COVID-19 Science Update released: October 16, 2020 Edition 57

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
COVID-19 and psychologic distress—changes in internet searches for mental health issues in New York during the pandemic. external iconStijelja et al. JAMA Internal Medicine (October 5, 2020).
Key findings:
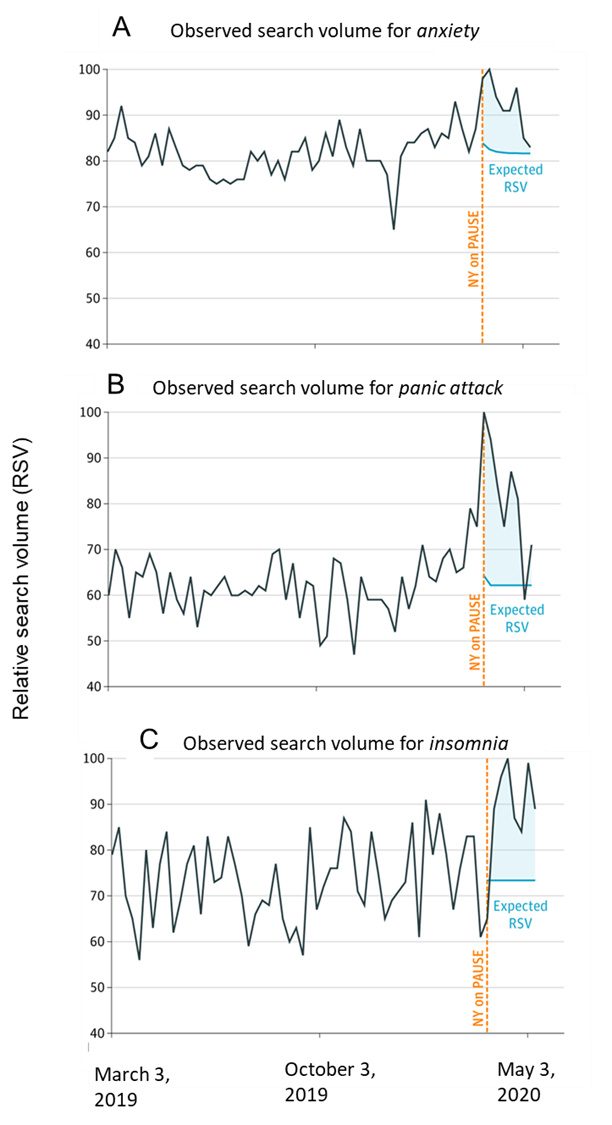
- Internet searches were higher than expected based on historical search data for the topics anxiety, 18% (95% prediction interval [PI] 5%-29%), panic attack, 56% (95% PI 37%-97%), and insomnia, 21% (95% PI 1%-55%) after March 22, 2020, and remained high for up to 5 weeks (Figure).
- Searches for the topics suicide and depression did not increase above expected.
- Searches for suicide increased during the week of April 25 to May 2, matching the time frame of a publicized suicide of a New York physician.
Methods: Google Trend was used to examine relative search volumes (RSV) and forecasts between March 23 and May 14, 2020 for internet search terms including suicide, anxiety, panic attack, insomnia, and depression in New York State. Weekly RSV forecasts were computed with 95% PIs. Limitations: Does not estimate the number of individuals experiencing symptoms; many people with mental health issues may not have access the internet.
Implications: There is the potential to monitor the mental health of a population during emergencies using internet search trends to identify areas needing targeted resources for mental health support.
Note: Adapted from Stijelja et al. Observed search volume and excess search volume for the terms anxiety (A), panic attack (B), or insomnia (C). Orange dotted line indicates start of New York State lockdown. Reproduced with permission from JAMA Internal Medicine. Stijelja et al., COVID-19 and psychologic distress—changes in internet searches for mental health issues in New York during the pandemic. DOI: 10.1001/jamainternmed.2020.3271. October 5, 2020. Copyright©2020 American Medical Association. All rights reserved.
The impact of the COVID-19 pandemic on healthcare acquired infections with multidrug resistant organisms.external icon Cole et al. American Journal of Infection Control (October 1, 2020).
Key findings:
- 25% increase in hand sanitizer and hand soap use among health care personnel in the second quarter (Q2) of 2020 compared to the first quarter (Q1).
- 35% decrease in hospital-acquired multidrug resistant infection rates from 0.3/1,000 person-days in Q1 to 0.2/1,000 person-days in Q2, (p = 0.03); specifically:
- 80% decrease in vancomycin-resistant Enterococcus.
