COVID-19 Science Update released: November 5, 2021 Edition 112

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 database, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 Delta variant. Zhang et al. Science (October 26, 2021).
Key findings:
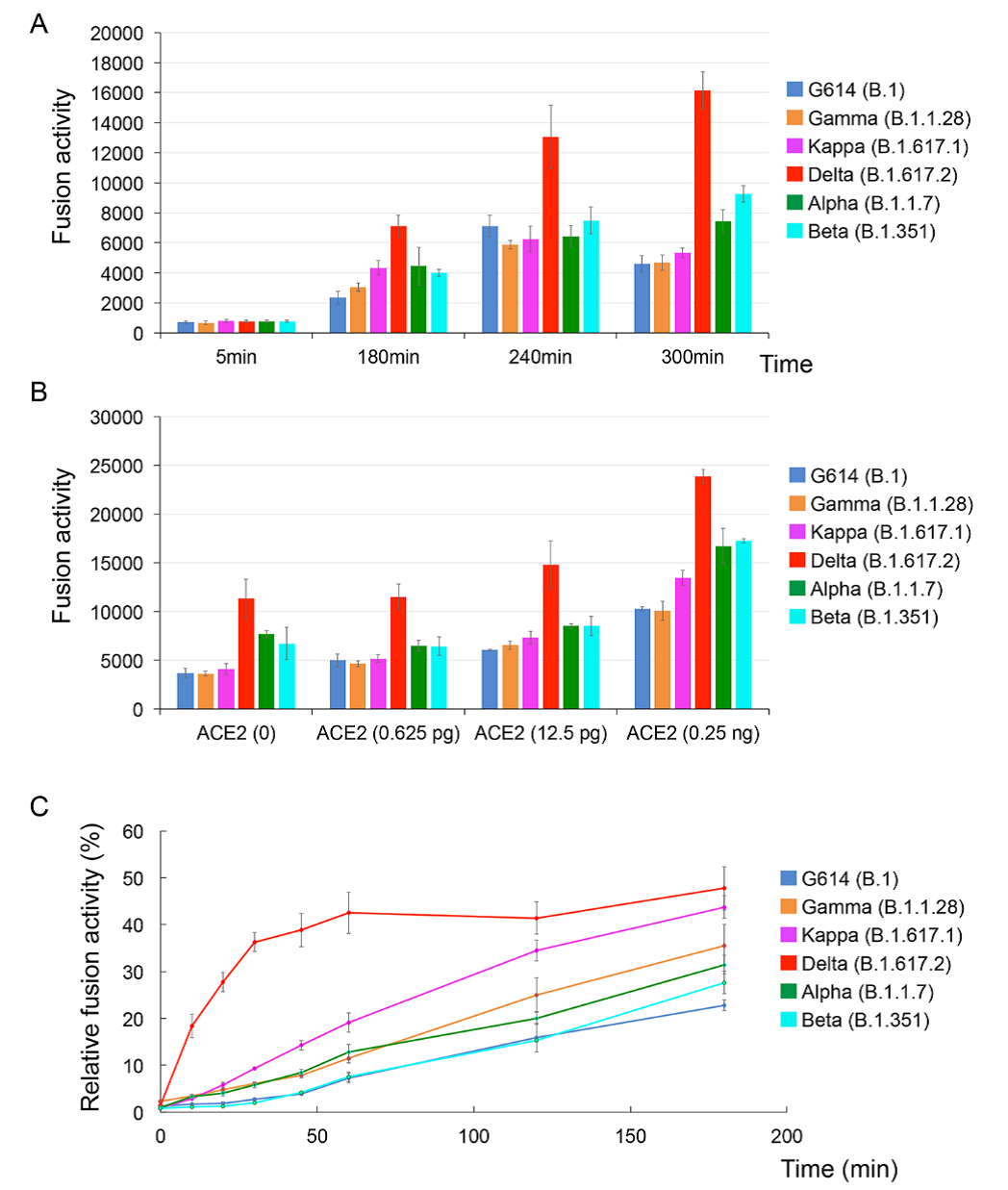
- In vitro, the spike (S) protein of the SARS-CoV-2 Delta (B.1.617.2) variant was more efficient at fusion for cell entry compared with S proteins of the G614, Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Kappa (B.1.617.1) variants (Figure).
- Delta variant fusion occurred more quickly and with lower receptor (ACE2) levels compared with other variants (Figure).
Methods: S protein functional characteristics of G614, Alpha, Beta, Gamma, Delta, and Kappa variants were assessed by binding, fusion, and pseudovirus assays using primarily ACE2, HEK-293 cells, and monoclonal antibodies. Limitations: No in vivo model; did not confirm with full-length authentic viruses.
Implications: The Delta variant has an in vitro advantage due to its spike protein that rapidly fuses with cells for entry, using low levels of ACE2 receptor. The relative ease of cellular entry may contribute to the Delta variant’s efficient person-to-person transmissibility and worldwide expansion.
