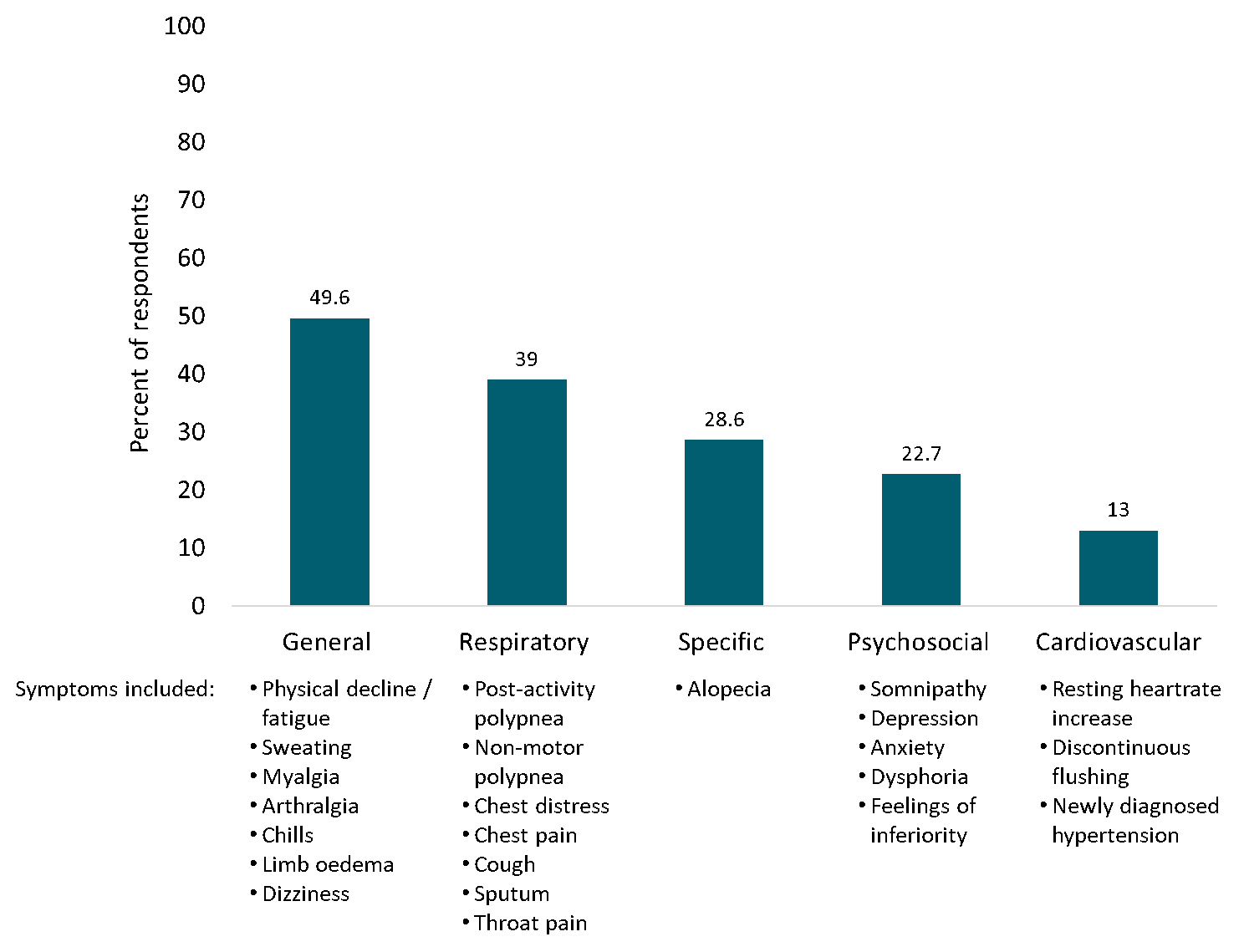COVID-19 Science Update released: October 9, 2020 Edition 55

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
US adults’ preferences for public allocation of a vaccine for coronavirus disease 2019external icon. Gollust et al. JAMA Network Open (September 29, 2020).
Key findings:
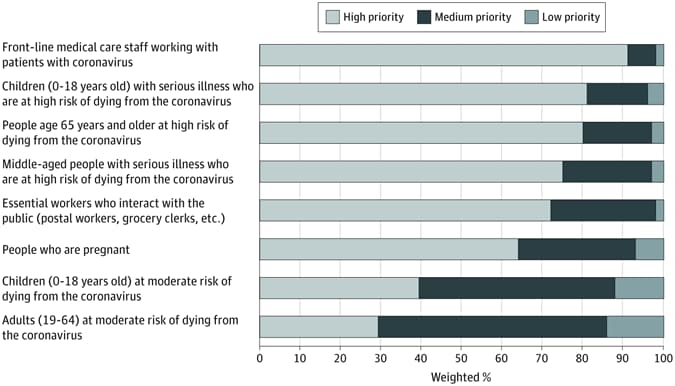
- Respondents rated front-line medical workers as highest priority (91.6%) followed by persons with highest risk of dying (72.2%–81.0%) (Figure).
- Persons at moderate risk of dying were more likely to receive a medium priority ranking (Figure).
