COVID-19 Science Update released: October 6, 2020 Edition 54

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adultsexternal icon. Anderson et al. NEJM (September 29, 2020).
Key findings:
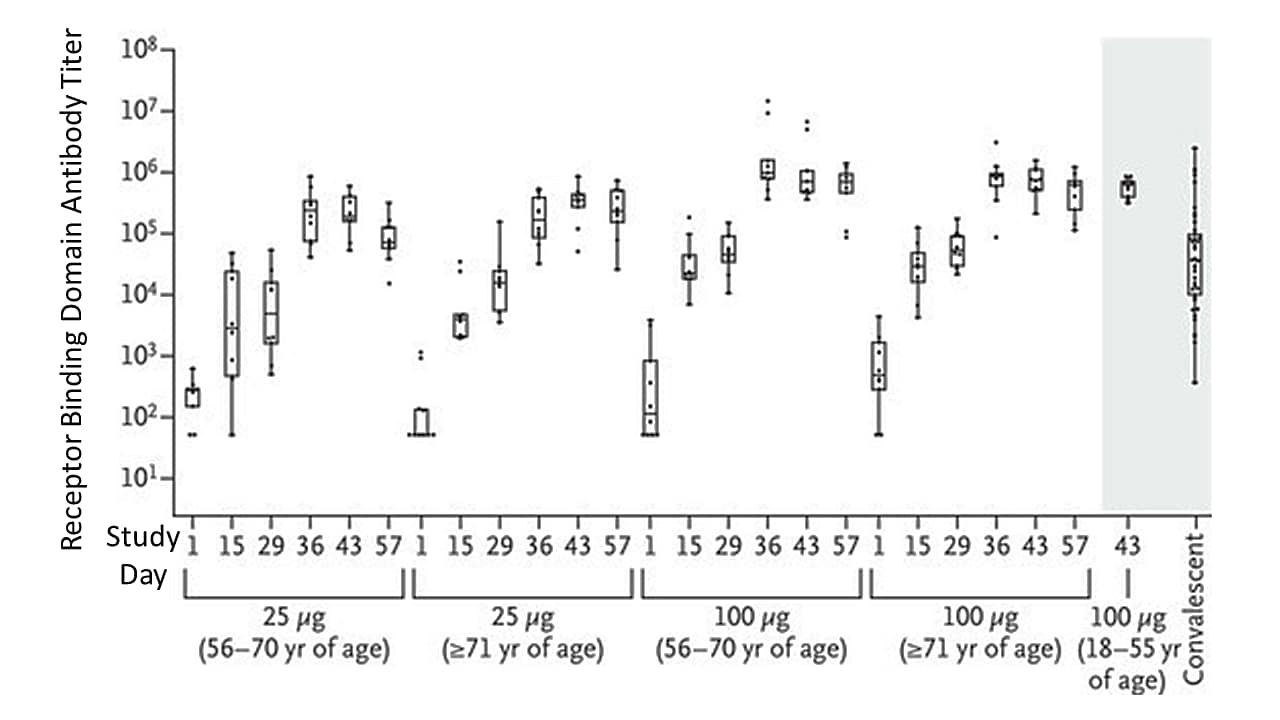
- After two-doses of an mRNA vaccine, antibody levels to the receptor binding domain (RBD) were higher in vaccinated individuals compared to the antibody levels among mostly mild and moderately ill COVID-19 individuals who donated convalescent serum (controls) (Figure 1).
- Antibody levels were similar to those in younger adults who received the vaccine in another study (Figure 1).
