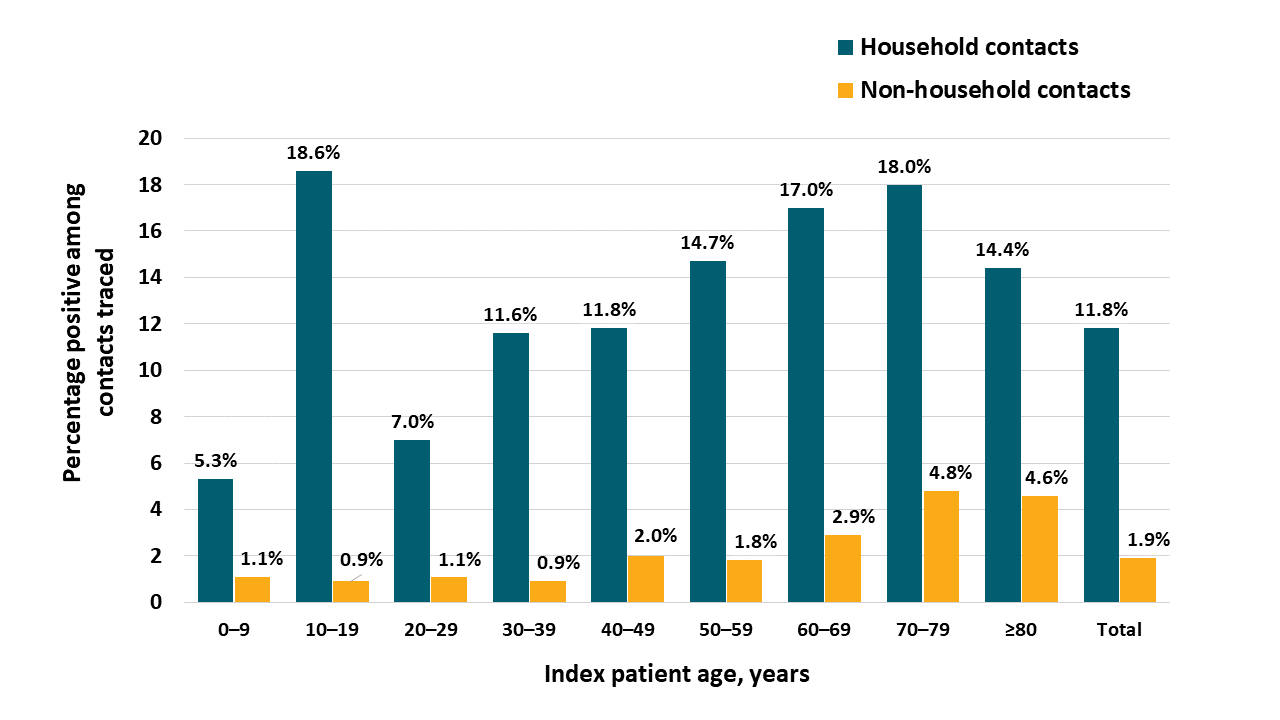COVID-19 Science Update released: July 28, 2020 Edition 34

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Contact Tracing during Coronavirus Disease Outbreak, South Korea, 2020. Park et al. Emerging Infectious Diseases (July 16, 2020).
Key findings:
- The detection SARS-CoV-2 among household contacts of index patients (11.8%) was much higher than non-household contacts (1.9%) (Figure).
- Among household contacts, the rate of SARS-CoV-2 was highest for contacts of children aged 10-19 years (18.6%), while the lowest rate was for those of children aged 0–9 years (5.3%) (Figure).
Methods: A descriptive analysis of South Korea’s contact tracing program that included 59,073 contacts of 5,706 COVID-19 index patients between January 20 and March 27, 2020. Limitations: Underreporting of asymptomatic persons.
Implications: Contact tracing is critical in assessing SARS-CoV-2 transmission patterns. Rates of household transmission from older children is important in discussions and policies around reopening of schools.
Figure:
Note: Adapted from Park et al. Proportion of COVID-19 among household (teal bars) and non-household (gold bars) contacts of index patients, by age. Open access journal; all content freely available.
SARS-CoV-2 failure to infect or replicate in mosquitoes: an extreme challengeexternal icon. Huang et al. Scientific Reports (July 17, 2020).