Methods: Telephone- and internet-based survey of 1,007 persons representative of US households from April 23 to 27, 2020, to assess preferences about SARS-CoV-2 vaccine allocation. Respondents assigned high, medium, and low priorities to 8 groups of potential vaccine recipients. Limitations: No limitations on the number of high-priority selections; 17.7% of respondents selected all 8 groups as high priority; 14.4% response rate; no non-response bias analysis conducted.
Implications: This survey highlights the public’s recognition of the risk to frontline medical workers and high-risk individuals in the COVID-19 pandemic and the need to prioritize these individuals for vaccines.
Figure:
Note: From Gollust et al. Prioritization for SARS-C0V-2 vaccine allocation. Licensed under CC-BY.
Evidence is insufficient to support treatment of COVID-19 with hydroxychloroquine (HCQ) and guidance from NIH recommends against its use. Here we present four papers evaluating HCQ for treatment of COVID-19, as pre-exposure prophylaxis, and on fetal outcomes when used during pregnancy.
PEER-REVIEWED
A. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19external icon. The RECOVERY Collaborative Group. NEJM (October 8, 2020).
Key findings:
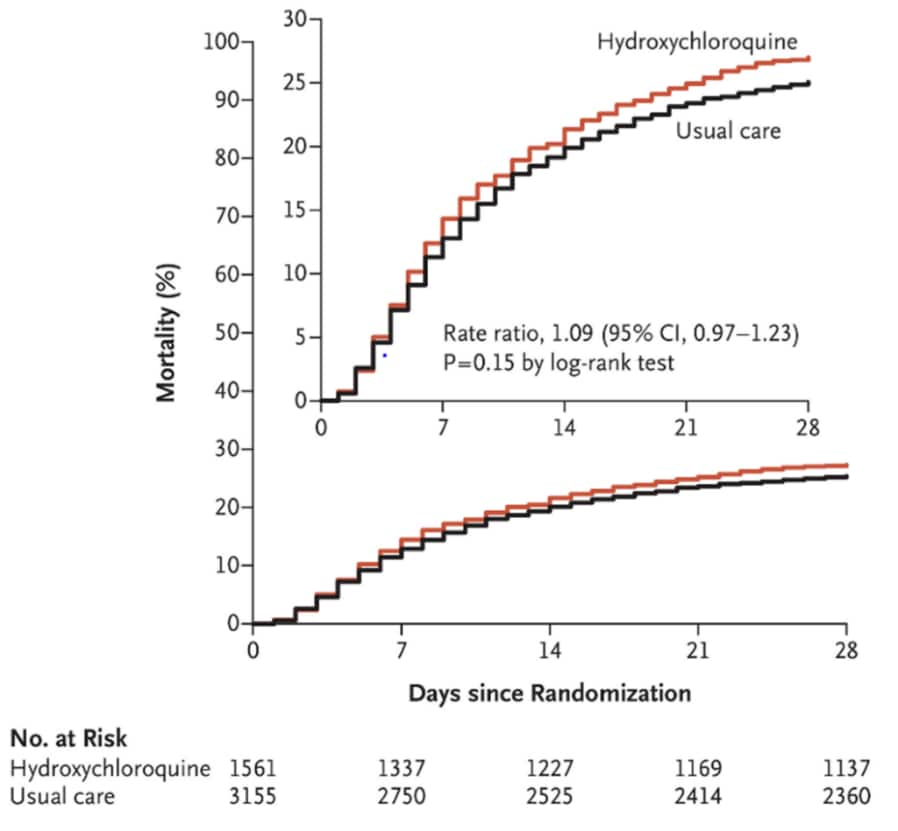
- Death within 28 days occurred in 421 patients (27.0%) in the hydroxychloroquine (HCQ) group and in 790 patients (25.0%) in the usual-care group, rate ratio 1.09, p = 0.15 (95% CI 0.97-1.23) (Figure).
- Among patients not undergoing mechanical ventilation at enrollment, those in the HCQ group had a higher frequency of invasive mechanical ventilation or death (30.7% vs 26.9%); risk ratio 1.14 (95% CI 1.03-1.27).
- An interim analysis determined lack of efficacy of HCQ, and enrollment closed early.
Methods: The RECOVERY trial is a randomized, controlled, open-label platform trial comparing a range of possible treatments with usual care in patients hospitalized with COVID-19 at 176 hospitals in the UK. In this phase of the study, 1,561 COVID-19 patients were randomly assigned to receive HCQ and 3,155 received usual care. The primary outcome was 28-day mortality.
Figure:
Note: From RECOVERY Collaborative Group. Mortality at 28 days. Inset is scaled on the y-axis to show slight differences in mortality later in the trial. From NEJM, The RECOVERY Collaborative Group. Effect of Hydroxychloroquine in Hospitalized Patient with COVID-19, DOI: 10.1056/NEJMoa2022926, October 8, 2020. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
B. Hydroxychloroquine early in pregnancy and risk of birth defectsexternal icon. Huybrechts et al. American Journal of Obstetrics and Gynecology (September 19, 2020).
Key findings:
- 54.8 per 1,000 infants exposed to hydroxychloroquine (HCQ) in the first trimester were born with a major congenital malformation compared with 35.3 per 1,000 unexposed infants, pooled unadjusted relative risk (RR) 1.51 (95% CI 1.27-1.81).
- The adjusted RR (aRR) for congenital malformation in infants exposed to HCQ compared to unexposed infants was 1.26 (95% CI 1.04-1.54).