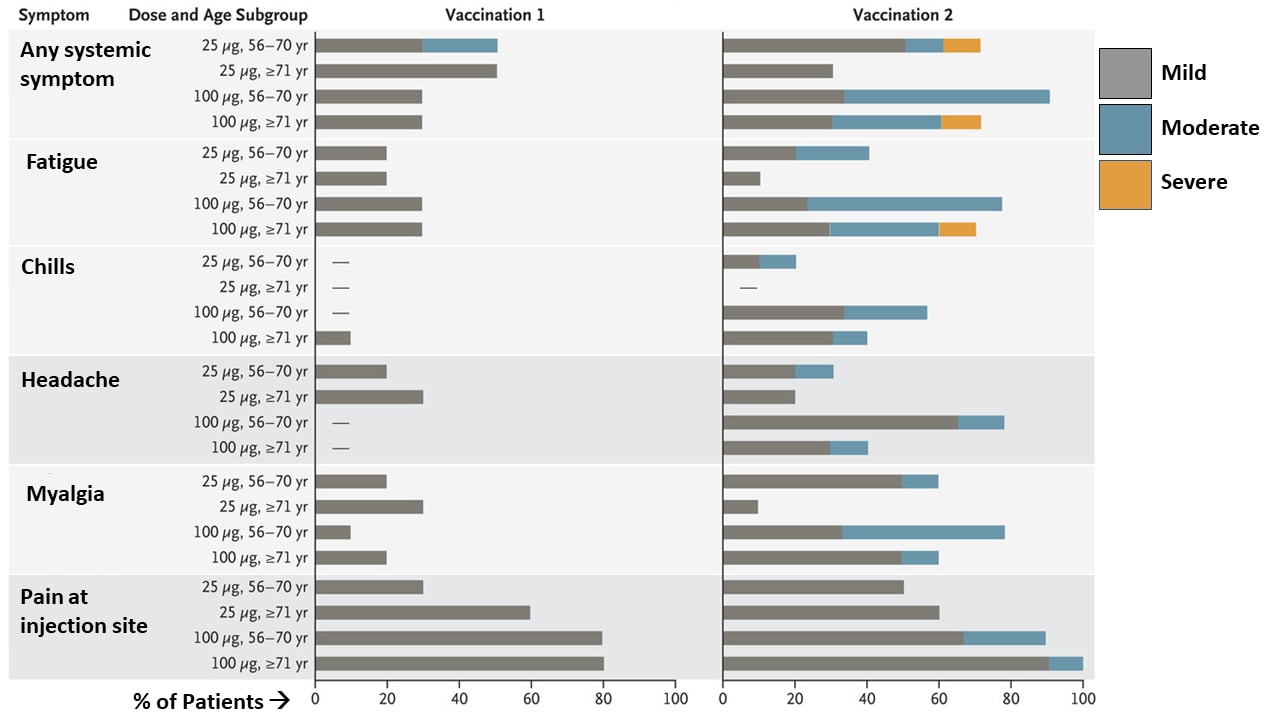
- Adverse events were generally mild or moderate, dose-dependent, and more commonly detected after second vaccine dose (Figure 2).
- Antiviral T cell responses to the vaccine were detected in most participants.
Methods: Phase 1 open-label vaccine trial of mRNA-1273, first reported in Jackson et alexternal icon., expanded to include 40 older adults stratified by age (56 to 70 years or ≥71 years) in the US. All participants received two doses of vaccine (25 μg or 100 μg) 28 days apart between April 16 and May 12, 2020. Immunoassays were used to quantify the binding IgG responses to spike protein RBD on days 1, 15, 29, 36, 43, and 57; and assays were performed to measure T-cell responses. Comparisons were made to previously reported antibody levels from persons 18–55 years with mild and moderate COVID-19 who donated convalescent serum. Limitations: Small sample size may not detect rare adverse events; duration of immune responses and effectiveness of immune responses to prevent infection not captured; limited ethnic or racial diversity of participants.
Implications: Similar to findings from the initial trial in adults 18–55 years reported by Jackson et al.,external icon the mRNA vaccine was immunogenic in older adults and produced only mild to moderate adverse reactions. More studies are warranted to further assess safety and efficacy in older adults who are at higher risk for severe illness.
Figure 1
Note: Adapted from Anderson et al. Anti-SARS-CoV-2 spike RBD IgG antibody titers at 2 doses in 3 age groups and 41 convalescent serum donors. From NEJM, Anderson et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults, DOI: 10.1056/NEJMoa2028436, September 29, 2020. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Note: Adapted from Anderson et al. Selected mild, moderate and severe adverse events within 7 days of vaccination. From NEJM, Anderson et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults, DOI: 10.1056/NEJMoa2028436, September 29, 2020. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Orally or intravenously administered dexamethasone has been shown to reduce the risk of death in people with severe COVID-19, probably through decreasing SARS-CoV-2 infection-associated inflammation. Inhaled corticosteroids (ICS) treat asthma and chronic obstructive pulmonary disease (COPD) by reducing lung inflammation, leading to a hypothesis that ICS might protect against severe SARS-CoV-2 infection. The following study assessed the effect of treatment with ICS at the time of SARS-CoV-2 infection on COVID-19-related mortality.
PEER-REVIEWED
Risk of COVID-19 related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: An observational cohort study using the OpenSAFELY platform.external icon Schultze et al. Lancet Respiratory Medicine (September 24, 2020).