Figure:
Note: Adapted from Zhang et al. A) Cell-cell fusion with full-length S proteins of different variants expressed in HEK293 cells measured by complementation of beta-galactosidase in the target HEK293 cells over time. B) Cell-cell fusion when target HEK293 cells are enriched with increasing amounts of ACE2. C) Pseudotyped viruses with different variants were used to infect HEK293-ACE2 cells in single cycle infections with viruses washed off at the time points shown. Licensed under CC BY 4.0.
PREPRINTS (NOT PEER-REVIEWED)
COVID-19 breakthrough infections and pre-infection neutralizing antibody. Yamamoto et al. medRxiv (October 25, 2021). Published in Clinical Infectious Diseases as Coronavirus Disease 2019 (COVID-19) breakthrough infection and post-vaccination neutralizing antibodies among healthcare workers in a referral hospital in Tokyo: A case-control matching study (December 24, 2021)
Key findings:
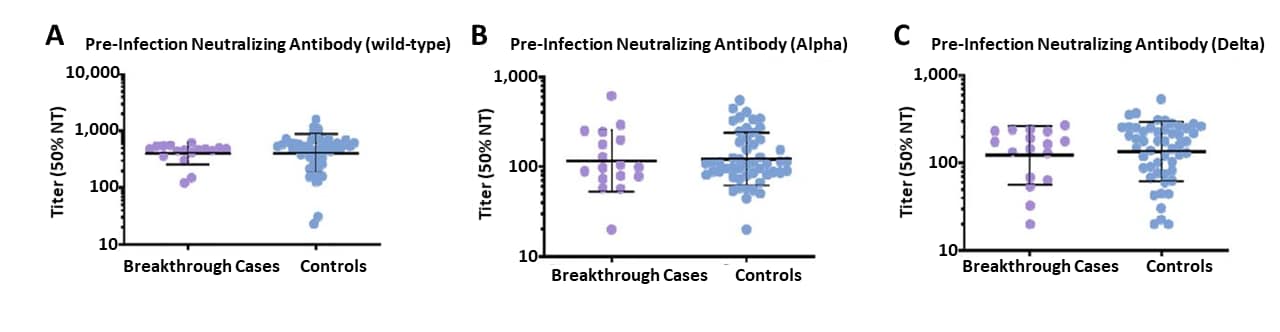
- Among vaccinated (BNT162b2 [Comirnaty, Pfizer/BioNTech]) hospital workers, persons with breakthrough infections during July–September 2021 had pre-infection neutralizing antibody titers that were similar to titers among uninfected persons (matched controls) (Figure).
- Neutralizing antibodies against Alpha and Delta variants were lower compared to wild type.
Methods: Matched case-control study conducted within longitudinal serological study, Japan. Cases (n = 17) and controls (n = 51) were matched on multiple factors, including worksite, sex, age, and time interval between the 2nd vaccination and blood specimen collection. Pre-infection neutralizing antibodies and anti-spike antibodies were assessed 5–10 weeks before infection. Limitations: Small sample size; antibodies might have waned before infection.
Implications: Neutralizing antibody titers did not distinguish between susceptibility or immunity to breakthrough infections in this small study. These data support CDC guidance, which does not currently recommend antibody testing to assess for immunity following COVID-19 vaccination.
Figure:
Note: Adapted from Yamamoto et al. Pre-infection neutralizing and anti-spike antibody titers among post-vaccination breakthrough cases and vaccinated uninfected controls. Neutralizing antibodies against wild-type strain (A), Alpha variant (B), and Delta variant (C) during the pre-infection period. Horizontal bars mark the geometric mean titers; vertical bars indicate geometric standard deviations. Licensed under CC-BY-NC-ND 4.0.
Predictors of SARS-CoV-2 infection following high-risk exposure. Andrejko et al. medRxiv (October 23, 2021). Published in Clinical Infectious Diseases (December 21, 2021).
Key findings:
- Among persons reporting a high-risk exposure, wearing a face mask during the exposure reduced the odds of infection by 48% (95% CI 9%-71%), and vaccination reduced the odds of infection by 77% (95% CI 59%-87%).