- aRR was 1.33 (95% CI 1.08-1.65) for a daily HCQ dose ≥400mg and aRR 0.95 (95% CI 0.60-1.50) for daily HCQ dose of <400mg.
- Increased risk for oral cleft, RR 3.70 (95% CI 1.55-8.82) and urinary malformations, RR 2.21 (95% CI 1.26–3.86) in infants exposed to HCQ compared to unexposed infants.
Methods: Analysis of pregnancy cohorts from two large US national health insurance databases composed of 2,045 live-born births exposed to HCQ in the first trimester and 3,198,589 unexposed live-born births from 2000-2015. Exposure was based on filled HCQ prescription during first trimester of pregnancy. The analysis was controlled for many potential confounders including concurrent medical conditions and medications. Limitations: Included only women with live births; congenital diagnoses and HCQ exposure based on claim reports; did not include uninsured or self-insured women; race/ethnicity not provided.
C. Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: A retrospective cohort study.external icon Gentry et al. Lancet Rheumatology (September 21, 2020).
Key findings:
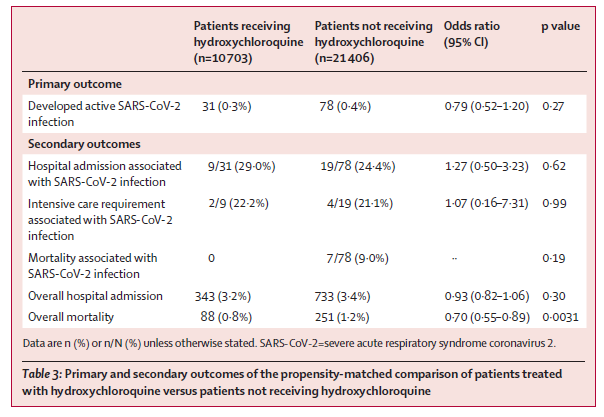
- The incidence of SARS-CoV-2 did not differ between patients receiving (31/10,703, [0.3%]) and not receiving (78/21,406, [0.4%]) hydroxychloroquine (HCQ), OR 0.79, p = 0.27 (95% CI 0.52-1.20).
- There were no differences in hospitalization, ICU admission or mortality between the two groups in patients who developed SARS-CoV-2 (Table).
Methods: A large, propensity score matched, retrospective cohort study across US Veteran Affairs Medical Centers of rheumatology patients receiving and not receiving HCQ from March to June 2020. The primary endpoint was the incidence of active SARS-CoV-2 infection. Secondary endpoints included hospital admission, ICU admission and mortality associated with SARS-CoV-2. Limitations: Women comprised only 24% of study participants; only the HCQ group was selected based on medication adherence; unmeasured between-group differences could have introduced bias which could have affected mortality outcomes.
Table:
Note: From Gentry et al. SARS-CoV-2 and COVID-19-related outcomes in patients receiving or not receiving hydroxychloroquine for rheumatic conditions. This article was published in Lancet Rheumatology, Gentry et al., Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: A retrospective cohort study., DOI: https://doi.org/10.1016/ S2665-9913(20)30305-2, September 21, 2020. Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource centre: https://www.elsevier.com/connect/coronavirus-information-center.
D. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers. A randomized clinical trial.external icon Abella et al. JAMA Internal Medicine (Sept 30, 2020).
Key findings:
- There was no significant difference in SARS-CoV-2 incidence between the hydroxychloroquine (HCQ) and placebo groups (6.3% vs 6.6%), p >0.99.
- There were more adverse events in the HCQ arm vs placebo (45% vs 26%), p = 0.03.
- 7.4% of individuals given HCQ had detectable IgG antibody against spike proteins compared with 3.7% given placebo, p = 0.40.
- There was no significant difference in the median change in QT interval between the HCQ and placebo arms, p = 0.98.
Methods: Single-health system, double-blind, placebo-controlled randomized trial of health care workers (64 in HCQ arm, 61 in control arm), comparing HCQ 600 mg daily to placebo taken orally for 8 weeks to prevent SARS-CoV-2 infection between April 9, 2020 and July 14, 2020, Pennsylvania. The primary outcome was infection with SARS-CoV-2; secondary outcomes were adverse events, SARS CoV-2 antibody positivity, electrocardiogram changes and clinical outcomes. Limitations: The study did not meet the target sample size of 200 and was terminated early for futility.
Implications for 4 studies (RECOVERY Collaborative Group, Huybrechts et al., Gentry et al., & Abella et al.): HCQ is not effective for treatment of COVID-19 or prevention of SARS-CoV-2 infection. The potential for congenital malformation may not be an acceptable level of risk for healthy women of childbearing age, especially as data to support HCQ use for treatment or prevention of COVID-19 are lacking. These additional data support previous data suggesting no role for HQC in the management of COVID-19 or prevention of SARS-CoV-2 infection.
PEER-REVIEWED
Seasonal coronavirus protective immunity is short-lastingexternal icon. Edridge et al. Nature Medicine (September 14, 2020).