Key findings:
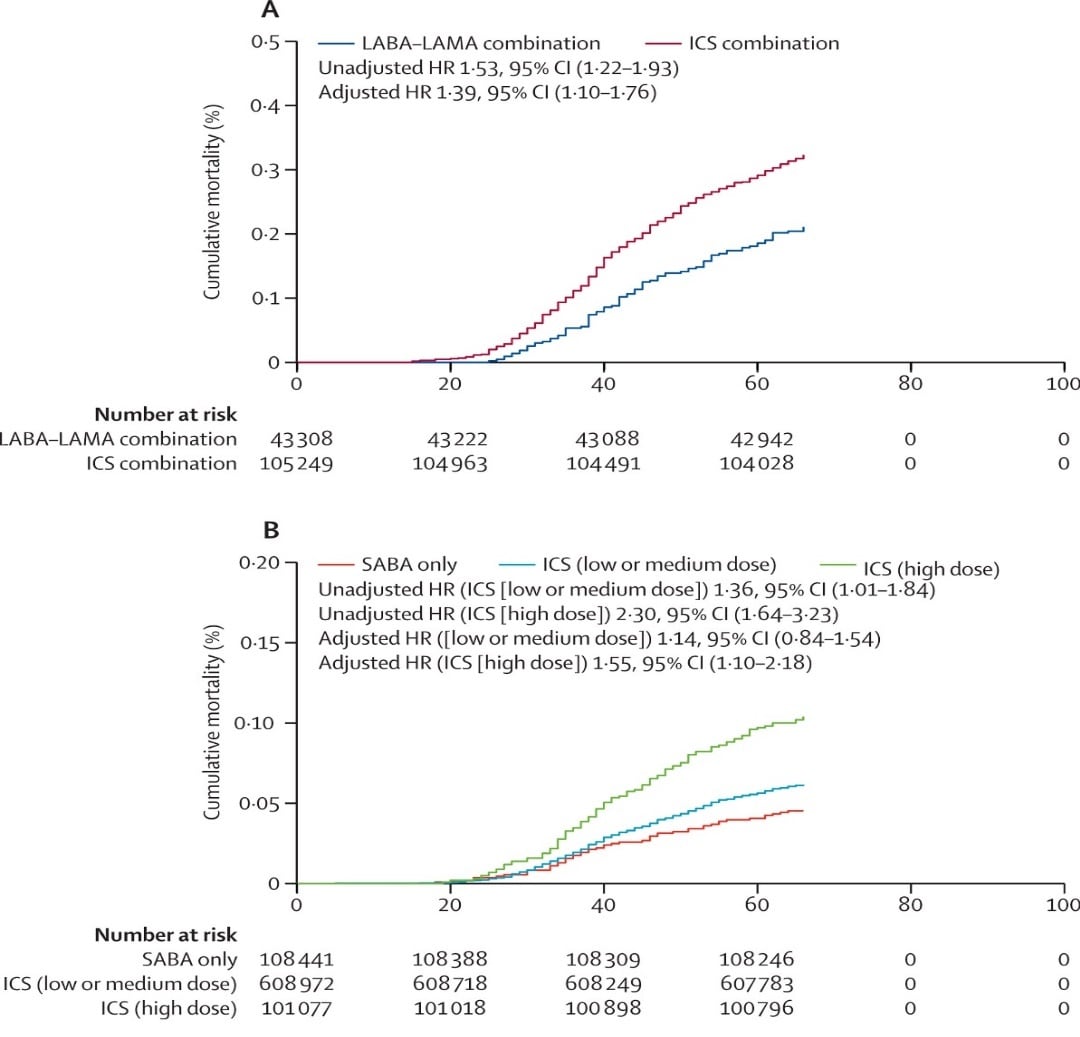
- People with COPD who were prescribed ICS with other inhaled medications were at increased risk of COVID-19-related death compared with those prescribed other inhaled medications (long-acting β agonists [LABA] and long-acting muscarinic antagonists [LAMA]), (adjusted HR [aHR] 1.39, 95% CI 1.10–1.76) (Figure 1A).
- People with asthma who were prescribed high-dose ICS were at an increased risk of COVID-19 related death compared with those prescribed short-acting β agonists (SABA) only (aHR 1.55, 95% CI 1.10–2.18) (Figure 1B).
- People with asthma prescribed low- or medium-dose ICS were not at increased risk of death (aHR14, 95% CI 0.85–1.54) compared with those prescribed SABAs only (Figure 1B).
- Sensitivity analyses found that the apparent associations between ICS and COVID-19-related death in persons with COPD or asthma could be explained by underlying health differences between people prescribed ICS and those prescribed other respiratory medications.
Methods: Analysis of data from 148,557 people with COPD and 818,490 people with asthma in the United Kingdom between March 1 and May 6, 2020 comparing risk for COVID-19-related death among those prescribed and those not prescribed ICS. Limitations: Risks of confounding due to unmeasured variables and to misclassification of exposures.
Implications: Regular ICS use for treatment of asthma or COPD does not appear to be protective against COVID-19-related death. Because sensitivity analyses suggest observed increases in mortality with ICS were likely due to underlying health differences, adjustments in ICS therapy among patients with asthma or COPD during outbreaks of SARS-CoV-2 are not supported.
Figure:
Note: Adapted from Schultze et al. Time to COVID-19-related death for A: Persons with COPD prescribed inhaled LABA/LAMA combinations or combinations including ICS, B: Persons with asthma prescribed SABA only, low- or medium-dose ICS, or high-dose ICS. Licensed under CC-BY.