Key findings:
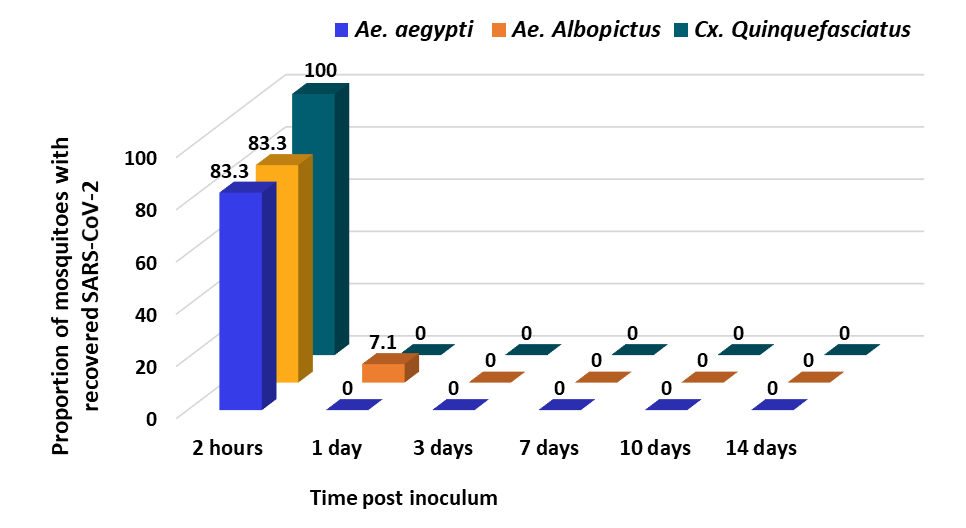
- SARS-CoV-2 was not detected in the 277 inoculated mosquitoes collected at any time point beyond 24 hours (Figure).
Methods: Three widely distributed species of mosquito, Aedes aegypti, Aedes albopictus and Culex quinquefasciatus were inoculated with SARS-CoV-2. To recover infectious virus at specified time points, inoculated mosquitoes were individually ground to fine powder in 1 ml of medium and cultured in Vero76 cells. Limitations: Not generalizable to all species.
Implications: Some of the most common species of mosquitos that can transmit viral infections in humans are not vectors for SARS-CoV-2. There remains no evidence that SARS-CoV-2 can be transmitted by mosquitos.
Figure:
Note: Adapted from Huang et al. Recovery rates of SARS-CoV-2 in mosquitoes receiving inoculum via intrathoracic injection. Licensed under CC-BY 4.0.
Individual susceptibility to repeat infection and population susceptibility to recurrent epidemics may depend in part on antibody dynamics among previously infected people. Below we summarize a few studies examining antibody levels and persistence among persons with prior SARS-CoV-2 infection.
PEER-REVIEWED
A. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild COVID-19external icon. Ibarrondo et al. NEJM (July 21, 2020; Correction external iconon July 24, 2020).
Key findings:
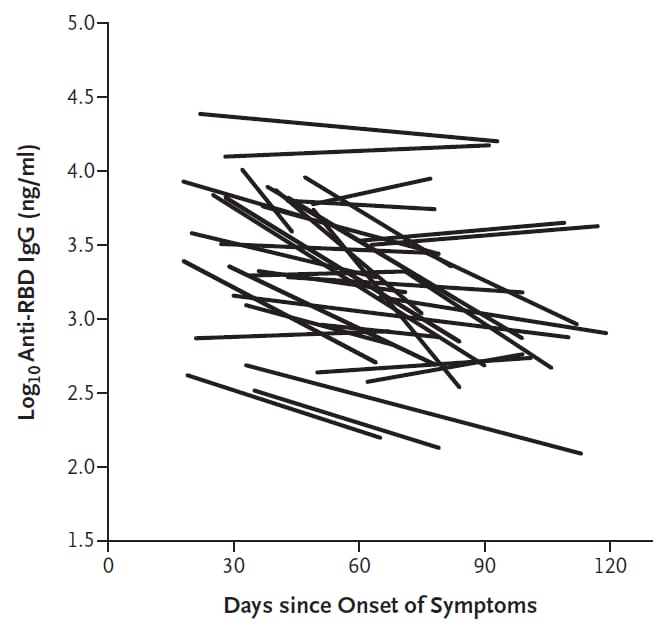
- Anti-SARS-CoV-2 antibodies have a half-life of approximately 36 days (95% CI 26-60 days) in recovered patients with mild illness (Figure).
Methods: Observational study of anti-SARS-CoV-2 IgG antibodies among 34 recovered individuals, most with mild illness. Initial and final blood samples were obtained at a mean of 37 (range 18-65) and 86 (46-119) days post symptom onset, respectively. Anti–SARS-CoV-2 receptor-binding domain serum IgG concentrations were quantified by enzyme-linked immunosorbent assay. Limitations: Most patients had only 2 specimens obtained; limited observation period.