Key findings:
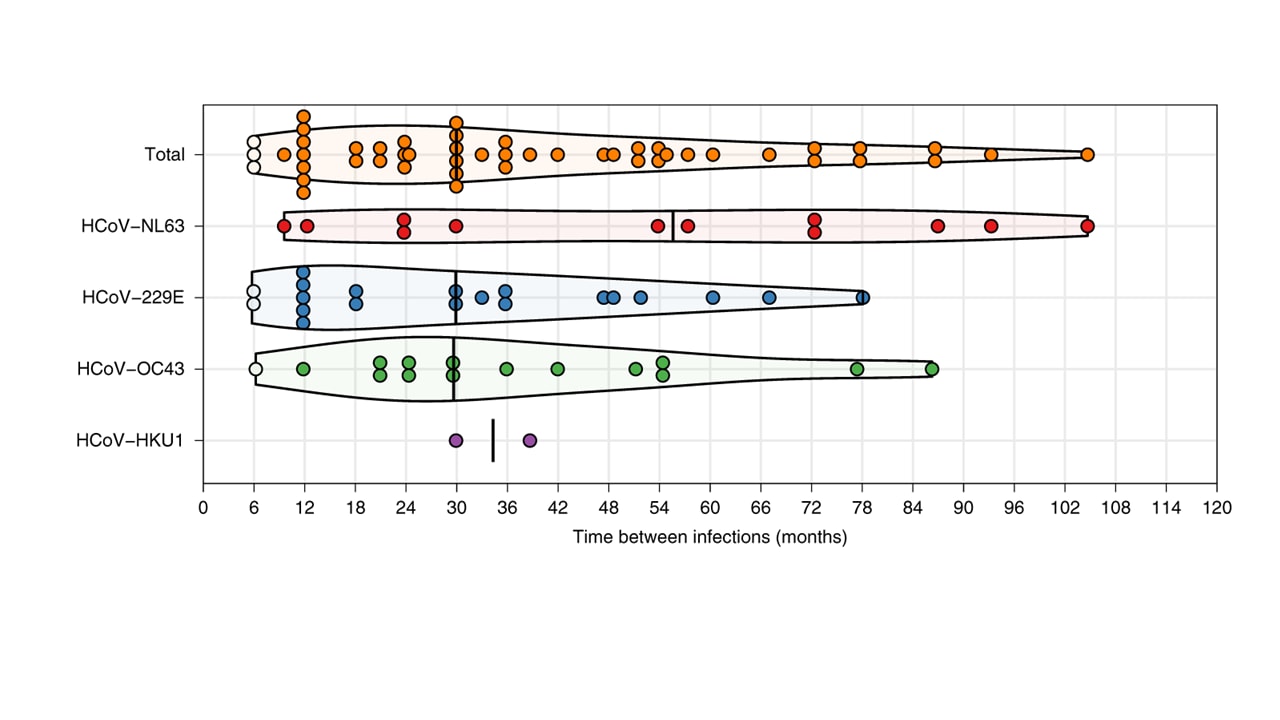
- Reinfections with seasonal coronaviruses (CoVs) frequently occurred at 12 months, and in a few cases as early as 6 months (Figure).
Methods: Testing for antibodies to seasonal CoV using stored serum from 10 healthy adult males who gave blood every 3 to 6 months for 12 to 35 years as part of the Amsterdam Cohort Studies on HIV-1 infection and AIDS. Increases in antibody levels to seasonal CoVs (HCoV-NL63, HCoV-229E, HCoV-OC43 and HCoV-HKU1) were used as a proxy for reinfection. Limitations: Ability to detect short-term reinfections was limited by the sampling interval; 1.4-fold increase in antibodies to CoV N antigen was used as a proxy for incident infection.
Implications: Natural reinfection occurred for 4 common seasonal endemic coronaviruses and may be a common feature of human coronaviruses including SARS-CoV-2. Calculations of herd-immunity and vaccine strategies may need to consider this and other studies when determining population-level protection and planning vaccination intervals.
Figure:
Note: Adapted from Edridge et al. Coronavirus reinfections for ten individuals. White dots represent reinfections without an intermediate decrease in antibody levels; black vertical lines represent median reinfection times. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Medicine, Edridge et al., Seasonal coronavirus protective immunity is short-lasting. DOI: https://doi.org/10.1038/s41591-020-1083-1, September 14, 2020, COPYRIGHT 2020.
Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in catsexternal icon. Bosco-Lauth et al. Proceedings of the National Academy of Sciences (September 29, 2020).
Key findings:
- Cats are highly susceptible to infection with SARS-CoV-2 and have oral and nasal viral shedding when experimentally or naturally infected (Figure).
- Infected cats did not display overt clinical signs and were able to infect other cats.
- Infected cats had mild pathologic changes of lungs, trachea, and nasal passages at different time points following inoculation.
- Cats rechallenged with virus after initial infection had a boosted immune response with high antibody titers but did not shed virus.
- Dogs mounted antibody responses but did not shed virus.
Methods: Animal infection and transmission study of seven cats and three dogs inoculated with SARS-CoV-2. Three cats were inoculated with SARS-CoV-2 and rechallenged on Day 28. Two cats were inoculated with SARS-CoV-2 and introduced to two uninfected cats 48 hours later. Viral shedding and antibody responses were assessed in all animals at multiple time points. Limitations: Animals were all from pathogen-free colonies and were in good health at time of infection; limited experimental study, replication with larger study size may be needed.
Implications: The high-titer viral shedding of cats along with the rapid transmission among cats may make them a good model for studies of kinetics of shedding and spread of SARS-CoV-2. Resistance to reinfection in cats may inform vaccine studies and strategies.
