COVID-19 Science Update released: November 6, 2020 Edition 63

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
*** Next week the CDC COVID-19 Science Update will only be produced on Tuesday, 11/10. ***
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
COVID-19 testing and cases in immigration detention centers, April–August 2020external icon. Erfani et al. JAMA (October 29, 2020).
Key findings:
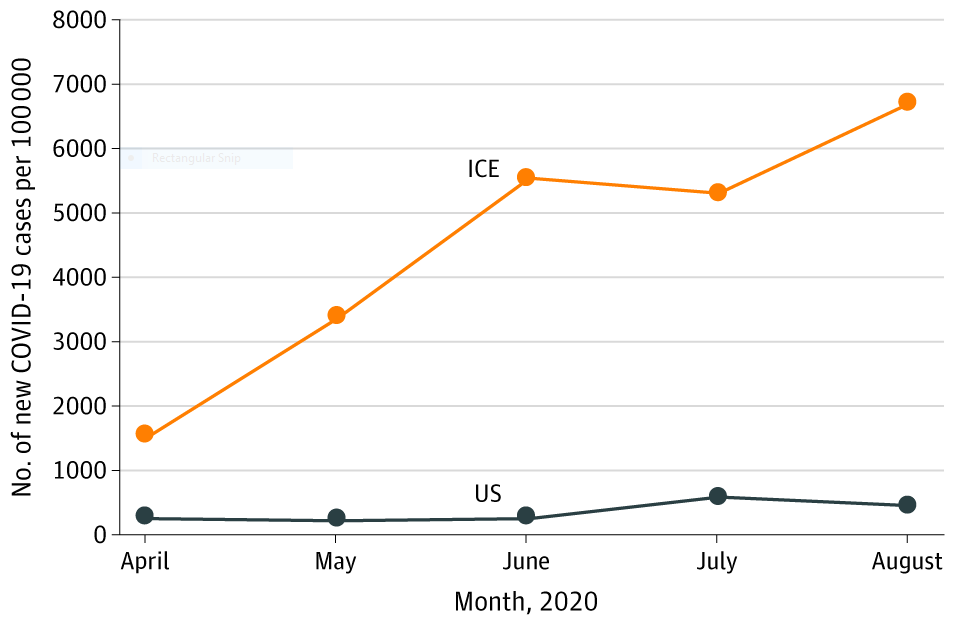
- The monthly case rate per 100,000 detainees increased from 1,527 to 6,683 from April 2020 to August 2020 (Figure).
- Cases increased despite mitigation measures put in place in April 2020, including social distancing and disinfection protocols, testing guidelines, and expedited detainee release (45% decrease in the detained population).
- US Immigration and Customs Enforcement (ICE) reported 5,379 cumulative COVID-19 cases and 6 deaths among detainees by August.
- Cases were reported in 92 of 135 facilities; 20 facilities accounted for 71% of cases.
Methods: Review of COVID-19 testing and deaths among ICE detainees from April 1 to August 31, 2020. Limitations: Relied on ICE publicly available data, which may be subject to reporting delays and missing data; asymptomatic detainees not routinely tested, which may underestimate case counts.
Implications: Detainees living in congregate settings are at increased risk for COVID-19 despite implementation of mitigation measures.
Figure:
Note: Adapted from Erfani et al. Monthly COVID-19 case rate per 100,000 persons for ICE detainee and US populations from April 2020 to August 2020. Reproduced with permission from JAMA. Erfani et al., COVID-19 testing and cases in immigration detention centers, April–August 2020. DOI: 10.1001/jama.2020.21473. Copyright©2020 American Medical Association. All rights reserved.
Association of state stay-at-home orders and state-level African American population with COVID-19 case ratesexternal icon. Padalabalanarayanan et al. JAMA (October 23, 2020).
Key findings:
- Implementation of state stay-at-home orders (SAHOs) was associated with reductions in case rates (β = -1.17, 95% CI -1.48 to -0.85, p <0.001) and fatality rates (β = -0.20, 95% CI -0.29 to -0.11, p <0.001) when compared to periods of no state-imposed SAHOs.
- Expected cumulative case rates would have been 219% higher (95% CI 134%-339%) and fatality rates 22.1% higher (95% CI 12.1%-34.3%) if there were no SAHOs when compared to rates during state-imposed SAHOs.
- States where the proportion of African American population was larger had higher case rates (β = 0.045, 95% CI 0.014-0.077, p = 0.001) and fatality rates (β = 0.068, 95% CI 0.044-0.091, p <0.001).