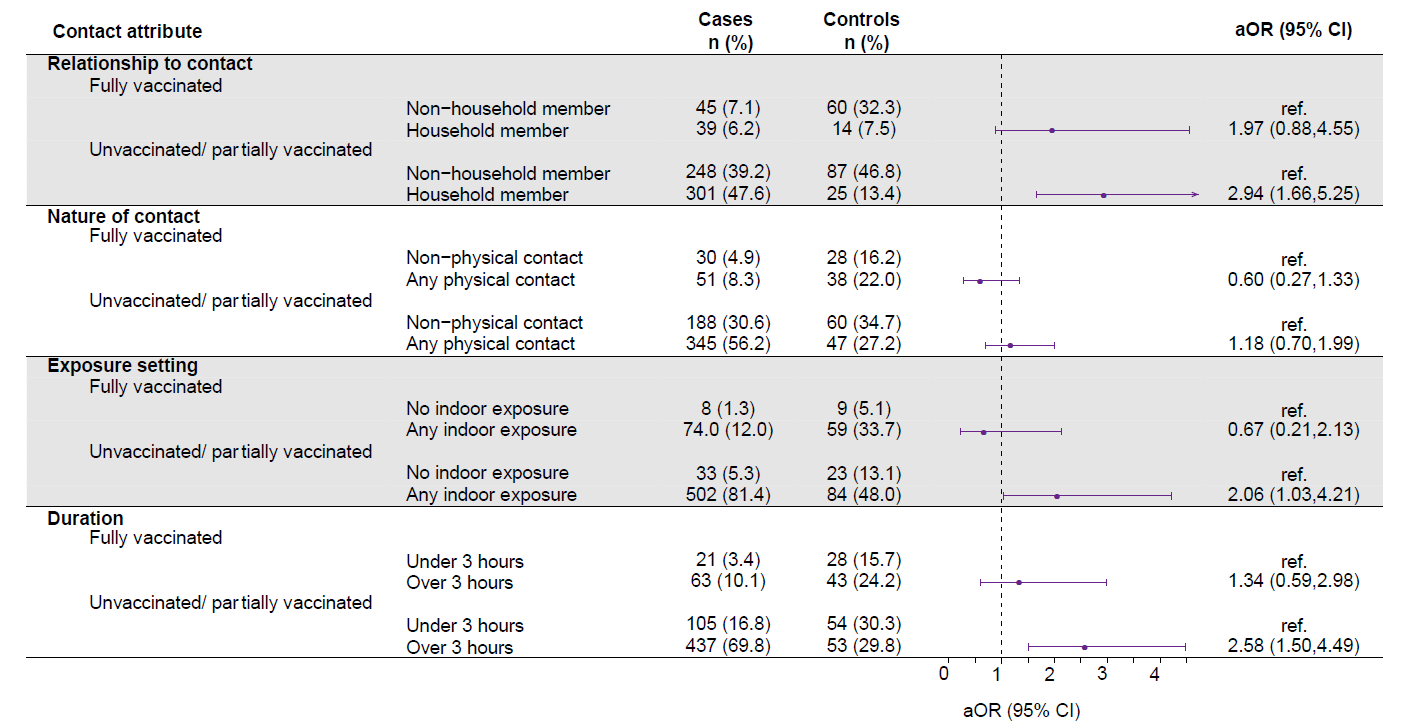
- Among unvaccinated and partially vaccinated persons, exposures that occurred indoors, lasted ≥3 hours, or were from a household member were associated with greater odds of infection (aOR 2.06 [95% CI 1.03-4.21], 2.58 [95% CI 1.50-4.49], and 2.94 [95% CI 1.66-5.25], respectively) (Figure).
- The excess risk associated with these exposures was attenuated among fully vaccinated individuals.
Methods: Case-control study among persons (643 cases and 204 controls) with molecular SARS-CoV-2 test results reported to California Department of Public Health during February–September 2021 and who reported a high-risk exposure (social contact with a person known or suspected to have SARS-CoV-2 infection) within 14 days prior to testing. Predictors of positive tests assessed using multivariable conditional logistic regression. Limitations: Vaccination coverage was low, and Delta (B.1.617.2) variant was not widespread during study; type of masks used not reported; underpowered to make certain comparisons.
Implications: Masks and vaccination significantly reduce the risk of infection in real world settings.
Figure:
Note: Adapted from Andrejko et al. High-risk exposure characteristics stratified by vaccination status with adjusted odds ratios (aOR) for testing positive for SARS-CoV-2 within each category. Adjusted for age, sex, region, mask wearing, and community exposures. Used by permission of authors.
PEER-REVIEWED
Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Singanayagam et al. The Lancet Infectious Diseases (October 29, 2021).
Key findings:
- Among symptomatic persons infected with the Delta (B.1.617.2) variant (index cases) and their household contacts, the secondary attack rate (SAR) was 25% (95% CI 18%-33%) among fully vaccinated household contacts and 38% (95% CI 24%-53%) among unvaccinated household contacts.
- The SAR among contacts of vaccinated index cases (25%) was similar to the SAR among contacts of unvaccinated index cases (23%).
Methods: Prospective longitudinal cohort study, United Kingdom, September 2020–September 2021. SARS-CoV-2 household transmission risk was evaluated by vaccination status for 231 contacts (aged ≥5 years) exposed to 162 epidemiologically linked Delta variant-infected index cases. Limitations: Only symptomatic index cases were recruited; young household members unlikely to have been vaccinated.
Implications: Vaccination of household contacts may provide modest protection against household transmission of the Delta variant. However, fully vaccinated individuals with Delta variant infection can transmit SARS-CoV-2 to vaccinated and unvaccinated household contacts.