Figure:
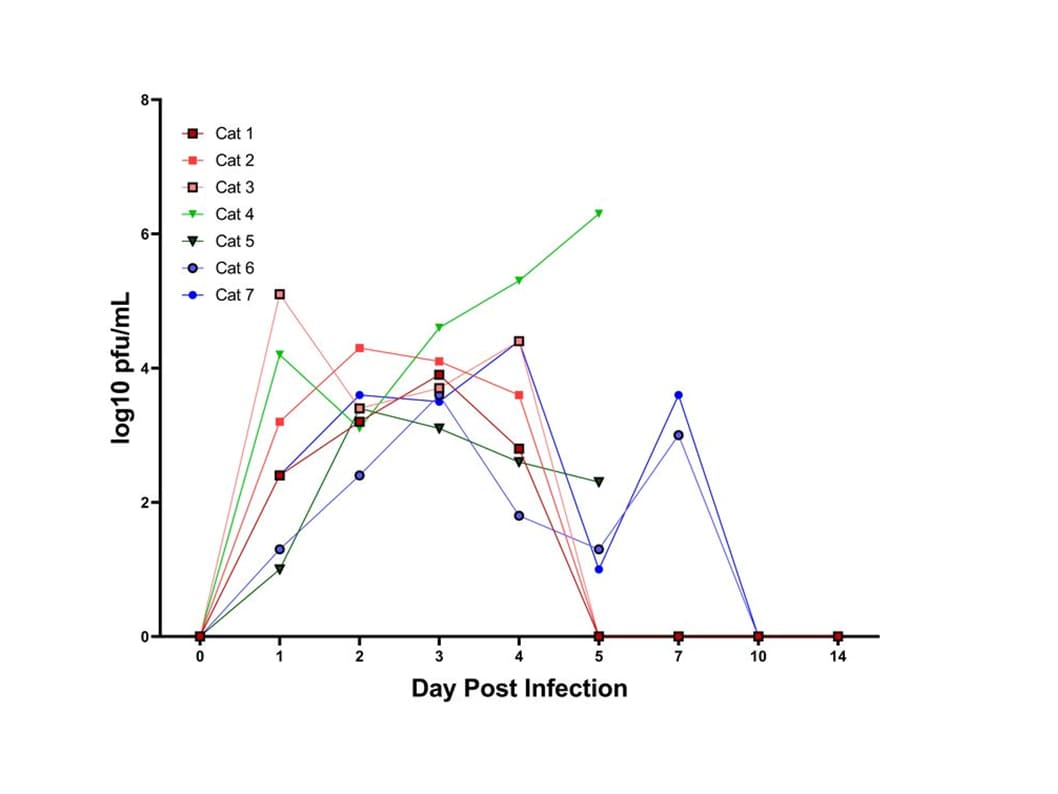
Note: Adapted from Bosco-Lauth et al. Shedding of SARS-CoV-2 detected by plaque assay from nasal secretions of cats. Viral titers expressed as log10 plaque-forming units (pfu)/mL. Used by permission of the National Academy of Sciences.
PEER-REVIEWED
Clinical outcomes of in-hospital cardiac arrest in COVID-19external icon. Thapa et al. JAMA Internal Medicine (September 28, 2020).
Key findings:
- Of 54 COVID-19 patients with in-hospital cardiac arrest (IHCA), none survived to discharge (95% CI 0-6.6).
- Pre-COVID-19 survival to discharge for IHCA was 25%.
- 42% had hypertension and 55.6% had diabetes.
Methods: A single institution, retrospective cohort study of hospitalized COVID-19 patients who underwent cardiopulmonary resuscitation (CPR) for IHCA, from March to April 2020. Primary outcomes were time to return of spontaneous circulation, overall survival and discharge. Limitations: Patient population had a high rate of comorbidities and although these constitute the vast majority of COVID-19 patients likely to suffer IHCA, may limit generalizability; observational data may be subject to unmeasured confounders.
Implications: CPR in hospitalized COVID-19 patients likely yields poor outcomes in patients with high rates of comorbidities.
Clinical sequalae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study.external icon Xiong et al. Clinical Microbiology and Infection (September 23, 2020).
Key findings:
- Almost half (49.6%) of COVID-19 survivors reported general symptoms compared with 12.0% of control group members, p <0.01 (Figure).
- Additional reported clinical sequelae included respiratory symptoms (39%), alopecia (28.6%), psychosocial symptoms (22.7%) and cardiovascular symptoms (13%).
- Three symptoms were more common in women than in men: physical decline/fatigue (34.1% vs. 21.2%) p <0.01, rapid breathing after activity (24.6% vs 17.6%) p <0.05, and alopecia (48.5% vs 4.9%) p <0.01).
Methods: Phone survey of 538 individuals with COVID-19 discharged from Renmin Hospital, Wuhan at least three months prior to the survey. Incidence of symptoms from five categories—general, respiratory, cardiovascular, psychosocial, and specific— were compared with those from 184 controls. Limitations: Self-reported data from a single hospital; control group had not been recently hospitalized; not all responded to psychosocial questions.
Implications: Given the prevalence of symptoms among convalescent COVID-19 patients, targeted investigation into the etiology, time course, and treatment of clinical sequelae is warranted. This is especially true for unique sequelae such as sleep disorders and alopecia, a symptom that disproportionately affected women in this study.