Methods: A cross-sectional study of daily, state-level data on COVID-19 cases, tests, and fatalities from March 1 to May 4, 2020, for all states (except Washington) and the District of Columbia. State-level SAHOs and the proportion of African American population in the state were evaluated. The primary and secondary outcomes were daily cumulative COVID-19 case rates and fatality rates, respectively. Limitations: Enforcement of and adherence to SAHOs not known; did not control for city or county level SAHOs.
Implications: State-imposed SAHOs appear to reduce COVID-19 case and fatality rates and should be considered as a control strategy as we see a resurgence of cases across the country. An understanding of drivers of racial disparities in COVID-19 outcomes is needed to mitigate the disproportionate risk faced by certain populations.
Coronavirus disease among workers in food processing, food manufacturing, and agriculture workplaces. Waltenburg et al. Emerging Infectious Diseases (October 19, 2020).
Key findings:
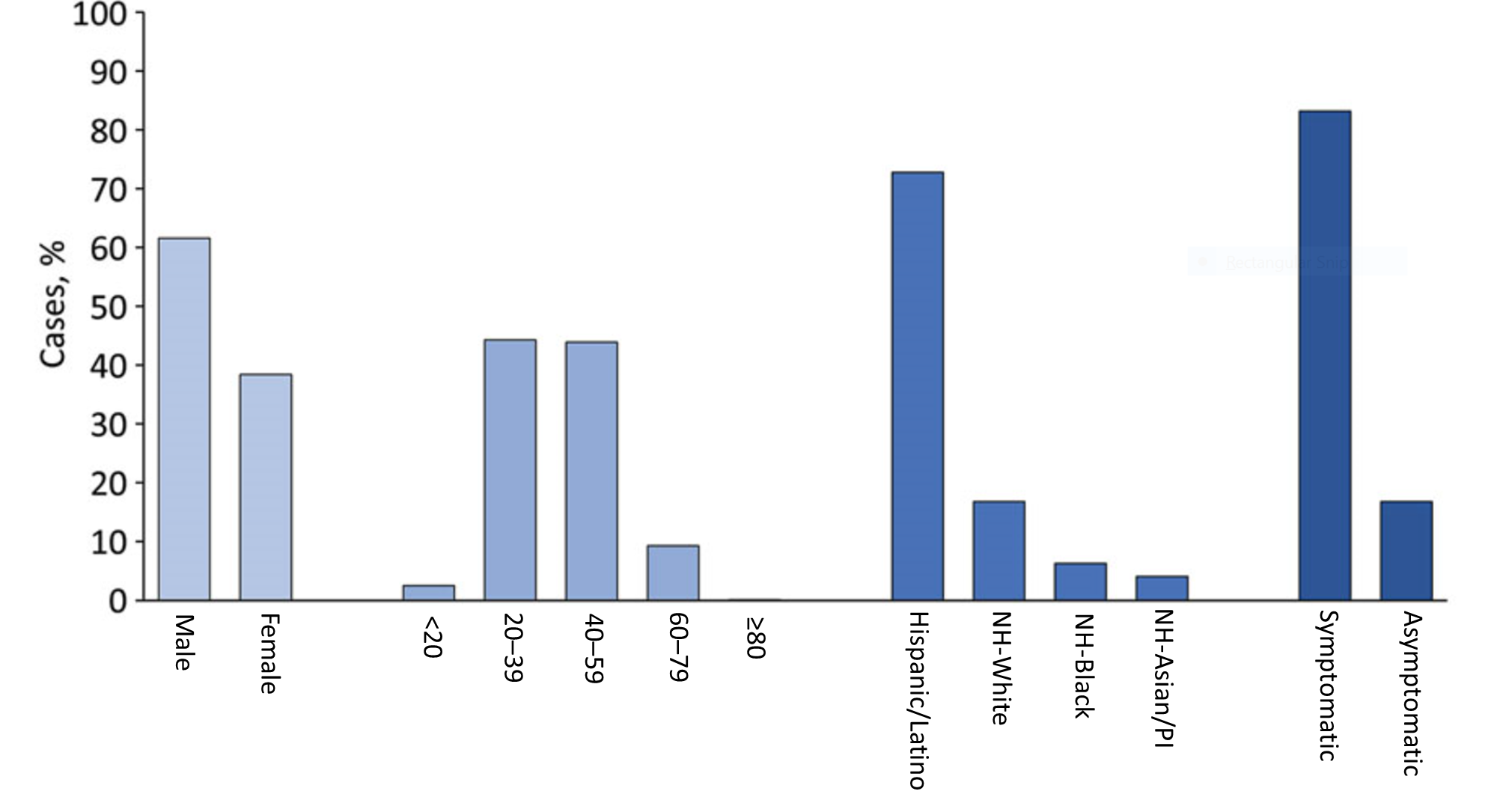
- 8,978 cases and 55 (0.6%) deaths were reported among workers in 742 food manufacturing and agriculture workplaces in 30 states.