PREPRINTS (NOT PEER-REVIEWED)
Post COVID-19 in children, adolescents, and adults: Results of a matched cohort study including more than 150,000 individuals with COVID-19. Roessler et al. medRxiv (October 22, 2021).
Key findings:
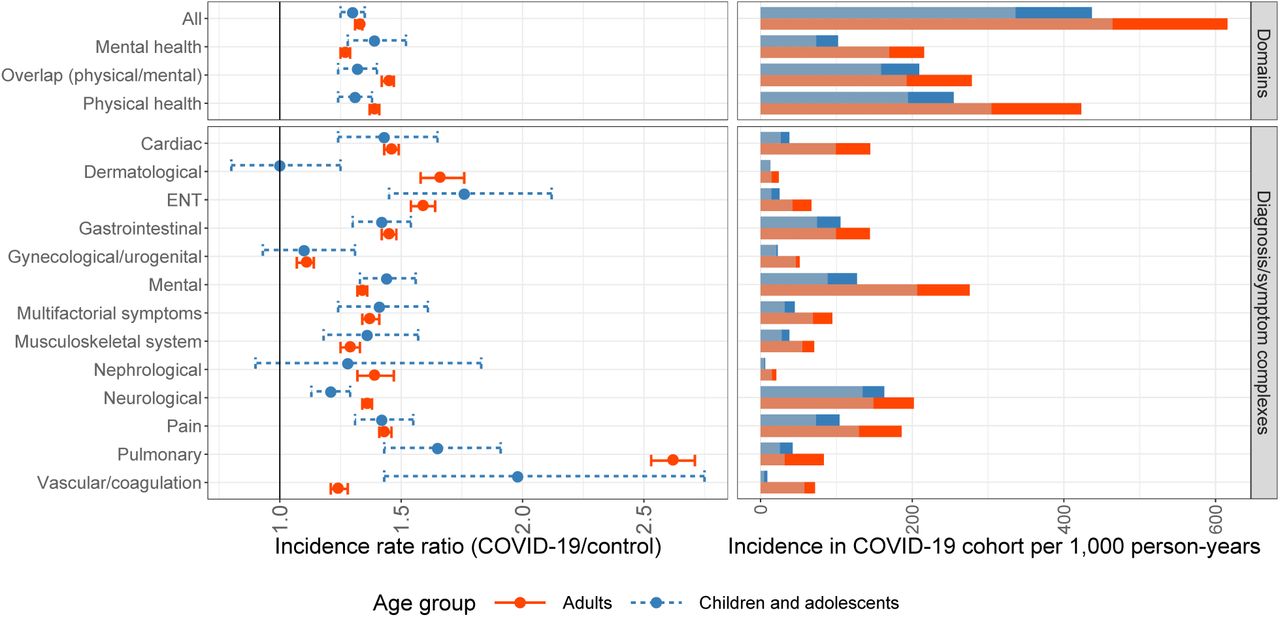
- Physical and mental health conditions that are considered “post COVID-19 conditions” were more common among children (IRR 1.30, 95% CI 1.25-1.35) and adults (IRR 1.33, 95% CI 1.31-1.34) who had COVID-19 within the prior 3 months than among matched controls who had no history of infection (Figure).
- Among children, the conditions with the highest IRRs were malaise/fatigue/exhaustion, cough, and throat/chest pain.
- Among adults, the conditions with the highest IRRs were dysgeusia, fever, and dyspnea.
- The overall incidence of post COVID-19 conditions was 41% higher among adults than children (615.82 vs. 439.91 per 1,000 person-years, respectively) within the 3 months after COVID-19.
Methods: Cohort study using health insurance data; each COVID-19 patient (11,950 children aged 0–17 years and 145,184 adults with COVID-19 in 2020) matched to 5 controls based on age, sex, and propensity score on prevalent medical conditions, Germany. 96 pre-defined outcomes were aggregated into 13 diagnosis/symptom complexes and 3 domains. Limitations: Short follow-up duration; might not be generalizable to the United States; persons with undiagnosed SARS-CoV-2 infections could have been misclassified as controls.
Implications: Post COVID-19 morbidity affects both children/adolescents and adults and indicates extended healthcare needs.
Figure:
Note: Adapted from Roessler et al. Estimated incidence rate ratios with 95% CIs and incidence rates in COVID-19 cohort for children/adolescents and adults by health outcome domain and diagnosis/system complex. Incidence rates in the control cohort are shown in pale color (right figure). Licensed under CC-BY-NC-ND 4.0.