PEER-REVIEWED
Mass screening of asymptomatic persons for SARS-CoV-2 using salivaexternal icon. Yokota et al. Clinical Infectious Diseases (September 25, 2020).
Key findings:
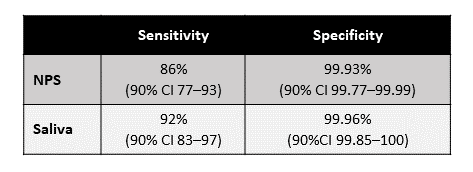
- Among asymptomatic persons, the sensitivity of nasopharyngeal swabs (NPS) and saliva specimen tests was 86% (90% CI 77-93%) and 92% (90% CI 83-97%), respectively (Table).
- Specificity for both specimen types was >99%.
- There was high concordance between results from using NPS and saliva specimens, ranging from 0.934 to 0.999 when SARS-CoV-2 prevalence was varied from 0% to 30%.
Methods: A mass-screening study (N=1,924) was conducted among asymptomatic persons from either contact tracing (n=161) or airport screening (n=1,763) in Japan. NPS and self-collected saliva specimens were tested for SARS-CoV-2 by RT-PCR. Test performance on paired samples was evaluated and concordance between NPS and saliva tests was determined. Limitations: No clinical information reported among positive cases.
Implications: Self-collected saliva may provide an opportunity to more efficiently test for SARS-CoV-2 infection in some community and healthcare settings due to the ease of collection, acceptability and decreased risk of exposure to healthcare workers.
Table:
Note: Adapted from Yokota et al. Sensitivity and specificity of RT-PCR tests using NPS and saliva specimens. Reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa1270/5898577external icon.
Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: A cross-sectional study.external icon Anand et al. Lancet (September 25, 2020).
Key findings:
- Less than 10% of US adults on dialysis had evidence of SARS-CoV-2 antibodies, less than 10% of those with antibodies were diagnosed with SARS-CoV-2 infection.
- Risk of seropositivity was increased for those who lived in (Figure):
- non-Hispanic Black and Hispanic neighborhoods (OR 3.9 95% CI 3.4-4.6)
- neighborhoods with highest population density compared to those with the lowest density (OR 10.3, 95% CI 8.7-12.2)
- When compared with descriptive measures of SARS-CoV-2 spread, there was a high correlation of seroprevalence with deaths per 100,000 population (Spearman’s ρ = 0.77).
Methods: Cross-sectional study of 28,503 randomly selected adult patients who underwent dialysis in July 2020 at 1,300 US dialysis facilities. Antibody to SARS-CoV-2 spike protein was tested in leftover plasma samples from dialysis. Demographic data from anonymized electronic health records were linked to patient-level residence data with cumulative and daily COVID-19 cases and deaths per 100,000. Standardized estimates of age, sex, region, and race and ethnicity in US dialysis and adult population were used. Limitations: Seroprevalence estimates from the US dialysis population may not be generalizable to the US adult population and did not account for state-level or county-level estimates.
Implications: Fewer than 10% of US adults had antibodies to SARS-CoV-2 with regional and demographic differences. As pointed out in a commentary by Flower et alexternal icon., even though persons who receive dialysis may not be representative of the general population, dialysis patients may be a good sentinel group for serosurveillance given regular blood tests, established vascular access, and a high proportion of patients with multiple risk factors for COVID-19.
Figure:
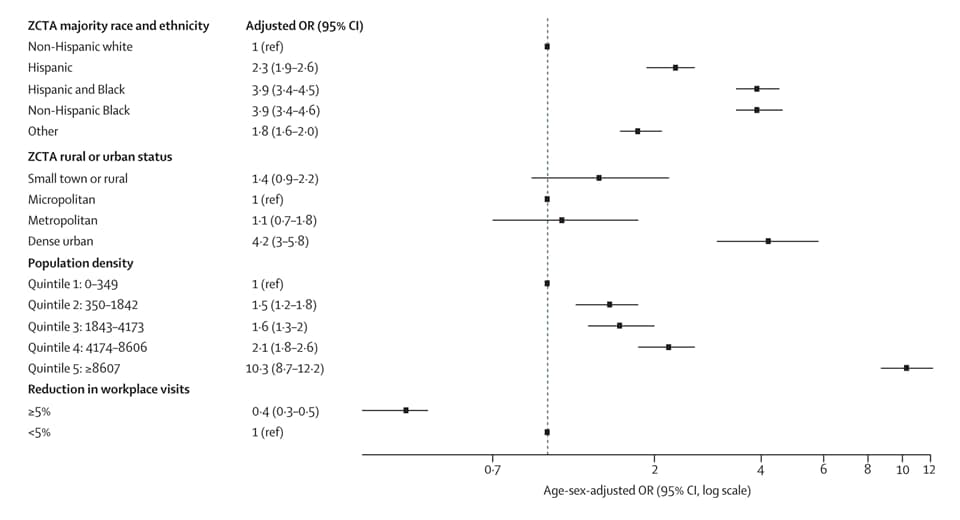
Note: Adapted from Anand et al. Odds for COVID-19 seropositivity. All variables are at a neighborhood level, except for reduction in workplace visits, which is at a county level. Reductions in workplace visits were measured during the first 2 weeks of March 2020, compared with a baseline in January–February 2020. ZCTA-ZIP code tabulation area. This article was published in Lancet, Vol 396, Anand, Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: A cross-sectional study, Page 1335-1344, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults.external icon Viner et al. JAMA Pediatrics (September 25, 2020).