- Median number of affected facilities per state was 12 (interquartile range 4–30).
- While only 36.5% of food manufacturing and agriculture workers are Hispanic or Latino, 72.8% of cases were found in this group (Figure).
- Of the 5,957 workers with symptom status reported, 4,957 (83.2%) were symptomatic and 1,000 (16.8%) were asymptomatic or presymptomatic.
Methods: Data from 36 state health departments on workers in US food processing, food manufacturing, and agriculture workplaces who had laboratory-confirmed COVID-19 from March 1 to May 31, 2020 were collected, including number and type of workplaces that reported ≥1 COVID-19 case among workers; the number of workers in affected workplaces; the number, demographics, and symptom status of workers with COVID-19; and the number of COVID-19 related deaths among workers. Limitations: Only 36 states reported data; varied testing strategies by workplace influenced number of cases detected and reported.
Implications: Given the disproportionate burden of COVID-19 among some racial and ethnic minority groups in the food manufacturing and agriculture workplaces, culturally and linguistically appropriate COVID-19 mitigation policies are needed to ensure equitable protection for these groups. Comprehensive testing strategies are needed in these high-density workplaces to rapidly detect cases, regardless of symptoms, and reduce transmission.
Figure:
Note: Adapted from Waltenburg et al. Characteristics of COVID-19 cases among workers in food manufacturing and agriculture workplaces in 28 US states, March 1 to May 31, 2020. NH- non-Hispanic; PI-Pacific Islander. Open access journal; all content freely available.
Incubation period of severe acute respiratory syndrome novel coronavirus 2 that causes coronavirus disease 2019: A systematic review and meta-analysisexternal icon. Wassie et al. Current Therapeutic Research (September 21, 2020).
Key findings:
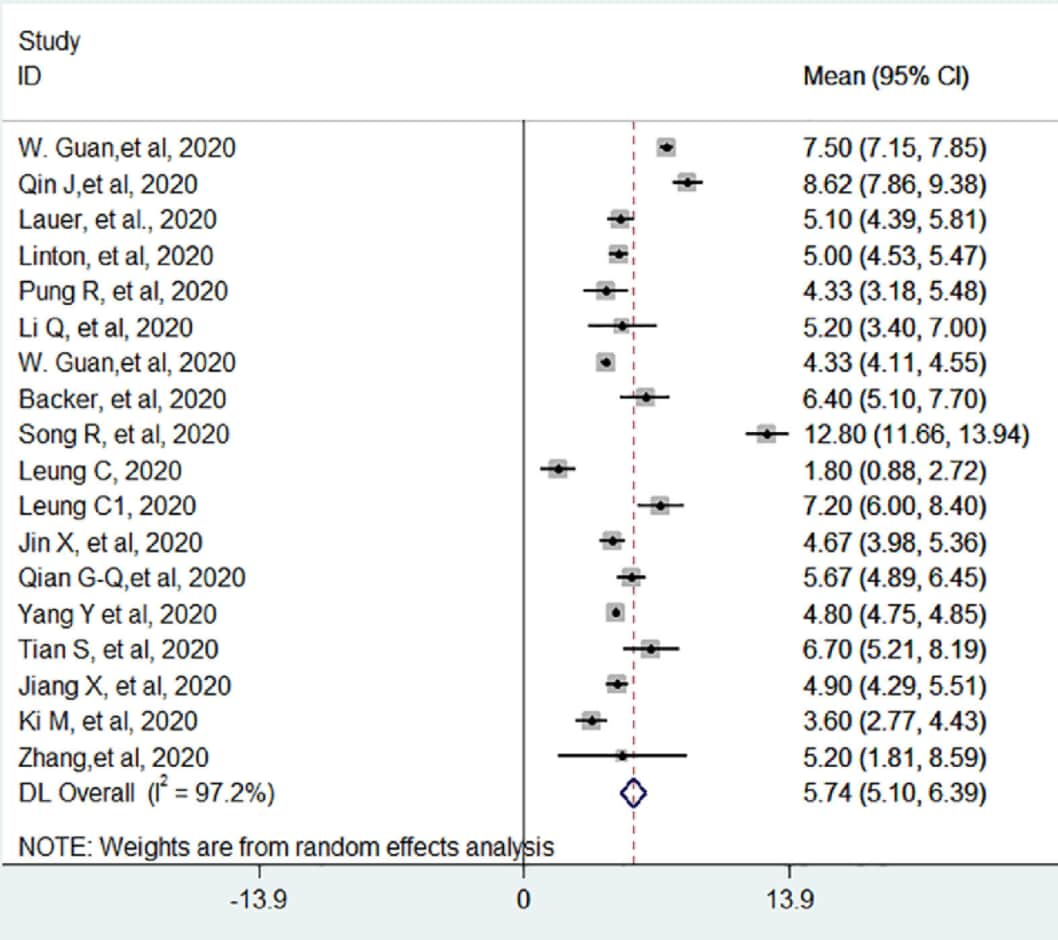
- The pooled estimate of the mean incubation period of SARS-CoV-2 was 5.7 days (95% CI 5.1-6.4) with significant heterogeneity (I2, p <0.05) (Figure).