Figure:
Note: Adapted from Xiong et al. Percent of COVID-19 patients reporting one or more symptom in each respective symptom category ≥3 months after hospital discharge. Polypnea = rapid breathing. Reprinted from Clinical Microbiology and Infection, Xiong et al., Clinical sequalae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study, DOI: https://doi.org/10.1016/j.cmi.2020.09.023, September 23, 2020. Copyright 2020, with permission from the European Society of Clinical Microbiology and Infectious Diseases.
PEER-REVIEWED
COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses.external icon Sahin et al. Nature (September 30, 2020).
Key findings:
- Highest levels of neutralizing antibody were seen 29 days after initial dose (virus neutralization titers [VNT50] 36–578) and were dose-dependent.
- A booster at day 22 after the initial dose was necessary to see a robust immune response.
- Levels of IgG binding to the receptor binding domain (RBD) and neutralizing antibodies were highly correlated, r = 0.9452, p <0.0001 (Figure 1).
- BNT162b1 had robust neutralizing titers against the common RBD variants, including D614G (the dominant spike variant).
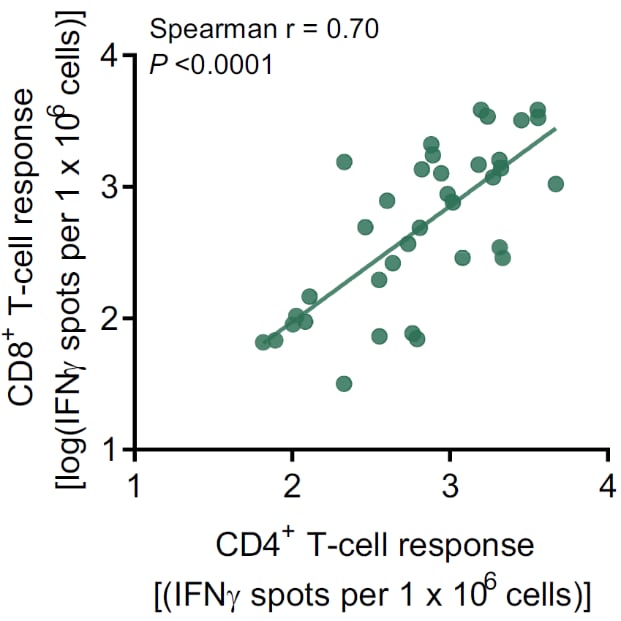
- A positive correlation between the frequency of RBD-specific CD8+ T cells and CD4+ T cells was seen (Figure 2).
Methods: Phase 1/2 clinical trial with 60 participants assigned to five 12-person groups and administered the BNT162b1 mRNA experimental vaccine at varying dose levels between April 23 and May 22, 2020. Groups were given the first dose on day one and boosted on day 22, except for one group that was administered only one dose. Antibody and T-cell immune responses were evaluated. Limitations: Small sample size and only included participants <55 years; short study did not allow testing persistence of immune response.
Implications: With a robust RBD-specific antibody and T-cell response, BNT162b1 mRNA vaccine is a promising SARS-CoV-2 vaccine candidate.
Figure 1
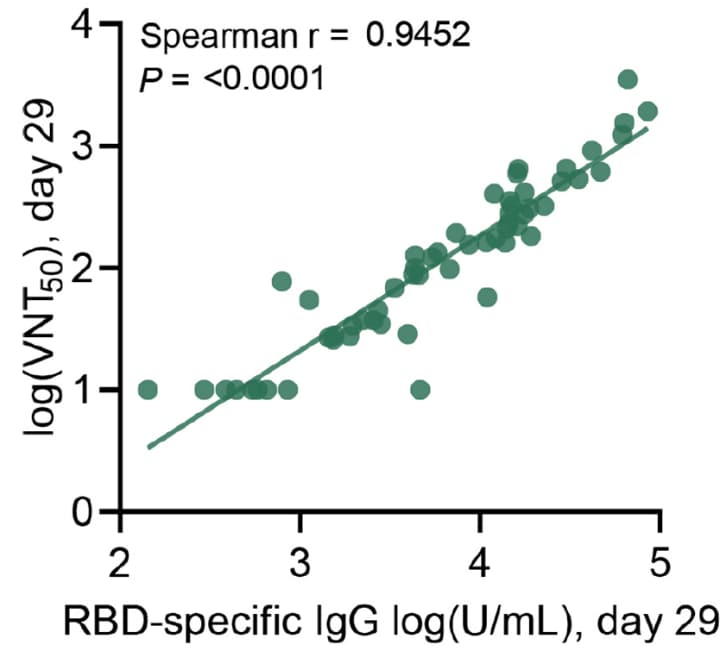
Note: From Sahin et al. Correlation of RBD-specific IgG titers versus VNT50 from day 29 sera of all immunized patients. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature, Sahin et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. DOI: https://doi.org/10.1038/s41586-020-2814-7, September 30, 2020, COPYRIGHT 2020.
Note: From Sahin et al. Correlation of CD4+ with CD8+ T-cell responses from blood collected on day 29 in all immunized patients. Data shown as number of T cells secreting interferon-gamma (IFNγ) per 106 cells. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature, Sahin et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. DOI: https://doi.org/10.1038/s41586-020-2814-7, September 30, 2020, COPYRIGHT 2020.
Epidemiological correlates of PCR cycle threshold values in detection of SARS-CoV-2external icon. Salvatore et al. Clinical Infectious Diseases (September 28, 2020).