- 41% decrease in methicillin-resistant Staphylococcus aureus.
- 21% decrease in extended-spectrum beta-lactamase Enterobacteriaceae
Methods: Analysis of multidrug resistant infections diagnosed in hospital patients ≥4 days following admission based on isolates from urine, blood, wound, and sputum specimens in four hospitals in Los Angeles County during the first six months of 2020. Limitations: demographics of patients and frequencies of type of specimens not included.
Implications: Increased awareness and use of basic infection control practices by health care providers, including effective hand hygiene, can reduce hospital-acquired and other healthcare-associated infections.
PEER-REVIEWED
SARS-CoV-2 outbreak investigation in a German meat processing plantexternal icon. Günther et al. EMBO Molecular Medicine (October 4, 2020).
Key findings:
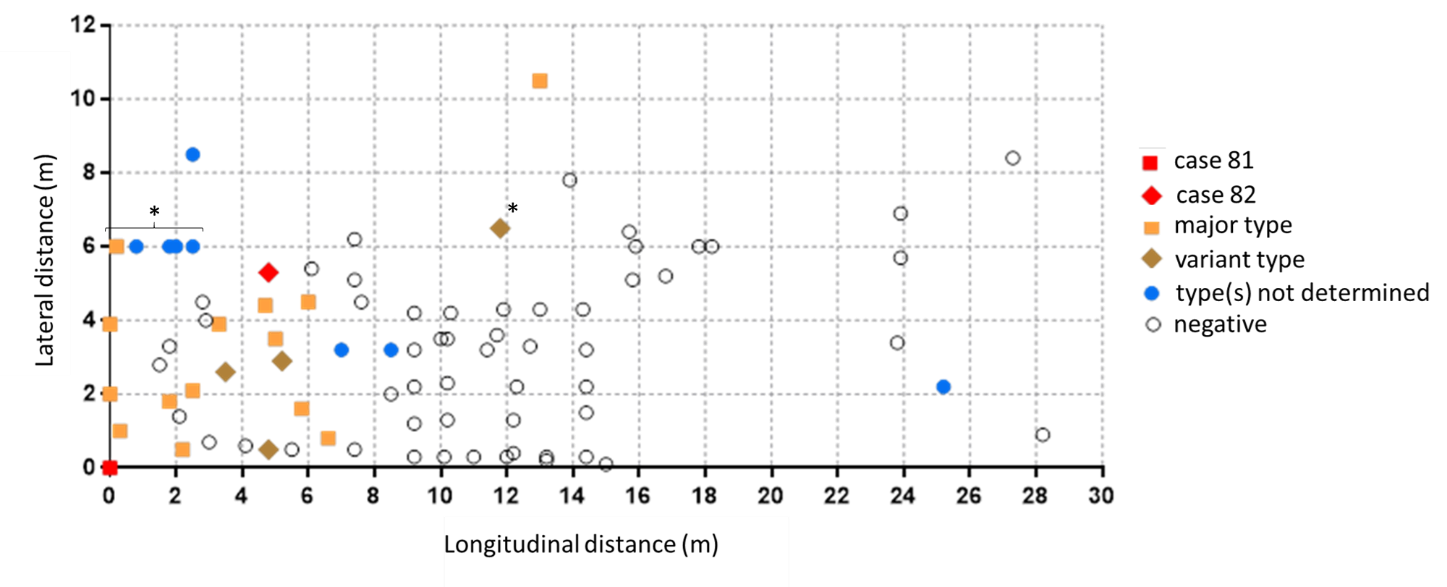
- Based on viral genome relatedness, a cluster of 29 infections among 147 workers from a single shift was attributable to a single index case (Figure).
- Follow-up screening and testing identified ongoing transmission with more than 1,400 cases identified through June.
- Workers within an 8-meter radius of the index case were at highest risk for infection (p <0.001).
- Infection was associated with working at fixed stations at which air conditioners recirculated air above workers, fans recirculating the air did not have filters and fresh-air exchange rates were low (<1 exchange per hour).
- Sharing a bedroom (p = 0.039) and sharing both an apartment and transportation (p = 0.048), were associated with infection.
Methods: Report of a large outbreak in a meat processing plant in Germany between May and June 2020, with genome sequencing of virus from cases. Limitations: All data on workers, including workplace location, sharing of housing, or transportation, were provided by the employer with no independent verification; genome type was not determined for all cases.