- Mean study-level incubation period ranged from 1.8 to 12.8 days (Figure).
- The pooled mean incubation period of SARS-CoV-2 stratified by geographic location was:
- China (14 studies): 6.1 days (95% CI 5.3-6.9)
- Other countries (4 studies): 4.5 days (95% CI 3.9-5.2)
Methods: A systematic review and meta-analysis of 18 studies published through May 2020 with 22,595 participants looked at incubation period for SARS-CoV-2. The majority of studies (72%) were rated as having a low risk of bias. Limitations: Short time period of study publication with 14 studies from China.
Implications: Public health policy makers should consider the incubation period when designing optimal prevention and control strategies such as quarantine periods for SARS-CoV-2.
Figure:
Note: from Wassie et al. Forest plot displaying the weighted DerSimonian-Laird random-effect, pooled average incubation period of SARS-CoV-2 denoted by the red vertical line. The area of each square is proportional to the weight that each individual study contributed to the analysis. I2 statistic indicates significant heterogeneity in the reported effect sizes. Licensed under CC-BY.
PEER-REVIEWED
Frequency of routine testing for coronavirus disease 2019 (COVID-19) in high-risk healthcare environments to reduce outbreaks.external icon Chin et al. Clinical Infectious Diseases (October 26, 2020).
Key findings:
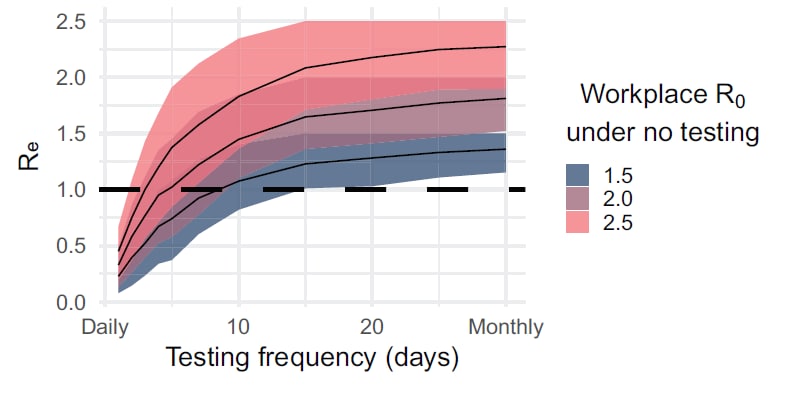
- Simulations of routine PCR testing showed reduction in the mean effective reproductive number (Re the number of infections transmitted from an infected person) to <1 in high-risk settings assuming the initial reproductive number (R0) = 2.5 (Figure).
- Daily testing resulted in Re = 0.44 (2% reduction, 95% CI 82.0-82.5).
- Testing every 3 days resulted in Re = 0.97 (64.4% reduction, 95% CI 61.2-61.7).
- Testing weekly or monthly did not result in Re <1 unless other interventions were in place.
- Optimal routine testing frequency to reduce transmission and bring Re to <1 depended on baseline R0 (Figure):
- Settings with an R0 of 2.5, optimal testing frequency was every other day.
- Settings with an R0 of 2.0, optimal testing frequency was twice weekly.
- Settings with an R0 of 1.5, optimal testing frequency was weekly.
Methods: Simulation model of SARS-CoV-2 transmission assessed the optimal frequency of routine PCR testing of persons in high-risk healthcare environments to reduce COVID-19 cases. Limitations: Assumptions included homogeneity in SARS-CoV-2 transmission, 24-hour test turnaround time, and rapid isolation of cases.
Implications: Implementation of frequent scheduled PCR testing can reduce Re among persons in high risk environments. The use of other prevention interventions (masks, distancing, improved ventilation, etc.) reduces Ro and can extend the time period between tests.