PREPRINTS (NOT PEER-REVIEWED)
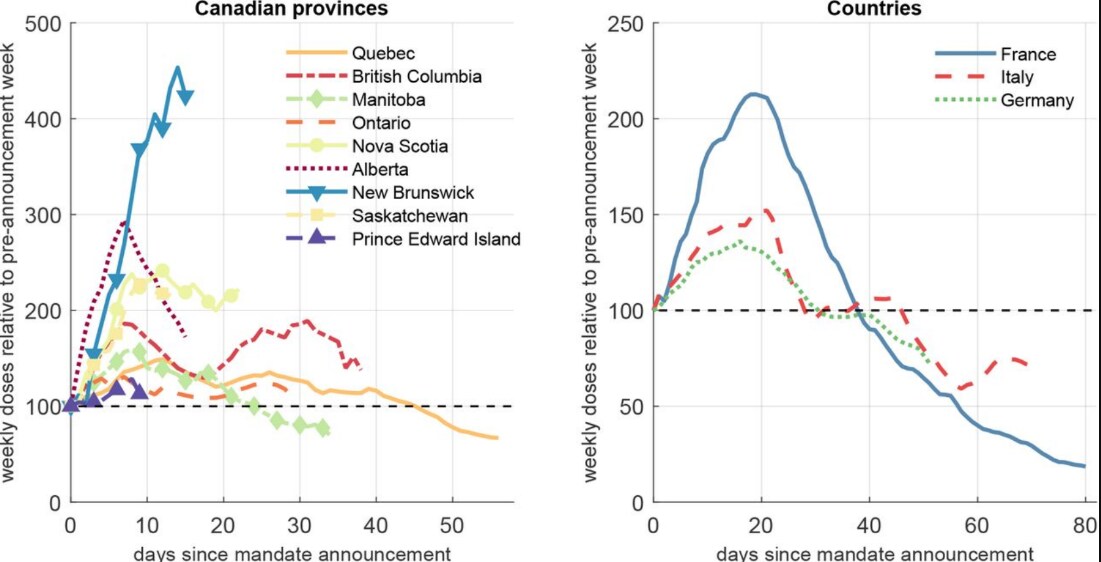
COVID-19 vaccination mandates and vaccine uptake. Karaivanov et al. medRxiv (October 25, 2021). Published in Nature (June 2, 2022).
Key findings:
- In Canada, announcements of governmental COVID-19 vaccination mandates (proof of vaccination requirements for access to public venues and non-essential businesses) were associated with an estimated 55% increase in 1st dose vaccinations in the 1st week and >100% increase in the 2nd week following the policy announcement.
- The mandates’ effect decreased over time and largely dissipated after 6–8 weeks.
- Similar trends were observed in France, Italy and Germany.
Methods: Variation in timing of provincial mandates (August 5 to September 21, 2021) allowed for a difference-in-differences model to determine the average effect of announcing vaccination mandates on 1st dose vaccine uptake in 9 Canadian provinces. Counterfactual simulations and time-series analyses were used to separately estimate the effect on uptake in each province and in France, Italy, and Germany. Limitations: Estimates may change as more data becomes available; cannot prove causality.
Implications: COVID-19 vaccination mandates appear to have a large but short-lived effect on 1st dose uptake.
Figure:
Note: Adapted from Karaivanov et al. Weekly 1st doses of COVID-19 vaccine by days since mandate announcement. Weekly 1st doses for the week just prior to the mandate announcement are normalized to 100 (horizontal dashed line) for each province (on the left) or country (on the right). Used by permission of authors.
PEER-REVIEWED
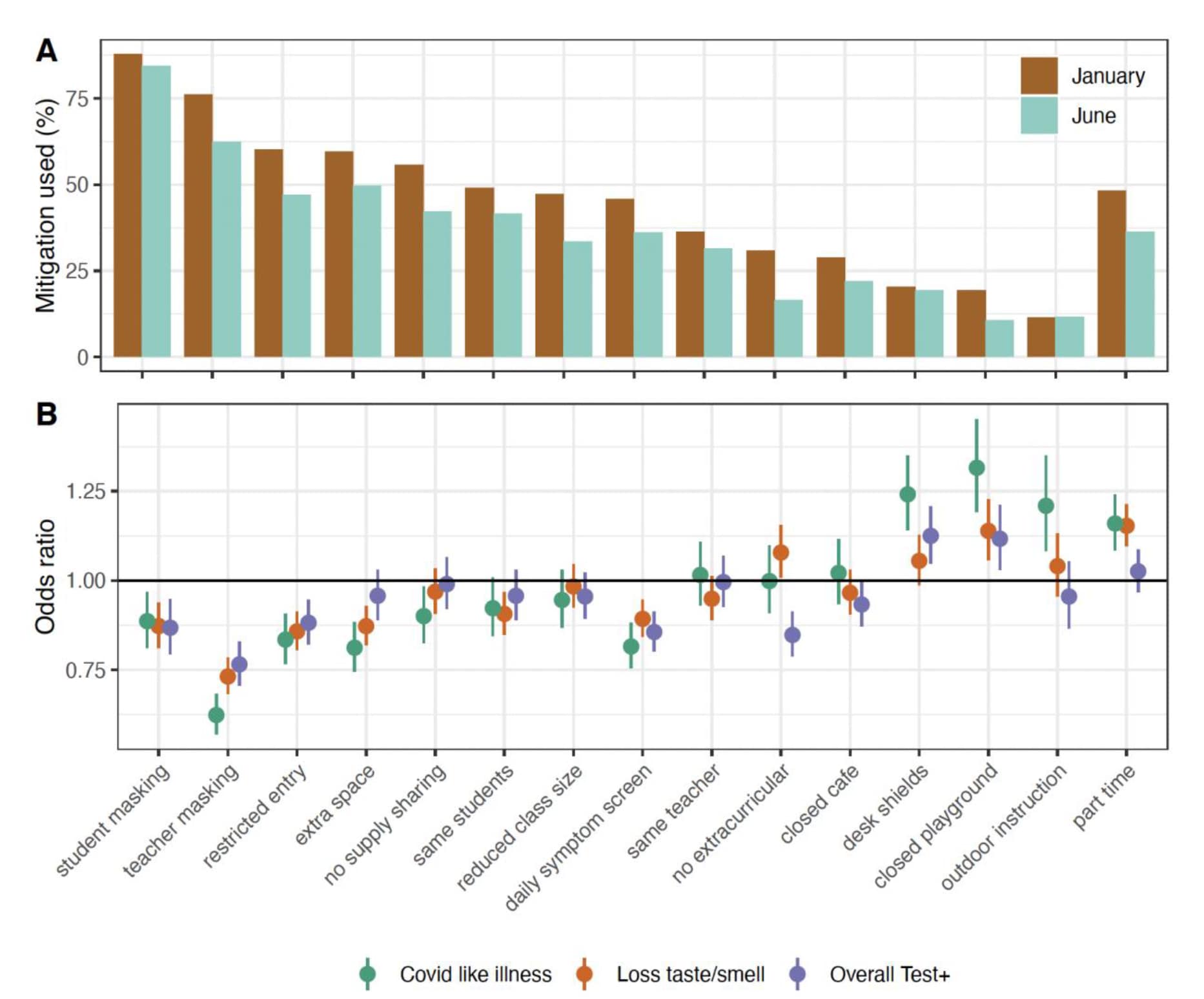
In-person schooling and associated COVID-19 risk in the United States over spring semester 2021. Wiens et al. medRxiv (October 23, 2021).
Key findings:
- During January–June 2021, full-time in-person schooling was associated with increased odds of COVID-19-like illness (CLI) (aOR 1.32, 95% CI 1.25-1.40) and a positive SARS-CoV-2 test (aOR 1.32, 95% CI 1.27-1.38) compared with no in-person schooling.
- The associations between in-person schooling and CLI or positive SARS-CoV-2 test results decreased as the number of school-based mitigation measures implemented increased.