Key findings:
- In 15 contact-tracing studies, children and adolescents (<20 years) had lower susceptibility to SARS-CoV-2 infection than adults (≥20 years), (pooled OR, 0.56 95% CI 0.37–0.85).
- Susceptibility for SARS-CoV-2 infection among children (<10-14 years) was significantly lower than for adults (pooled OR 0.52, 95% CI 0.33–0.82) (Figure 1).
- Susceptibility for SARS-CoV-2 infection adolescents (10–19 years) was not significantly different than for adults (OR 1.23, 95% CI 0.64–2.36).
- Most population screening studies found lower seroprevalence in children than in adults.
- Seroprevalence appeared similar in adolescents and adults.
Methods: Systematic review of 32 studies up till July 28, 2020 on the prevalence of SARS-CoV-2 among children and adolescents compared with adults. Limitations: Most studies were medium or low quality, with only 2 high-quality studies; different age cutoffs to define children and adolescents used in different studies; wide confidence intervals for individual studies.
Implications: Pooled evidence suggests children may have lower susceptibility to SARS-CoV-2 infection than adults. Data specific to adolescents are sparse but suggest susceptibility and prevalence similar to adults. Informative data on transmission risk from children are needed to inform prevention strategies, particularly in schools and childcare settings.
PEER-REVIEWED
Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS-CoV-2external icon. Gupta et al. Clinical Infectious Diseases (September 23, 2020).
Key findings:
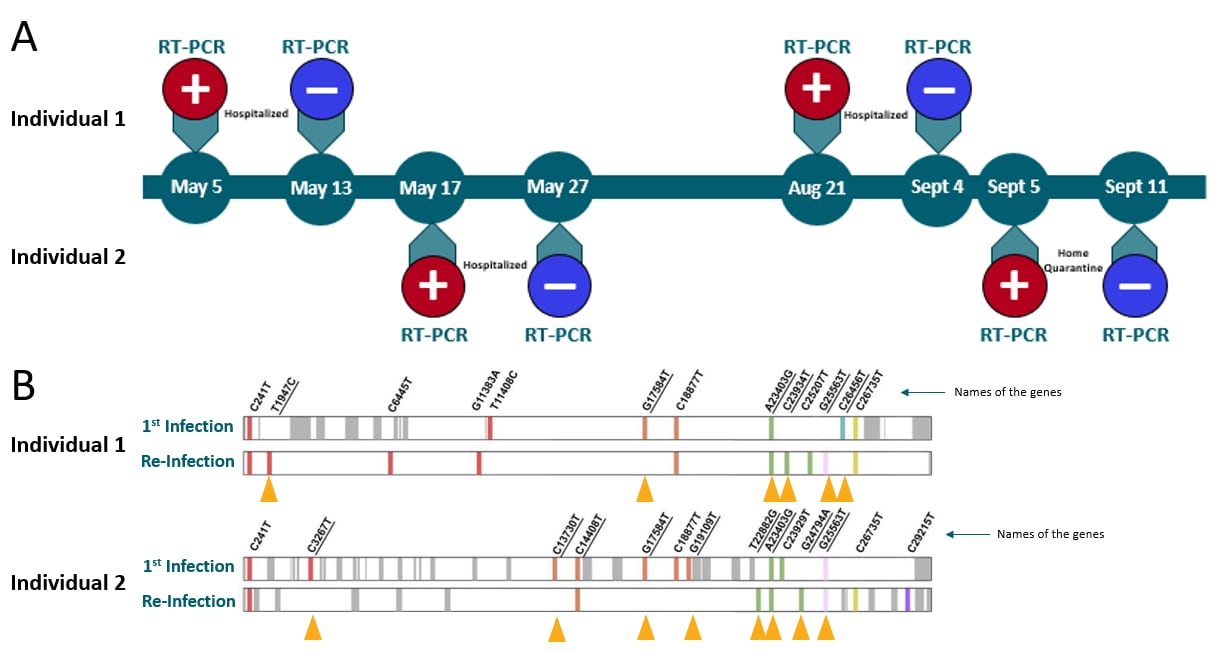
- Asymptomatic reinfection was identified in two healthcare workers (HCWs) with genetically distinct SARS-CoV-2 viruses (Figure 1).