Figure:
Note: Adapted from Chin et al. Lower baseline R0 values permitted longer time periods between tests (x-axis) compared to higher R0 values to achieve Re<1 (y-axis). Reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://doi.org/10.1093/cid/ciaa1383external icon.
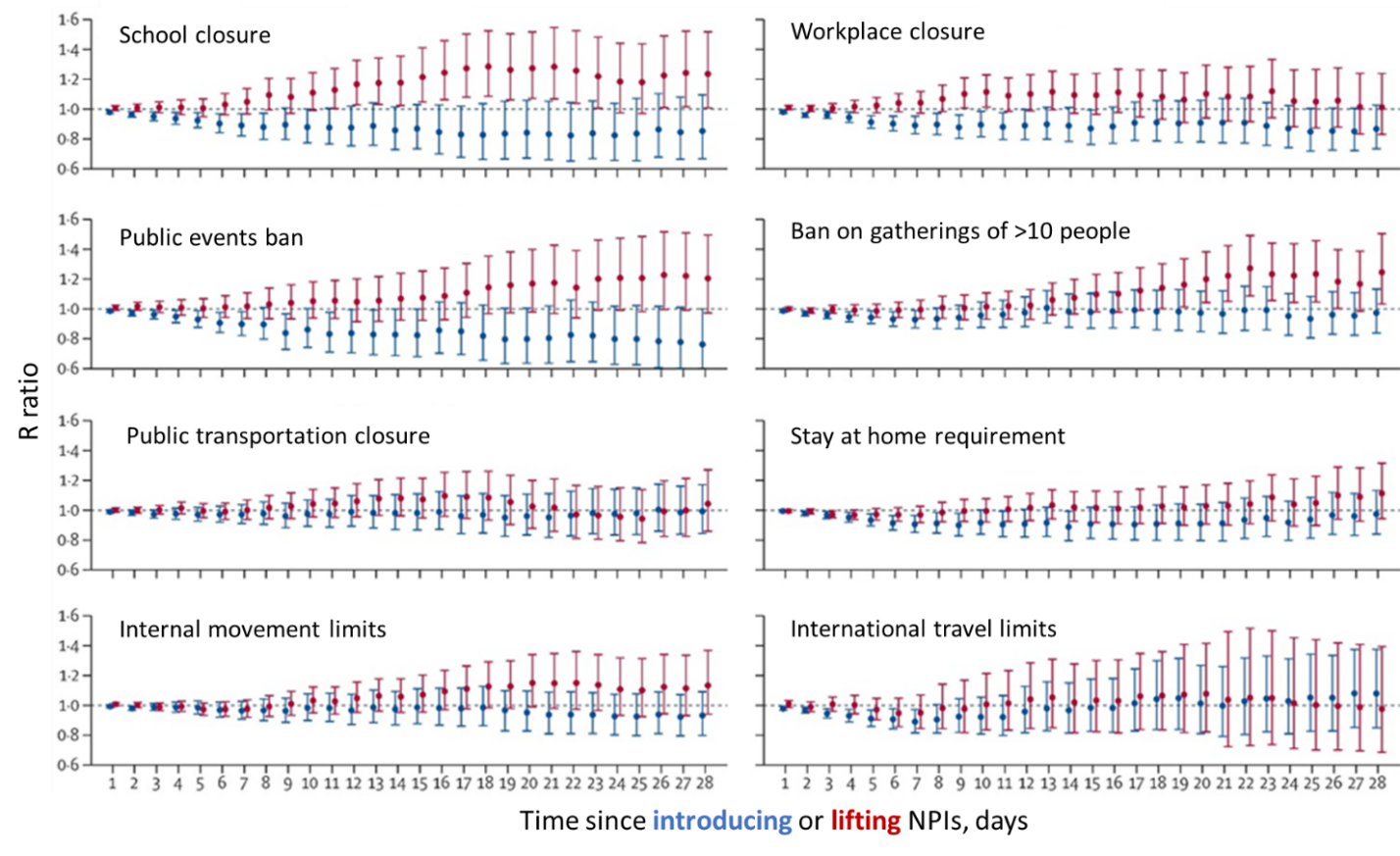
The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: A modelling study across 131 countries.external icon Li et al. Lancet Infectious Diseases (October 22, 2020).
Key findings:
- There was a 3%–24% reduction in the time-varying reproductive number (R) at day 28 following introduction of non-pharmaceutical interventions (NPIs).
- Public events ban was most effective at reducing R (R ratio 0.76, 95% CI 0.58-1.00) (Figure).
- R reached 60% of maximum reduction in a median of 8 days (interquartile range [IQR] 6–9 days).
- There was an 11%–25% increase in R at day 28 following relaxation of NPIs.