- Teacher masking appeared to be the most protective school-based mitigation measure, followed by daily symptom screens, student masking, and restricted entry (Figure).
Methods: Weighted analyses of US COVID-19 Trends and Impact Survey (CTIS) data, administered with Facebook using 2-stage sampling (n = 1,082,733 respondents living with school-aged children). Survey elicited demographics, COVID-19 symptoms, test results, vaccination, and schooling for any children in the household. Primary outcomes included CLI, loss of taste and/or smell, and positive test results in the past 24 hours. Limitations: Data were self-reported and subject to recall bias; unclear validity of respondents’ description of school policies and adherence to mitigation strategies; CLI is subjective; may not be representative of U.S. population.
Implications: School-based mitigation measures appear to reduce transmission risk associated with in-person schooling and are important steps for protection of the health of students and school staff.
Figure:
Note: Adapted from Wiens et al. Individual mitigation measures in schools and odds of COVID-19-related outcomes. A) Percent of respondents living with children in in-person schooling that reported each school-based mitigation measure being used in January and June, weighted using U.S. CTIS survey weights. B) Odds ratio of COVID-19-related outcomes among respondents who reported each mitigation measure (January 12–June 12, 2021). Licensed under CC BY 4.0.
Vaccines
- Guaranteed financial incentives for COVID-19 vaccination: A pilot program in North Carolina. Wong et al. JAMA Internal Medicine (October 25, 2021). In 4 counties in North Carolina (April–June 2021), vaccine initiation declined less at sites offering $25 cash card incentives to vaccine recipients (n = 2,890) and drivers (n = 1,374), compared with other sites in the same counties (−26.4% vs. −51.1%, p <0.001) and the overall state (−48.6%, p <0.001). Among those surveyed (n = 401), vaccine recipients who were Hispanic/Latino, Black or African American, or who had lower income were more likely to report that incentives were important for vaccine decision-making.