- Both individuals had higher viral loads during reinfection than during the first infection.
- Ct values during the first and second infections were 36 and 16.6 for the first individual, and 28.2 and 16.9 for the second individual, respectively.
Methods: Case studies of SARS-CoV-2 reinfections among two asymptomatic HCWs (25 and 28 years of age) in a COVID-19 hospital unit in North India, between May and September 2020. Viral isolates underwent genome sequencing. Limitations: Case study in two persons; there are no consensus genetic criteria for distinguishing intra-host evolution and selection of minor variants from reinfection.
Implications: This study of two individuals shows that reinfection may be asymptomatic and could potentially lead to underreporting. Further research is needed to help determine how common asymptomatic reinfections are, the clinical course of reinfection, and the risk of transmission from such cases.
Figure:
Note: Adapted from Gupta et al. A: Timeline of RT-PCR test results in two young asymptomatic HCWs reinfected with SAR-CoV-2. B: Genomes of the first infection and re-infection for both. Important genetic differences are shown by yellow arrows. Reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa1270/5898577external icon.
Extracorporeal membrane oxygenation support (ECMO) is a therapeutic intervention where blood from the body is externally oxygenated and returned to the body either through an artery (venoarterial ECMO that also assists circulation as well) or a vein (venovenous ECMO that does not assist circulation) and is used when there is life-threatening heart or lung failure. It has been recommended by WHO for the management of life-threatening COVID-19. Given high rates of mortality among patients receiving ECMO early in the pandemic, and the invasiveness of this resource-intensive intervention, it is important to understand how ECMO is impacting patient survival. The following describes findings from patients who required ECMO to support severe COVID-19-related acute respiratory failure.
PEER-REVIEWED
Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registryexternal icon. Barbaro et al. Lancet (September 25, 2020).
Key findings:
- The estimated cumulative incidence of in-hospital mortality 90 days after initiation of ECMO was 37.4% (95% CI 34.4–40.4) (Figure).
- Venoarterial ECMO support was significantly associated with in-hospital mortality compared with venovenous ECMO (hazard ratio (HR) 1.89, 95% CI 1.20–2.97).