- School re-openings (R ratio 1.24, 95% CI 1.00-1.52) and lifting ban on public gatherings (R ratio 1.25, 95% CI 1.03-1.51) were most likely to increase R (Figure).
- R reached 60% of maximum increase in a median of 17 days (IQR 14–20 days).
Methods: Country-level estimates of R and data on NPIs (January to July 2020) were used to model the change in R values expressed as a R ratio between day 1 and day 28 after introducing or relaxing NPIs in 131 countries. The R ratio is the degree of association of introducing or relaxing NPIs with transmission of SARS-CoV-2. Limitations: Study does not account for within-country differences in R values or implementation of NPIs; R estimated through day 28.
Implications: The effect of introducing and relaxing NPIs on SARS-CoV-2 transmission is delayed by 1–3 weeks. These findings can inform decisions on the timing of introducing and lifting NPIs.
Figure:
Note: From Li et al. Change over time in the R ratio following the introduction and lifting of individual NPIs. For each NPI, the reference period is the day before introduction or relaxation of that NPI. An R ratio >1 indicates increased transmission, an R ratio <1 indicates decreased transmission. Error bars represent the 95% CIs of the R ratios. Reprinted from The Lancet Infectious Diseases, Li et al., The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: a modelling study across 131 countries. DOI: https://doi.org/10.1016/S1473-3099(20)30785-4external icon, Copyright 2020, with permission from Elsevier.
PEER-REVIEWED
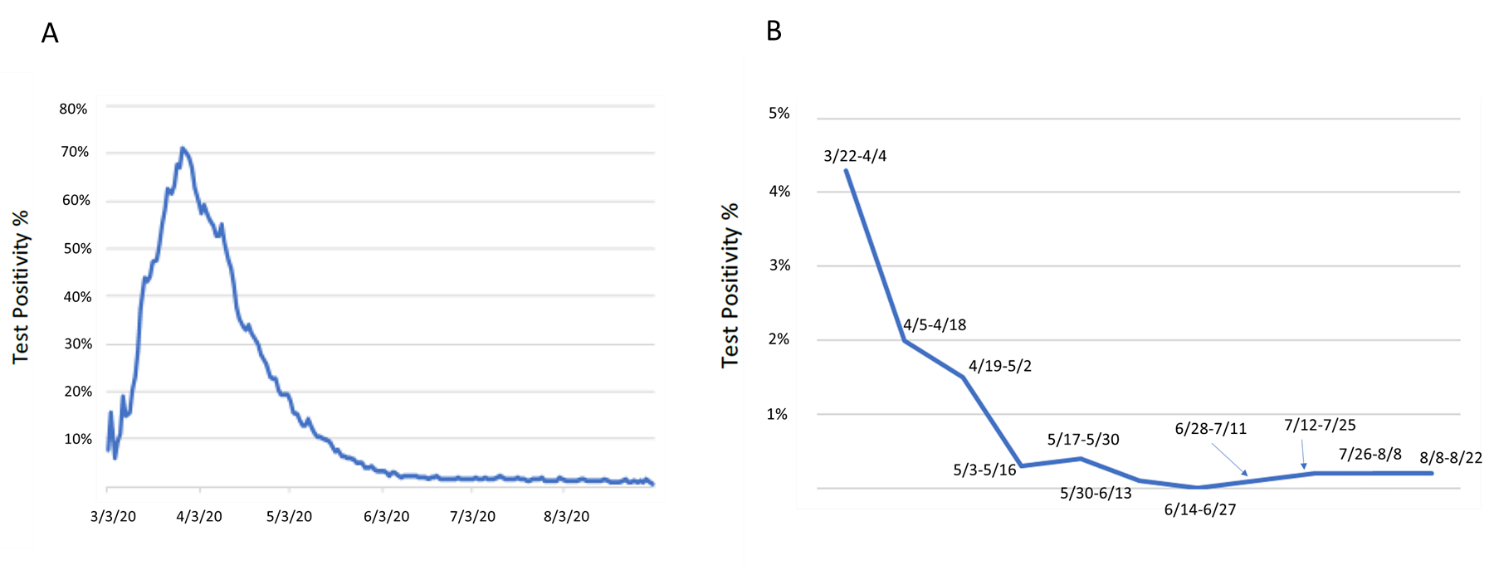
SARS-CoV-2 surveillance and exposure in the perioperative setting with universal testing and personal protective equipment (PPE) policies. external iconAslam et al. Clinical Infectious Diseases (October 22, 2020).
Key findings:
- Rate of positive tests in patients mirrored the rate of positive tests for the New York City area but at a much lower rate (Figure).