Implications: SARS-CoV-2 super spreading events have occurred in meat processing plants. In the absence of good ventilation, physical distance of 2 meters (6 feet) did not prevent transmission of SARS-CoV-2.
Figure:
Note: Adapted from Günther et al. Distance (in meters) of co-workers from the suspected index case 81. For workers without fixed position in the beef processing plant (marked with an asterisk) coordinates indicate estimated average location. Squares and diamonds denote major or variant SARS-CoV-2 genotypes, respectively. Filled blue circles denote cases for which viral genomes were not sequenced. Case 82 tested positive at the same time as case 81, but genome sequencing did not link this case to the outbreak. Licensed under CC-BY-4.0.
PEER-REVIEWED
Follow-up of adults with non-critical COVID-19 two months after symptoms’ onsetexternal icon. Carvalho-Schneider et al. Clinical Microbiology and Infection (October 5, 2020).
Key findings:
- Two-thirds of non-critical COVID-19 patients experienced prolonged symptoms, and some felt worse later in the disease course than at symptom onset.
- Loss of smell and taste were the most frequent persistent symptoms at day 60 (22.7%).
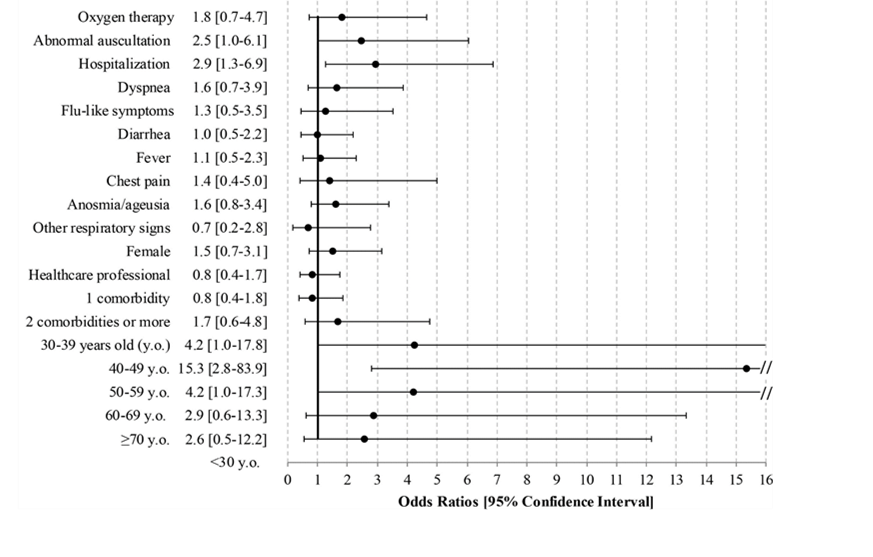
- Persisting clinical symptoms were significantly associated with being admitted to the hospital, having an abnormal chest exam (auscultation), and being 40–60 years old at time of symptom onset (Figure).
Methods: Follow-up of 150 patients with non-critical RT-PCR-confirmed COVID-19 admitted to hospitals or seen as outpatients up to 60 days after symptom onset in France, between March 17 and June 3, 2020. Demographic, clinical, and laboratory data were collected from medical records and phone interviews. Limitations: Baseline characteristics partially collected retrospectively; some data for contributing factors were missing limiting analysis; smoking status was not available.
Implications: Clinicians and patients should be aware of the potential need for prolonged medical follow-up even among those with mild initial clinical courses.
Figure:
Note: Adapted from Carvalho-Schneider et al. Odds Ratios and 95% CIs of acute-infection and demographic related factors associated with persisting symptoms at day 60 after symptom onset. This article was published in Clinical Microbiology and Infection, Carvalho-Schneider et al., Follow-up of adults with non-critical COVID-19 two months after symptoms’ onset. DOI: https://doi.org/10.1016/j.cmi.2020.09.052. October 5, 2020. Copyright European Society of Clinical Microbiology and Infectious Diseases 2020. This article is currently available at the Elsevier COVID-19 resource centre: https://www.elsevier.com/connect/coronavirus-information-center.
Neuropathology of patients with COVID-19 in Germany: A post-mortem case seriesexternal icon. Matschke et al. Lancet Neurology (October 5, 2020).
Key findings:
- There was substantial evidence of destruction of neurons (astrogliosis) in all autopsied patients.
- 86% had astrogliosis in all brain regions tested.
- Infiltration by cytotoxic T lymphocytes and microglia (cells that act as immune defense for the brain and spinal cord) were most prominent in the brainstem and cerebellum.