Figure:
Note: From Ibarrondo et al. Initial and repeat levels of anti-SARS-CoV-2 receptor binding domain IgG antibodies on a log scale plotted against the time since the onset of symptoms in each participant. From NEJM. Ibarrondo et al. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. 383:1085-1087. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
PREPRINTS (NOT PEER-REVIEWED)
B. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection.external icon Seow et al. medRxiv (July 11, 2020). Publishedexternal icon in Nature Microbiology (October 26, 2020).
Key findings:
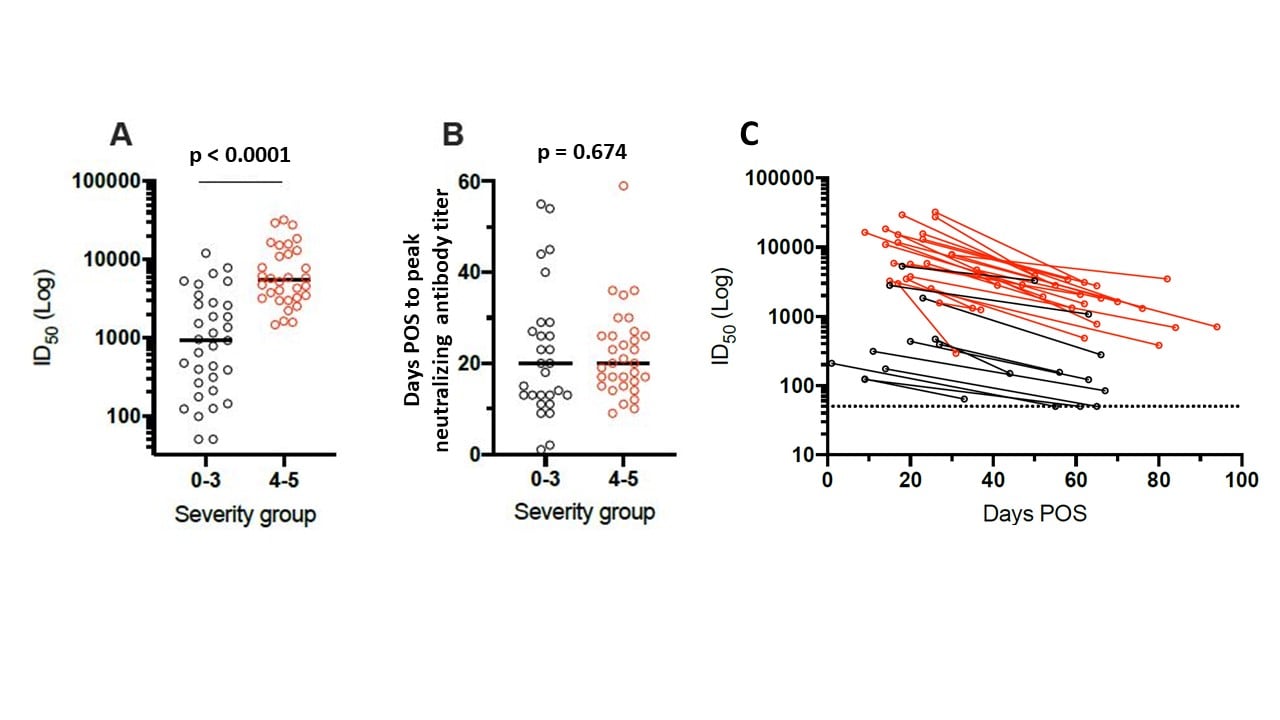
- Patients with increased disease severity demonstrated an increase in neutralizing antibody levels, but disease severity does not impact time to neutralizing antibody peak (Figure).
- Neutralizing antibody levels peaked on average 23 days post onset of symptoms (POS), decreased 2- to 23-fold from 1 to 3 months POS, and became undetectable for some patients after 50 days (Figure).
- Antibody levels to multiple viral proteins varied between individuals.
- 6% of individuals showed synchronous development of IgG, IgM and IgA antibodies.
- 1% showed synchronous development of antibodies to viral S, RBD and N proteins.
Methods: Evaluation of binding and neutralizing antibodies from sequential samples up to 94 days POS from 65 persons with PCR-confirmed SARS-CoV-2 infection, and 31 seropositive healthcare workers by severity of illness. Antibodies to multiple viral proteins (S, RBD, N) and antibody isotypes (IgA, IgG and IgM) were assayed by enzyme-linked immunosorbent assay. Limitations: Results are limited to antibody response and do include other aspects of the immune response such as cell mediated immunity.