- Increasing age was associated with a higher risk of in-hospital mortality for those 70 years or older compared with patients aged 16–39 years (HR 3.07, 95% CI 1.58–5.95).
Methods: Retrospective analysis of 1,035 COVID-19 patients ≥16 years of age who had ECMO support between January 16 and May 1, 2020, from 36 countries. Patient outcome was measured at 90 days after ECMO initiation and with evaluation of risk factors for mortality. Limitations: Represents subset of facilities and finding may not be generalizable; no comparison to rate of COVID-19 acute respiratory distress syndrome-associated deaths without ECMO.
Implications: This study supports existing recommendations from the WHO to consider use of ECMO in refractory COVID-19-related respiratory failure in experienced centers.
Figure:
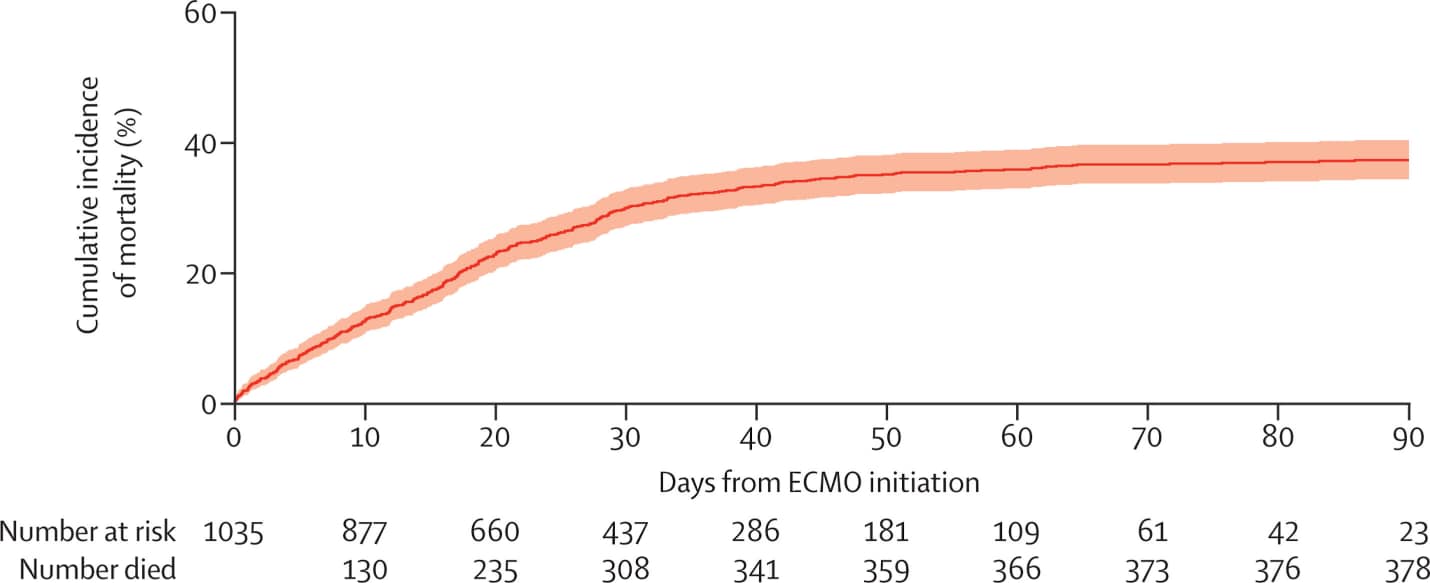
Note: Adapted from Barbaro et al. Cumulative incidence of mortality from time of ECMO initiation. The solid line represents the estimated cumulative incidence of mortality and the shaded area represents the 95% CI. This article was published in Lancet, Vol 396, Barbaro et al., Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry, Page 1071-1078, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon
Since early in the emergence of COVID-19, the increased risk for both venous and arterial thromboembolic disease in those with SARS-CoV-2 infection has been recognized. While the use of low-dose anticoagulants for prophylaxis is widely used in general for hospitalized patients who are at increased risk of thromboembolic disease, there is increasing interest in the use of higher, therapeutic dosing as a preventive measure for those with COVID-19.
PEER-REVIEWED
Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID)external icon. Lemos et al. Thrombosis Research (September 20, 2020).
Key findings:
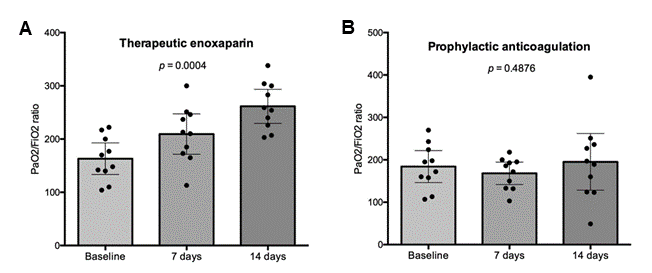
- PaO2/FiO2 was increased at 7 and 14 days compared with baseline in patients receiving therapeutic enoxaparin (p = 0.0004) (Figure 1A).
- There was no observed improvement in the standard thromboprophylaxis group over this time period (Figure 1B).
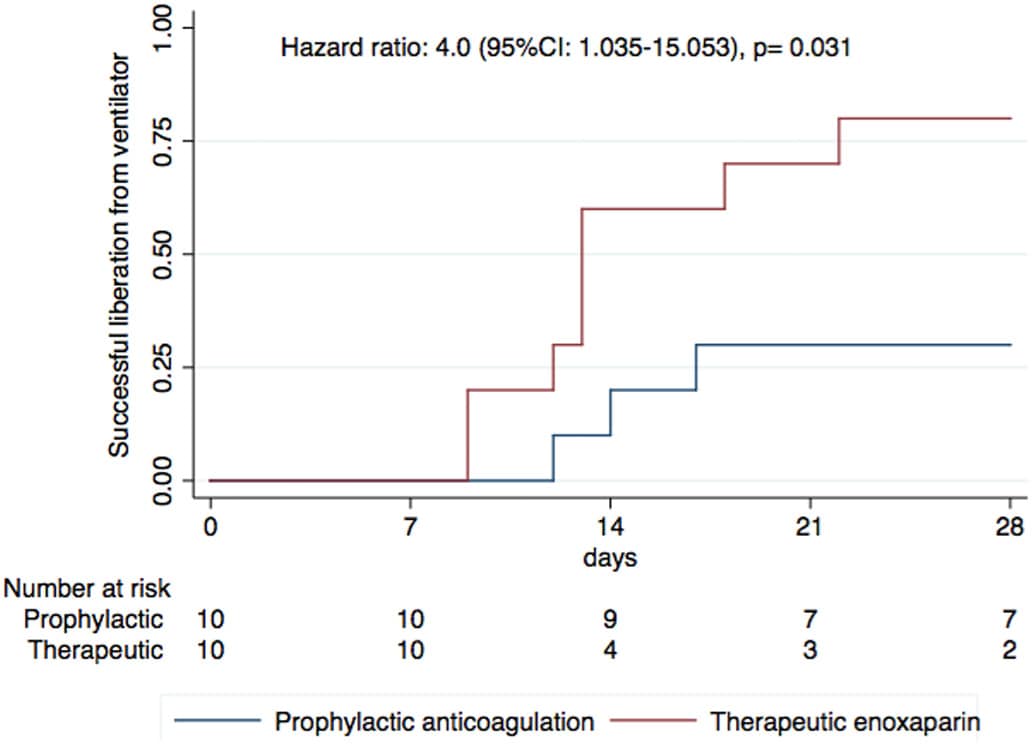
- The therapeutic group had a higher rate of mechanical ventilation compared with the prophylactic group (hazard ratio 4.0, 95% CI 1.04–15.05, p = 0.031) (Figure 2).