- COVID-19 vaccine perceptions and uptake in a national prospective cohort of essential workers. Lutrick et al. medRxiv (Preprint; October 23, 2021). Published in Vaccine (January 24, 2022). In a multicenter prospective cohort of essential workers (n = 2,017) who completed 2 surveys 3 months apart (December 2020–May 2021), 25% (n = 94) of those initially classified as vaccine-reluctant, 56% (n = 195) of those classified as reachable, and 83% (n = 1,232) vaccine-endorsers were vaccinated at the time of the follow-up survey. Among endorsers, unvaccinated participants were more likely to be male, younger, and firefighters than endorsers that were vaccinated. Sixteen percent of firefighters and 21% of other first responders were vaccine-reluctant.
Natural History, Reinfection, and Health Impact
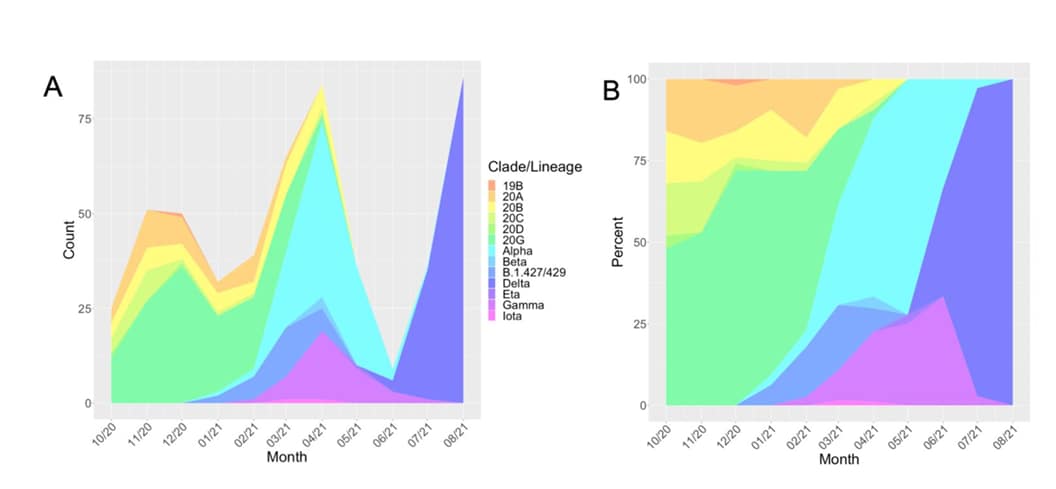
- Severity of illness caused by Severe Acute Respiratory Syndrome Coronavirus 2 variants of concern in children: A single-center retrospective cohort study. Edward et al. medRxiv (Preprint; October 26, 2021). Among 499 children infected with SARS-CoV-2 whose isolate was sequenced (October 2020–August 2021, Chicago), 46.5% of infections were Delta, 37.5% were Alpha, and 14.8% were Gamma variants. After adjusting for confounding variables, Gamma was associated with severe COVID-19 (OR 7.7, 95% CI 1.0-78.1), but Alpha and Delta were not.
Note: Adapted from Edward et al. Frequencies (A) and proportions (B) of lineages of SARS-CoV-2 identified in pediatric patients over time, Chicago, October 2020–August 2021. Variants of concern identified include Alpha, Beta, Gamma, and Delta. Licensed under CC-BY-NC-ND 4.0.
- Evaluation of COVID-19 mortality and adverse outcomes in US patients with or without cancer. Chavez-MacGregor et al. JAMA Oncology (October 28, 2021). In a cohort of 507,307 patients with COVID-19, those with cancer who received anticancer treatment within 3 months before COVID-19 diagnosis had an increased risk of death (aOR 1.74, 95% CI 1.54-1.96), intensive care unit admission (aOR 1.69, 95% CI 1.54-1.87), and hospitalization (aOR 1.19, 95% CI 1.11-1.27) compared with patients without cancer. Cancer patients without recent cancer treatment had similar or better outcomes than patients without cancer.
Prevention Strategies and Non-Pharmaceutical Interventions
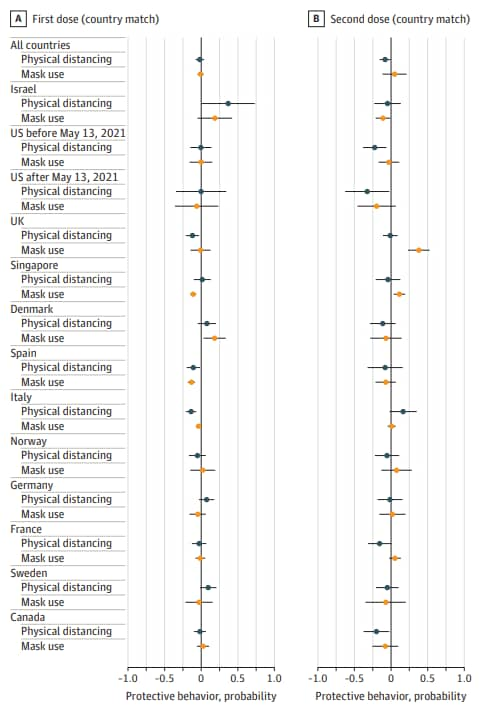
- Protective behaviors against COVID-19 by individual vaccination status in 12 countries during the pandemic. Goldszmidt et al. JAMA Network Open (October 26, 2021). Surveys of nationally representative samples in 12 countries (n = 80,305) found few differences in self-reported protective behaviors (i.e., masking, physical distancing) by vaccination status during February–June 2021. Fully vaccinated respondents physically distanced less than the unvaccinated (β = −0.07; 95% CI −0.13 to <−0.01), but there were no differences in mask use.