- Mild (n = 28) and moderate (n = 6) infiltration of meningeal compartments by cytotoxic T lymphocytes was identified in 79% of patients.
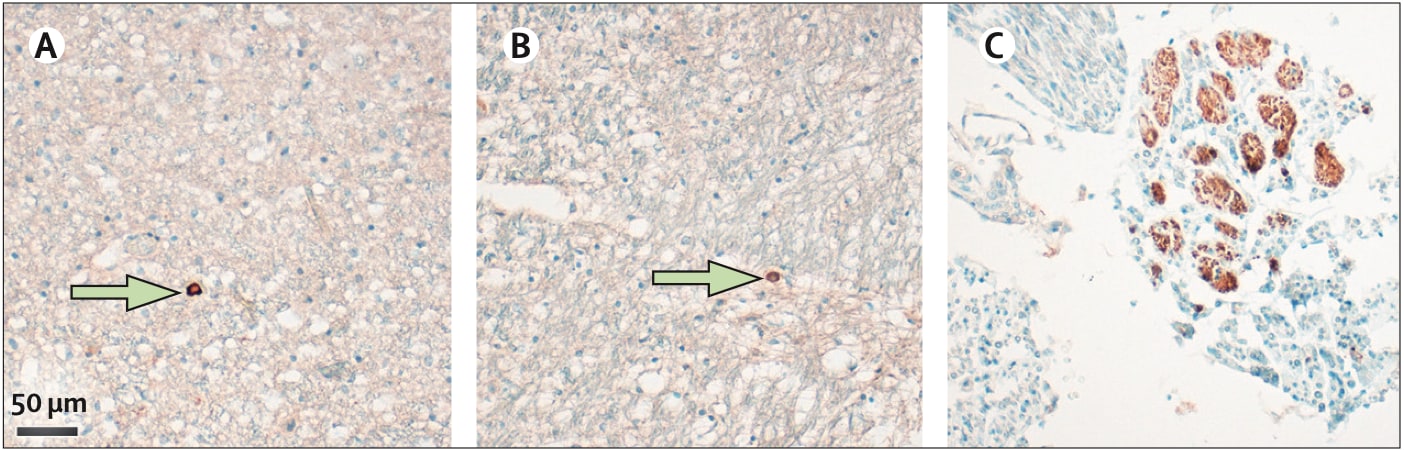
- SARS-CoV-2 RNA or proteins were detected in the brain tissues of 53% of patients tested (21/40), (Figure).
- Both SARS-CoV-2 RNA and protein were detectable in 20% of patients.
- SARS-CoV-2 presence did not correlate with the severity of neuropathological alterations.
Methods: Post-mortem case series of neural autopsies of 43 SARS-CoV-2-confirmed patients performed between March 13 and April 24, 2020 in Hamburg, Germany. Neural tissue from six brain regions were evaluated for pathology and for the presence and distribution of SARS-CoV-2 mRNA and protein. Limitations: 93% of patients had relevant pre-existing chronic medical conditions, and 30% had pre-existing neurological disease; no controls; small sample.
Implications: These data show that SARS-CoV-2 can infiltrate the central nervous system. Frank et al.,external icon discuss the importance of understanding if neuropathological alterations with COVID-19 are from viral-induced cell destruction or from sequelae of an overstimulated systemic immune response, since these different causes would require different treatments.
Figure:
Note: From Matschke et al. Viral protein-positive cells (green arrows) in the medulla oblongata detected by anti-nucleocapsid protein antibody (A) or anti-spike protein antibody (B). (C) SARS-CoV-2 nucleoprotein (brown staining) in cranial nerves. This article was published in Lancet Neurology, Matschke et al., Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. DOI: https://doi.org/10.1016/ S1474-4422(20)30308-2. October 5, 2020. Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource centre: https://www.elsevier.com/connect/coronavirus-information-center.
PREPRINTS (NOT PEER-REVIEWED)
Temporal analysis of COVID-19 convalescent plasma donations reveals significant decrease in neutralizing capacity over time.external icon Girardin et al. medRxiv (October 6, 2020).
Key findings:
- Of the initial donations of plasma from COVID-19 convalescent patients,79.5% of specimens that neutralized 50% of virus in a plaque reduction neutralization test (PRNT50) did so at a plasma IgG titer of at least 1:80, the US Food and Drug Administration (FDA) minimum titer for convalescent plasma (CP).
- 51.4% of specimens with a PRNT50 met the FDA recommended plasma titer of at least 1:160.