Methods: Randomized, open-label, phase II study comparing effect of therapeutic anticoagulation with enoxaparin vs standard anticoagulant thromboprophylaxis on lung function and duration of mechanical ventilation among 20 COVID-19 patients requiring mechanical ventilation. Lung function over time was measured by PAO2/FiO2 (ratio of oxygen in arterial blood to the percent of oxygen in inspired air) at baseline, 7 days, and 14 days after randomization; time until successful removal of mechanical ventilation, and number of ventilator-free days were also measured. Limitations: Small, single-center study; not blinded.
Implications: Early data show that therapeutic enoxaparin appears promising for patients with severe COVID–19 and respiratory failure. Larger randomized trials are needed to confirm these results.
Figure 1
Note: Adapted from Lemos et al. PaO2/FiO2 at baseline, 7 days and 14 days after randomization in the patients of the therapeutic enoxaparin group (A) and the prophylactic anticoagulation group (B). . This article was published in Thrombosis Research, Vol 196, Lemos et al., Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID), Page 359-366, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-center.external icon
Figure 2
Note: Adapted from Lemos et al. Cumulative incidence of successful removal from mechanical ventilation in the therapeutic enoxaparin and prophylactic anticoagulation groups to 28 days of follow-up. This article was published in Thrombosis Research, Vol 196, Lemos et al., Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID), Page 359-366, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
- Van Rijn et al. Reducing aerosol transmission of SARS-CoV-2 in hospital elevators.external icon International Journal of Indoor Environment and Health. Investigates aerosol persistence in elevators by mimicking a single cough using a specially designed spray nozzle to disperse a controlled quantity of droplets.
Note: Adapted from van Rijn et al. Average number of aerosol droplets as a function of time since production in large (15‐20 m3) elevator cabins [triangles] and medium‐sized (8‐12 m3) cabins [diamonds] during normal operation, with permanently open doors, and permanently closed doors. Reproduced by permission of John Wiley and Sons. ©John Wiley & Sons A/S.
Vaccines
- Krammer F. SARS-CoV-2 vaccines in development.external icon Review of SARS-CoV-2 vaccine development including visual description of accelerated timelines and types of vaccines being developed.
- Burki T. The online anti-vaccine movement in the age of COVID-19.external icon Lancet Digital Health. Discusses the extent of anti-vaccine content on social media and presents the impact of anti-vax groups using different social media platforms to strengthen their movement.
- Mallapaty et al. COVID-vaccine results are on the way – And scientists’ concerns are growing. external iconOutlines researcher concerns that vaccines could stumble in safety trials but still be fast-tracked.
Care and Treatment
- Tikkinen et al. COVID-19 clinical trials: Learning from exceptions in the research chaosexternal icon. Nature Medicine. Summarizes the RECOVER and SOLIDARITY trials highlighting the speed of assessing the effects of several treatments while maintaining rigorous yet flexible study designs to allow for rapid generation of high-quality data to guide recommendations for COVID-19.
- Bull et al. SARS-CoV-2 challenge studies: Ethics and risk minimisation. external iconJournal of Medical Ethics. Explores approaches to risk minimization and reasonableness in SARS-CoV-2 controlled human infection studies with a particular focus on whether effective treatment is always necessary for the ethical acceptability of such studies.
- Tao et al. Re-detectable positive SARS-CoV-2 RNA tests in patients who recovered from COVID-19 with intestinal infection. Protein & Cell. external iconInvestigates whether the intestine might be a “reservoir” of SARS-CoV-2 and a potential source of re-positive tests; also notes alteration of the intestinal microbiota of patients who were re-positives.
- Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. external iconDescription of “long haulers,” who have not fully recovered from COVID-19 weeks or even months after first symptoms with testimony from providers and individuals infected with SARS-CoV-2 on challenges of persistent symptoms.
- Ahlberg et al. Association of SARS-CoV-2 test status and pregnancy outcomes. external icon A study from Sweden comparing pregnant persons in labor infected with SARS-CoV-2 with uninfected persons. SARS-CoV-2 test positivity in individuals in labor was associated with a higher prevalence of preeclampsia and lower prevalence of induction of labor.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.

![1006_FIG10 Average number of aerosol droplets as a function of time since production in large (15‐20 m3) elevator cabins [triangles] and medium‐sized (8‐12 m3) cabins [diamonds] during normal operation [orange], with permanently open doors [green], and permanently closed doors [red].](/library/covid19/images/1006_FIG10.png?_=17380)