- 65 of 11,540 (0.6%) patients tested prior to procedures had positive SARS-CoV-2 RT-PCR tests.
- Three (4.6%) patients were pre-symptomatic, 38 were asymptomatic; and 24 were symptomatic.
- Five (0.04%) patients with a negative SARS-CoV-2 test pre-procedure tested positive within two days following the procedure; all were pre-symptomatic (n = 1) or symptomatic (n = 4) prior to procedure.
- 84 health care workers (HCWs) were in close contact with these five patients; 48 were tested and four (8.3%) were RT-PCR-positive for SARS-CoV-2.
- Follow-up with these positive HCWs suggested only one likely nosocomial transmission, in a situation where the patient was not masked.
Methods: Surveillance for SARS-CoV-2 infection in patients tested within 72 hours of a procedure at Memorial Sloan Kettering Cancer Center from March 22 to August 22, 2020. Patients with infection were characterized as pre-symptomatic, asymptomatic or symptomatic. Limitations: Data from one setting; transmission may have been missed in 84 exposed HCWs as only 48 (59%) were tested.
Implications: With standard universal personal protective equipment precautions, risk to HCWs from surgical patients was low, even at height of the pandemic in March and April 2020 in New York City. In areas of low community spread of SARS-CoV-2, universal pre-surgical testing may divert resources needed elsewhere.
Figure:
Note: Adapted from Aslam et al. A: New York City regional test positivity rate during study time frame. B: Memorial Sloan Kettering pre-procedural test positivity rate. Dates represent 2-week time frames represented by data points. Reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://doi.org/10.1093/cid/ciaa1607external icon.
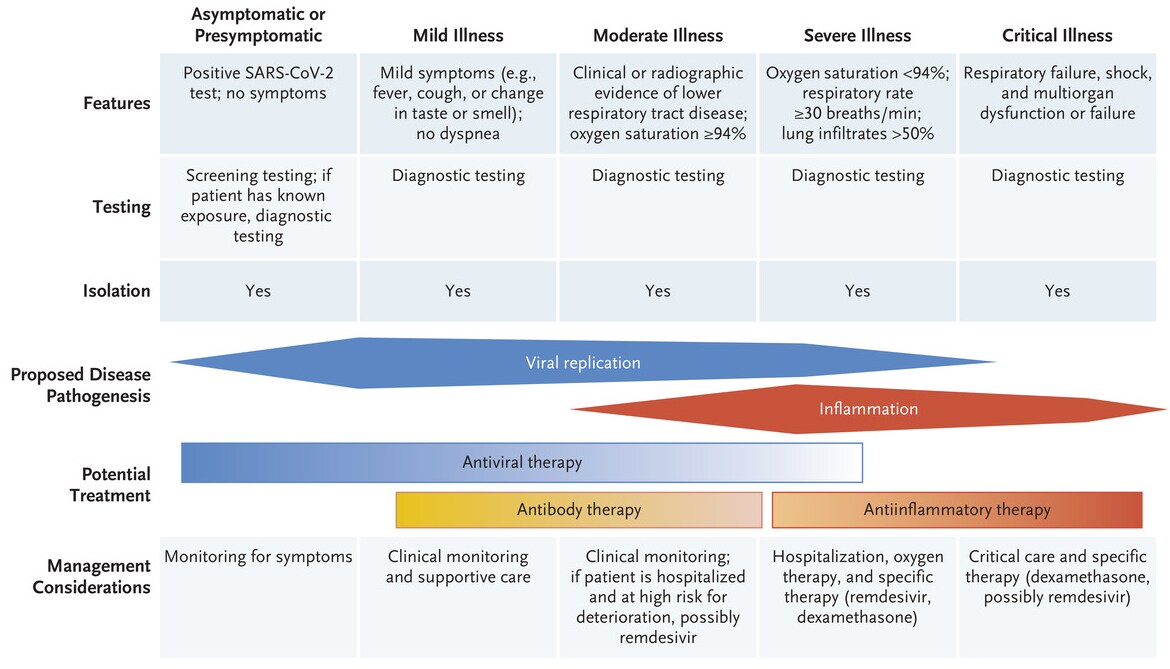
- Gandhi et al. Mild or moderate COVID-19external icon. Summary of clinical features, testing, and management of COVID-19 symptoms based on symptoms and illness severity.
Figure:
Note: From Gandhi et al. Clinical features and management of COVID-19 by disease severity. From NEJM. Ghandi et al., Mild or Moderate COVID-19. DOI: 10.1056/NEJMcp2009249. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Rasmussen et al. & Brosseau et al. Facial masking for COVID-19external icon. NEJM. Two letters caution against suggesting that masks provide benefits beyond reducing transmission; concern relates to an opinion piece by Gandhi & Rutherfordexternal icon suggesting that face masks can reduce viral load and therefore disease severity, as current evidence does not suggest a clear relationship between infectious dose of SARS-CoV-2 and disease severity.