Key findings:
- Cycle threshold (Ct) values were positively correlated with time elapsed since symptom onset, p <0.001.
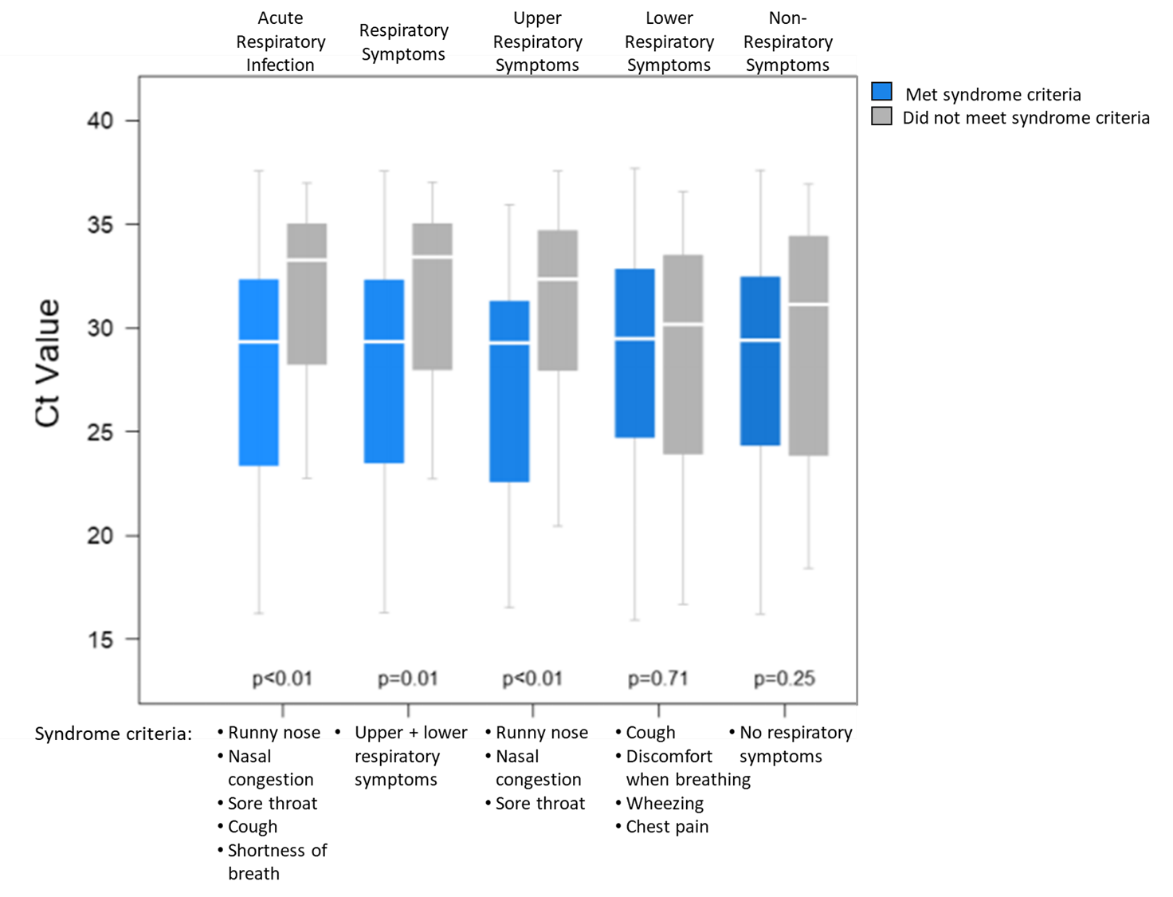
- Ct values were lowest (indicating highest viral RNA concentration) among those who tested positive <7 days after symptom onset and highest among those who tested positive ≥21 days after onset (Figure 1).
- Ct values were lower among those ≤17 years old than among those ≥18 (Figure 1).
- Low Ct values were associated with a set of symptoms meeting the criteria of various respiratory syndromes (Figure 2) rather than individual symptoms, aside from runny nose.
Methods: Demographic and self-reported symptom data on 93 SARS-CoV-2-positive participants during a prospective household transmission investigation of mild COVID-19 cases between March 23 and May 13, 2020. Associations between demographic and clinical factors and RT-PCR Ct values were determined. Limitations: Study not powered to test relationship between Ct values and severe COVID-19 symptoms; small sample size.
Implications: Testing for SARS-CoV-2 soon after symptom onset, particularly symptoms meeting respiratory syndrome definitions, will improve the chances of identifying and isolating individuals when they may be most infectious. Lower Ct values, reflecting higher viral loads, in symptomatic children suggests that children can contribute to SARS-CoV-2 transmission.
