in rates of COVID-19-associated hospitalization, intensive care unit admission, and in-hospital death in the United States from March 2020 to February 2021. Acosta et al. JAMA Network Open (October 21, 2021). During March 2020–February 2021 in the COVID-19-Associated Hospitalization Surveillance Network, age-adjusted rates of COVID-19 hospitalization, ICU admission, and in-hospital death were higher among American Indian or Alaska Native, Hispanic or Latino, Black or African American, and Asian or Pacific Islander persons compared with White persons.
Note: Adapted from Acosta et al. Cumulative age-adjusted hospitalization, intensive care unit (ICU) admission, and in-hospital death rates per 100,000 population by race and ethnicity, United States, March 1, 2020 to February 28, 2021. Licensed under CC BY.
From the Morbidity and Mortality Weekly Report (November 12, 2021).
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.