- Liu et al. Seropositive prevalence of antibodies against SARS-CoV-2 in Wuhan, Chinaexternal icon. JAMA. A serosurvey conducted between March 27 and May 26, 2020 of over 35,000 >18-year-olds with no history of COVID-19 showed 3.9% seropositivity to SARS-CoV-2-specific IgG.
- Strassle et al. COVID-19 vaccine trials and incarcerated people – the ethics of inclusionexternal icon. NEJM. Perspective discussing if including incarcerated populations in COVID-19 vaccine trials with early access to efficacious vaccines would outweigh concerns about consent regarding inclusion of incarcerated people in studies.
- Xu et al. The effect of prior ACEI/ARB treatment on COVID-19 susceptibility and outcome: A systematic review and meta-analysis.external icon Clinical Infectious Diseases. A review of 49 published studies showed no effect of prior use of angiotensin converting enzyme inhibitors or angiotensin receptor blocker on the risk of SARS-CoV-2 infection.
- Lazarus et al. A global survey of potential acceptance of a COVID-19 vaccine.external icon Nature Medicine. A 2020 survey of >13,000 individuals in 19 countries showed that 71.5% of participants reported they would be very or somewhat likely to take a COVID-19 vaccine.
- Marquez et al. Response to the COVID-19 pandemic among people experiencing homelessness in congregant living settings in San Diego, CAexternal icon. Clinical Infectious Diseases. From April to August 2020, a testing strategy combined with accessible isolation and symptom screening among people experiencing homelessness in congregant living settings in San Diego contributed to a 0.9% incidence of SARS-CoV-2 infection.
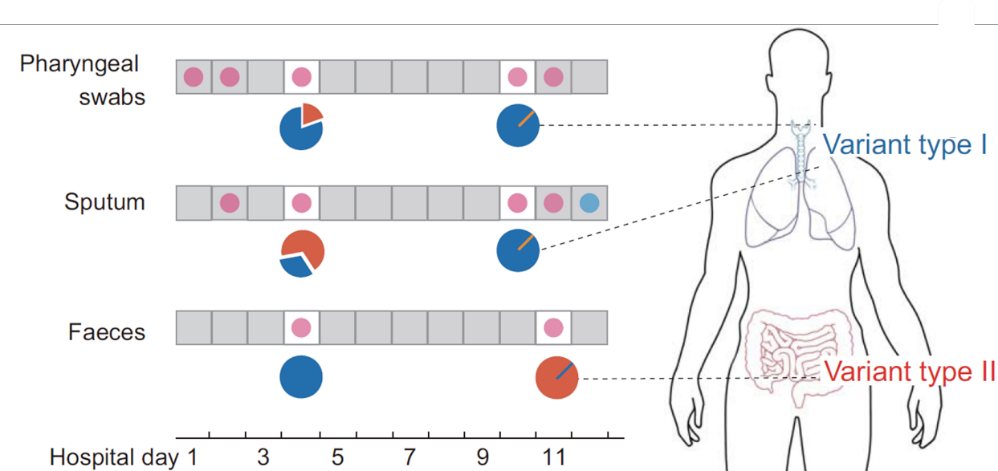
- Du et al. Specific re-distribution of SARS-CoV-2 variants in the respiratory system and intestinal tract.external icon Clinical Infectious Diseases. Case study of a patient with different SARS-CoV-2 variants in pharyngeal swab and sputum vs feces
- Kobayashi et al. COVID-19 serial testing among hospitalized patients in a Midwest tertiary medical center, July–September 2020.external icon Clinical Infectious Diseases. All patients with a negative SARS-CoV-2 test upon hospital admission were serially tested, and rapidly isolated upon becoming positive; 1% became positive.
- Overbaugh, J. Understanding protection from SARS-CoV-2 by studying reinfection.external icon Nature Medicine. Lessons from HIV are used to illustrate how understanding why some people can get re-infected can lead to better vaccines.
- Fang et al. COVID-19 – lessons learned and questions remainingexternal icon. Clinical Infectious Diseases. Review detailing what we know about the epidemiology, clinical features, diagnosis, treatment and prevention of SARS-CoV-2 infection, as well as unanswered questions about COVID-19.
Figure:
Note: Adapted from Du et al. Different SARS-CoV-2 variants found in respiratory (pharyngeal swabs and sputum) and fecal specimens of the same individual. Permission request in process.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.