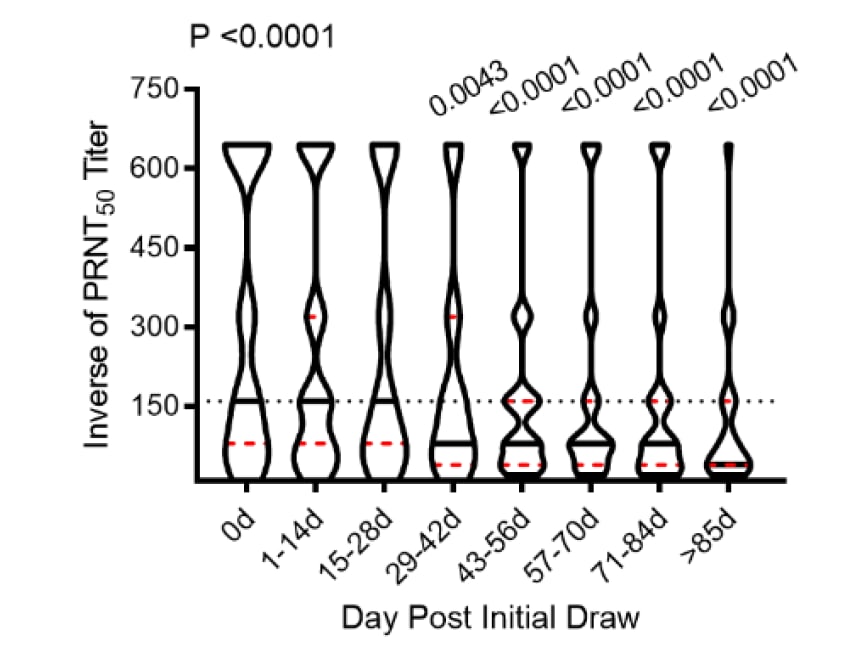
- At 85 days after the initial donation, only 48.8% of specimens with PRNT50 met the minimum plasma titer cutoff (Figure 1).
- The most significant decreases in PRNT50 titer occurred at ≥43 days after initial donation.
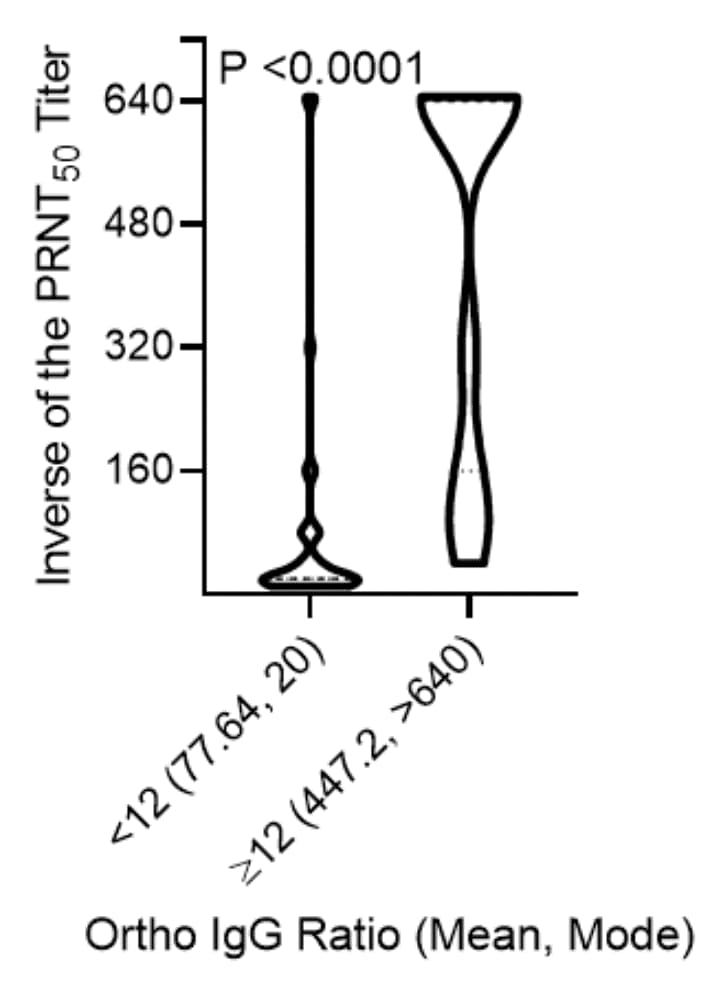
- When using an FDA-approved IgG test that is a suggested surrogate for virus neutralization, 100% of samples with an IgG signal-to-noise ratio ≥12 (the qualifying cutoff for CP) neutralized virus at a titer ≥1:80 (Figure 2).