Note: Adapted from Goldszmidt et al. Estimated associations between vaccination and COVID-19 protective behaviors. Countries are ordered by the proportion of the population who received 2 doses as of June 2021. A) individuals who received 1 dose (n = 5,229) compared to matched unvaccinated persons (n = 8,220). B) Individuals who received 2 doses (n = 2,893) compared to matched persons who received 1 dose (n = 3,249). May 13, 2021 = date when CDC released guidance about easing of non-pharmaceutical interventions if fully vaccinated. Licensed under CC BY.
Transmission Risk and Dynamics
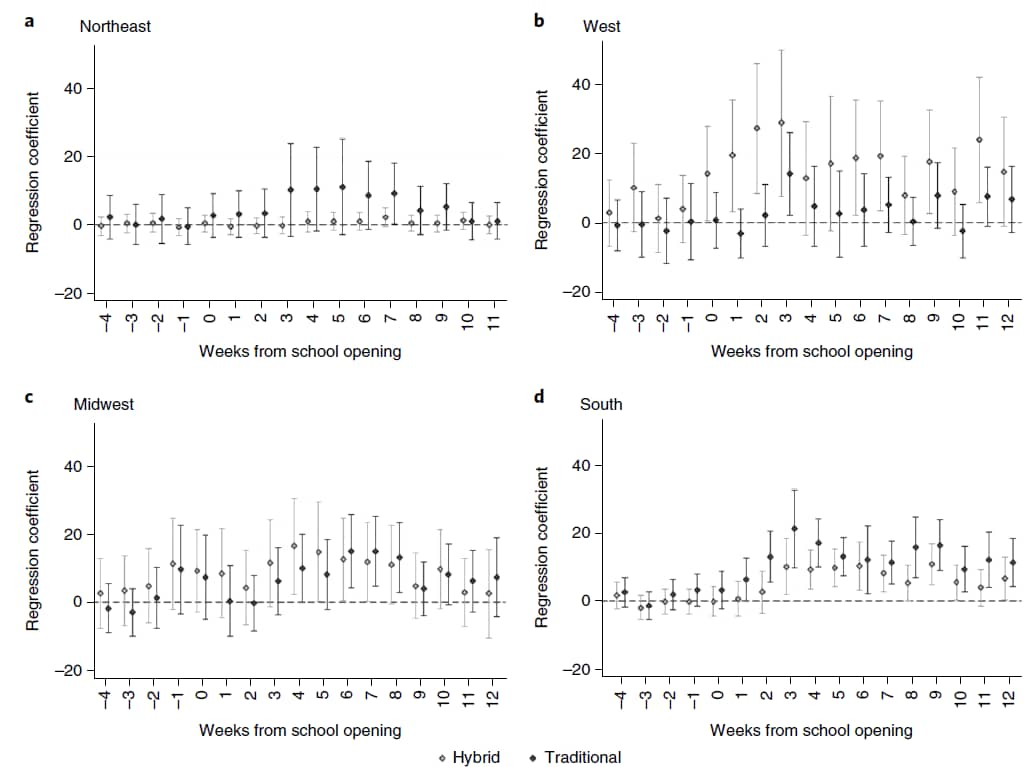
- The impact of school opening model on SARS-CoV-2 community incidence and mortality. Ertem et al. Nature Medicine (October 27, 2021). In the U.S. Midwest and Northeast regions, county-level incidence of SARS-CoV-2 did not vary with school mode (virtual, hybrid, or traditional) based on data from 895 school districts in 459 counties in the pre-Delta period (July–December 2020). In the South, weekly cases increased in counties with hybrid or traditional learning compared with virtual (9.8 to 21.3 additional cases per 100,000 persons); in the West, higher cases were found in hybrid models.
Note: Adapted from Ertem et al. Adjusted absolute difference between SARS-CoV-2 cases in counties with hybrid and traditional school modes relative to virtual (dashed line) for each week, with week 0 being the week when school started for each county, stratified by region: Northeast (a), West (b), Midwest (c), and South (d). U.S. Government work not subject to copyright.
From the Morbidity and Mortality Weekly Report (November 5, 2021).
- Laboratory-Confirmed COVID-19 Among Adults Hospitalized with COVID-19–Like Illness with Infection-Induced or mRNA Vaccine-Induced SARS-CoV-2 Immunity — Nine States, January–September 2021
- The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines — United States, 2021
- Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19–Associated Hospitalizations Among Immunocompromised Adults — Nine States, January–September 2021
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.