Figure 1
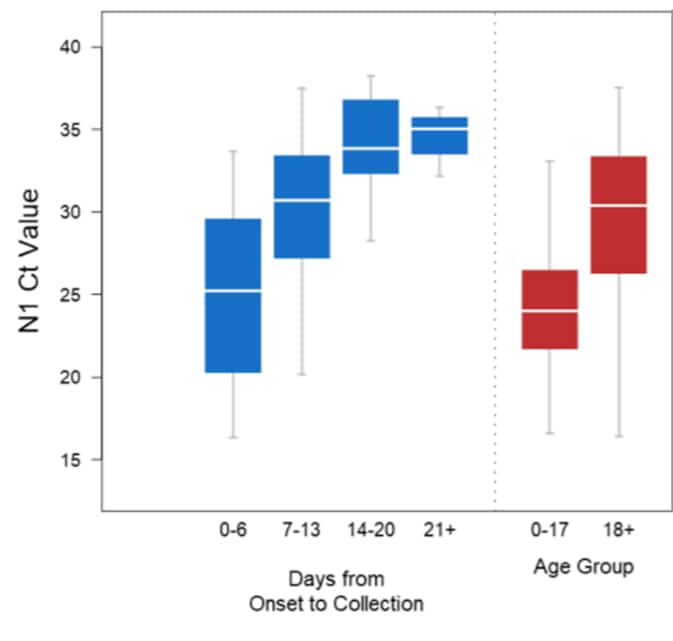
Note: Adapted from Salvatore et al. Ct values by days from symptom onset and age group. Medians are highlighted in white; boxes show the interquartile range and whiskers depict 95% CIs. US Government work not subject to copyright.
Figure 2
Note: Adapted from Salvatore et al. Ct values of patients meeting syndrome criteria or not meeting syndrome criteria. US Government work not subject to copyright.
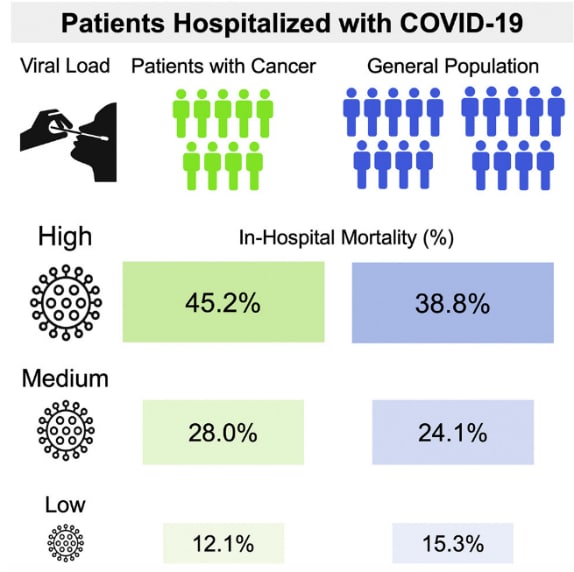
- Westblade et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19.external icon Cancer Cell. A retrospective analysis of over 3,000 individuals (100 with cancer) evaluating the relationship between viral load (represented by PCR cycle threshold [CT] value) and mortality.
Figure:
Note: From Westblade et al. In-hospital mortality of patients with COVID-19 with or without cancer. Reprinted from Cancer Cell, Westblade et al., SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19., DOI: https://doi.org/10.1016/j.ccell.2020.09.007, September 15, 2020. Copyright 2020, with permission from Elsevier.
- Laxminarayan et al. Epidemiology and transmission dynamics of COVID-19 in two Indian statesexternal icon. Science. Identifies high prevalence of infection among children who were contacts of cases, suggesting that social interactions among children may be conducive to transmission of SARS-CoV-2.
- Rali et al. Incidence of venous thromboembolism in coronavirus disease 2019: An experience from a single large academic centerexternal icon. Journal of Vascular Surgery. Reports on the incidence rate of venous thromboembolism (VTE) in COVID-19 patients and notes a higher all-cause mortality for COVID-19 patients with VTE than in those without VTE.
- Webb et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: A cohort study.external icon Lancet Rheumatology. Proposes and validates criteria for hyperinflammatory syndrome in COVID-19 among adults (≥18 years old), which is commonly associated with progression to mechanical ventilation and death.
- Colaneri et al. Severe acute respiratory syndrome coronavirus 2 RNA contamination of inanimate surfaces and virus viability in a health care emergency unit.external icon Clinical Microbiology and Infection. Environmental sampling of an emergency room unit showed little SARS-CoV-2 RNA on surfaces, suggesting environmental contamination might be less extensive than previously suggested.
- Zeberg et al. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals.external icon The only known genetic risk associated with severe COVID-19 is a region on chromosome 3; genetic analysis shows this risk haplotype came from Neanderthals and not from other hominins.
- Helfand et al. The exclusion of older persons from vaccine and treatment trials for coronavirus disease 2019–Missing the targetexternal icon. JAMA Internal Medicine. Exclusion of older age groups from clinical trials is common, which precludes the determination of an intervention’s effectiveness, dosage or frequency, and adverse effects in the group most vulnerable to COVID-19.
- Mina et al. Rethinking COVID-19 test sensitivity–A strategy for containment.external icon Authors advocate that use of inexpensive point-of-care tests that can detect the infectious period, be used frequently and can have a larger public health impact than a sensitive lab-based test.
- Fan et al. Effect of acid suppressants on the risk of COVID-19: A propensity score matched study using UK biobank acid suppressants and the risk of COVID-19external icon. No association of SARS-CoV-2 infection and mortality was seen in 1,516 users of acid suppressants compared to matched controls, and only patients with upper gastrointestinal diseases taking omeprazole were more susceptible to SARS-CoV-2 infection.
- Service, R. One number could help reveal how infectious a COVID-19 patient is. Should test results include it?external icon Ct values, which are proportional to viral loads, theoretically could be useful to guide patient care, but variations in Ct values due to assay design or instrument performance make standardization challenging.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.