Methods: A total of 196 specimens meeting the FDA standard of a minimum PRNT50 titer of 1:80 or more were selected at random from CP pools from the Wadsworth Center, New York. Neutralizing capacity of donated CP was measured by PRNT assay. IgG antibodies were detected using the Ortho VITROS SARS-CoV-2. Limitations: Information is based on time since initial donation rather than time since infection
Implications: The decrease in virus neutralization potential in donor CP suggests a limited time frame for CP donation because of potential loss of immunity over time. This also suggests that the donors themselves have waning immunity in this time frame. As assays to measure neutralization are cumbersome and require biosafety level-3 facilities, an antibody test that can be used as a surrogate will facilitate more laboratories to provide rapid turnaround of CP neutralization results. The Ortho IgG ELISA used here is the only FDA-approved surrogate for viral neutralization, and these data confirm the correlation between this test and live SARS-CoV-2 neutralization.
Figure 1
Note: From Girardin et al. Change in PRNT50 titers over time within two-week strata. Data are presented as inverse titers (i.e. a titer of 1:640 is graphed as 640, so that samples with more neutralizing antibody are higher on the y-axis). Dotted horizontal line represents the FDA recommended titer of 1:160 for use as convalescent plasma. Solid black lines represent median titers, dashed red lines represent 95% CI. Licensed under CC-BY-NC-ND 4.0.
Figure 2
Note: From Girardin et al. Distribution of PRNT50 titers in groups versus IgG ratios (signal to noise) using the FDA cut-off for CP. Licensed under CC-BY-NC-ND 4.0.
PEER-REVIEWED
Upper respiratory tract levels of SARS-CoV-2 RNA and duration of viral RNA shedding do not differ between patients with mild and severe/critical COVID-19.external icon Yilmaz et al. Journal of Infectious Diseases (October 6, 2020).
Key findings:
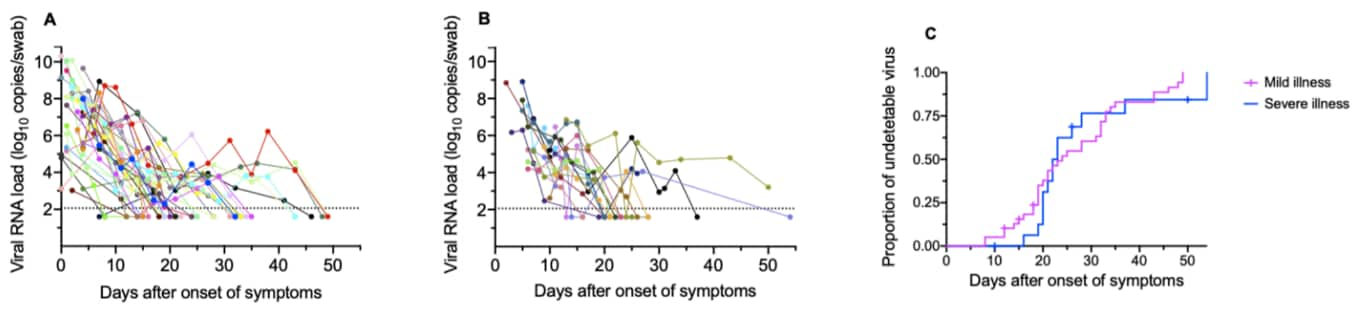
- Highest viral loads were observed early after symptom onset in persons with mild and severe COVID-19 and decreased over time (Figure).
- Median duration of viral shedding in individuals with mild and severe COVID-19 was similar, 24 vs 22.5 days, respectively (Figure).
- Most mild symptomatic cases (26/34) continued to be PCR-positive for 2 to 14 days after resolution of symptoms.
Methods: Retrospective cohort study of 59 individuals diagnosed with either mild or severe COVID-19 between February 25 and April 23, 2020, in Gothenburg, Sweden. Serial NP and throat swabs were collected, relative viral load was calculated, and statistical analysis was performed. Limitations: Small sample size from only one location; may not be generalizable.
Implications: Viral shedding was not associated with the severity of disease, indicating the potential for equal transmissibility of virus regardless of disease severity. However, further studies would have to be performed to determine if this is the case.
Figure:
Note: From Yilmaz et al. Viral load in nasopharyngeal and throat swabs in patients with COVID-19. Longitudinal viral loads for 39 patients with mild disease (A), and 17 with severe/critical disease (B). Horizontal dotted line in (A) and (B) mark detection limit of PCR assay. C: Time to viral RNA clearance for those with mild and severe/critical COVID-19. Yilmaz et al., Upper respiratory tract levels of SARS-CoV-2 RNA and duration of viral RNA shedding do not differ between patients with mild and severe/critical COVID-19, Journal of Infectious Diseases, 2020, volume, issue number, pagination; used by permission of the Infectious Diseases Society of America.
An effective vaccine will be key in controlling the COVID-19 pandemic, and multiple clinical trials are underway testing SARS-CoV-2 vaccine candidates. Some of these candidates have been given Fast-Trackexternal icon status by the US Food and Drug Administration (FDA) to accelerate licensing. While Fast-Track is an existing systematic approach to accelerate evaluation of vaccines and therapeutics, there is public concern about “rushing” a SARS-CoV-2 vaccine through this process. These two articles document public attitudes towards potential SARS-CoV-2 vaccines.
PEER-REVIEWED
Caregivers’ willingness to accept expedited vaccine research during the COVID-19 pandemic–A cross sectional survey.external icon Goldman et al. Clinical Therapeutics (October 3, 2020).
Key findings:
- Less than half (43%) of caregivers reported they were willing to accept a COVID-19 vaccine that is approved via Fast-Track status by the US Food and Drug Administration.
- Independent factors associated with willingness to accept expedited vaccine research included:
- Children up to date on their vaccination schedule (OR 1.72, 95% CI 1.29-2.31, p <0.001).
- Being worried that the caregivers themselves were sick with COVID-19 (OR 1.1, 95% CI 1.05-1.15, p <0.001).
- Mothers were less likely to accept expedited vaccine development than fathers (OR 0.64, 95% CI 0.53-0.78).
Methods: A cross-sectional survey of 2,557 caregivers arriving with their children (0–18 years of age) to 17 pediatric Emergency Departments in 6 countries between March 26 and June 30, 2020 to determine caregivers’ level of support of abridged COVID-19 vaccine testing. Limitations: Caregivers completed the survey before the approval of any COVID-19 vaccine; levels of acceptability may change as new information on candidate vaccines becomes available.
Influences on attitudes regarding potential COVID-19 vaccination in the United States.external icon Pogue et al. Vaccines. (October 3, 2020).
Key findings:
- 69% of respondents indicated they were open to receiving a COVID-19 vaccine.
- Positive attitudes towards a COVID-19 vaccine were higher if the vaccine spent longer in clinical testing, had high efficacy, and was developed in the US.
- Respondents who are current on vaccines recommended by their physician, whose children are current on vaccines, and who perceive that COVID-19 is an important problem in the US were more likely to indicate that they would take a COVID-19 vaccine.
- COVID-19 vaccine attitudes were unrelated to understanding of vaccines, demographics, COVID-19 knowledge, and number of people known with COVID-19 diagnosis.
Methods: Anonymous online survey on attitudes and behaviors related to potential COVID-19 vaccine in 316 respondents selected by age, race, and sex to reflect US population. Factor analysis and structural equation modeling were used. Limitations: Survey questions were not piloted or tested for understandability; vaccine history was self-reported; attitudes and beliefs are likely to evolve as more information about investigational vaccines emerge.
Implications for 2 studies (Pogue et al. & Goldman et al.): In both surveys, people already willing to vaccinate themselves or their child were more accepting of an accelerated SARS-CoV-2 vaccine, suggesting that motivation for COVID-19 vaccines may be driven by attitudes about vaccines in general more than control of the pandemic. Public health messaging around what “Fast Track” means may help quell some fears, but overall vaccine messaging will be needed to reap the full benefits of a COVID-19 vaccine, fast-tracked or not.
Face masks:
- Peeples, Face masks: What the data sayexternal icon. Nature. Reviews anecdotal and experimental evidence supporting a role for masks in decreasing SARS-CoV-2 transmission and debates the need for a randomized controlled trial.
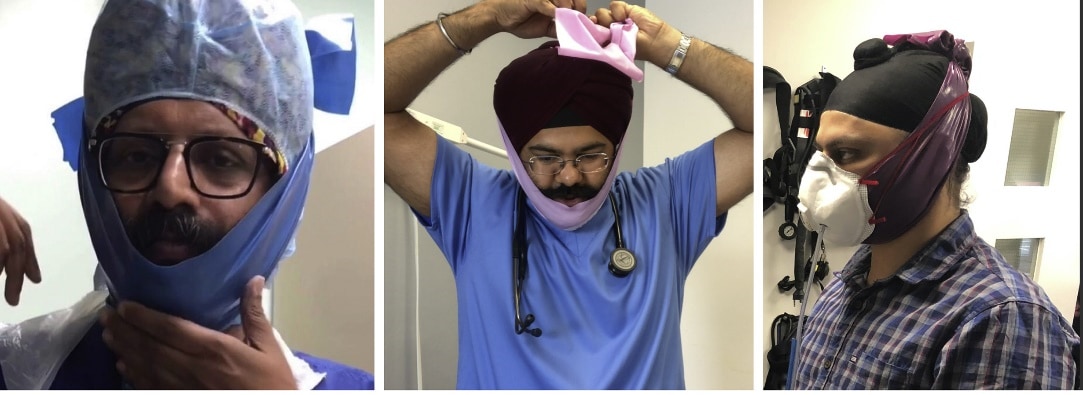
- Singh et al. Bearded individuals can use an under-mask beard cover ‘Singh Thattha’ for donning respirator masks in Covid-19 patient care.external icon Journal of Hospital Infection, The Singh Thattha technique, covering the beard with a cloth that ties atop the head, was used by 27 male bearded dentists with an N95 respirator and 25/27 (92.6%) passed a fit test.
Note: From Singh et al. The Singh Thattha technique to secure respirators over a beard. This article was published in Journal of Hospital Infection. Singh et al., Bearded individuals can use an under-mask beard cover ‘Singh Thattha’ for donning respirator masks in Covid-19 patient care. DOI: https://doi.org/10.1016/j.jhin.2020.09.034. October 3, 2020. Copyright Elsevier 2020 on behalf of The Healthcare Infection Society. This article is currently available at the Elsevier COVID-19 resource centre: https://www.elsevier.com/connect/coronavirus-information-center.
Clinical complications and sequelae:
- Rajdev et al. Acute ischemic and hemorrhagic stroke in COVID-19: Mounting evidenceexternal icon. Three case reports describing stroke in SARS-CoV-2-positive patients.
- Bazuaye-Ekwuyasi et al. Intussusception in a child with COVID-19 in the USAexternal icon. Emergency Radiology. The fourth case overall and first seen in the US of a child with COVID-19-related intestinal obstruction.
- Schwartz, M. MIS-C: post-infectious syndrome or persistent infection?external icon Lancet Infectious Diseases. Letter discussing the possibility that COVID-19-related multisystem inflammatory syndrome in children (MIS-C) is more consistent with a subacute infection than a post-infectious syndrome.
Other:
- Pombal et al. Risk of COVID-19 during air travelexternal icon. Air flow patterns and filtration of recirculated air in modern aircraft decreases the risk of SARS-CoV-2 transmission, with only 42 known flight-based cases; passengers can take additional steps to decrease their risks.
- Hojyo et al. How COVID-19 induces cytokine storm with high mortality.external icon Inflammation and Regeneration. Describes a potential pathway in which initiation of an IL-6 amplification loop in non-immune cells drives immune hyperreactivity in COVID-19 patients.
- Sallard et al. Clinical trial protocols of repurposed prophylaxis for COVID-19: A reviewexternal icon. Médecine et Maladies Infectieuses. Lists the known clinical trials, with rationales, to use existing drugs for COVID-19 treatment, including chloroquine derivatives, tuberculosis and measles vaccines, lopinavir, interferons, azithromycin, ivermectin, passive immunotherapies, and others.
- Lipsitch et al. Cross-reactive memory T cells and herd immunity to SARS-CoV-2.external icon Nature Reviews Reviews current knowledge of T-cell responses to SARS-CoV-2 and describes scenarios in which pre-existing CD4+ T-cell immunity may provide cross-protection against SARS-CoV-2 infection.
- Hosangadi et al. Current state of mass vaccination preparedness and operational challenges in the United States, 2018–2019.external icon Health Security. Summary of 37 interviews through March 2019 highlighting the unique operational needs of mass vaccination campaigns and the significant level of advanced planning that will be required for rollout of a SARS-CoV-2 vaccine.
- Wang et al. Inference of person-to-person transmission of COVID-19 reveals hidden super-spreading events during the early outbreak phaseexternal icon. Nature Communications. Phylogenetic analysis of early transmission in Wuhan, China, with identification of super spreader events.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.