Figure:
Note: Adapted from Seow et al. (A) Comparison of peak neutralizing antibody titers (Log of dilution of sera inhibiting 50% of in vitro infection (ID50)) by disease severity. (B) Days POS to reach peak ID50 neutralizing antibody titer by severity of disease. Peak neutralization titer was reached after an average of 23.1 days after POS. Severity Group 0-3, less severe disease shown with open black circles; Severity Group 4-5, more severe disease shown with open red circles. (C) Log of ID50 of in vitro infection by days POS when blood was collected; Severity Group 0-3 shown with black lines; Severity Group 4-5, more severe disease shown with red lines. Licensed under CC-BY-NC-ND 4.0.
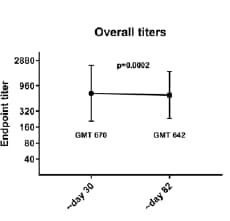
C. SARS-CoV-2 infection induces robust, neutralizing antibody responses that are stable for at least three months.external icon Wajnberg et al. medRxiv (July 17, 2020). Updated analysis published in external iconScience (December 4, 2020).
Key findings:
- Among persons who recovered from COVID-19, >90% had detectable neutralizing antibodies that lasted in some cases at least three months.
- Among a subset of plasma donors, neutralizing IgG titers were stable for approximately three months after symptom onset (Figure).
- Binding titers of anti-spike antibodies significantly correlated with neutralization of SARS-CoV-2.
Methods: Anti-SARS-CoV-2 spike protein IgG was measured in 19,763 persons from the NYC area who recovered from mild-to-moderate COVID-19. Longevity of antibody response was assessed among 121 persons with prior COVID-19 who donated plasma 30 and 82 days post symptom onset. Limitations: Self-reported symptom onset and previous PCR positive results; limited subset of plasma donors assessed for antibody over time.
Figure:
Note: From Wajnberg et al. SARS-CoV-2 antibody titers over time. Used by permission from author.
Implications for 3 studies (Ibarrondo et al., Seow et al., & Wajnberg et al.): The results show antibody response increased with severity of disease with possible decline over time. Further studies are needed to quantify correlates of protection from SARS-CoV-2 over longer periods of time, assess the magnitude and durability of the immune response, and understand how this impacts protection from recurrent infection in order to develop correlates of protection after natural infection.
PEER-REVIEWED
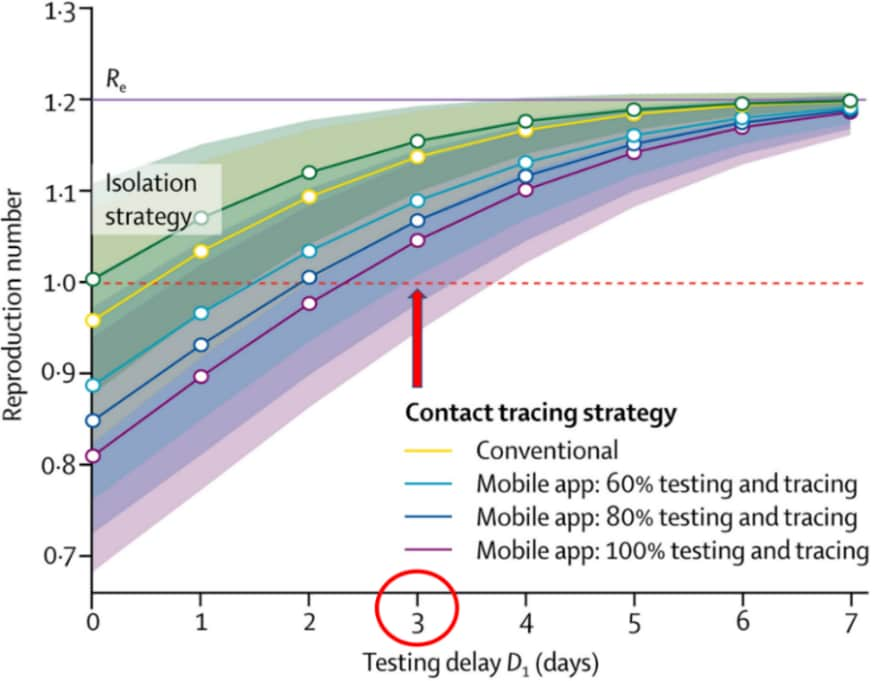
Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling studyexternal icon. Kretzschmar et al. Lancet Public Health (July 16, 2020).
Key findings:
- Reducing testing delay is the most important factor in preventing the spread of SARS-CoV-2.
- Optimizing testing and contract tracing coverage and minimizing contract tracing delays with app-based technology enhanced contact tracing effectiveness, with the potential to prevent 80% of transmissions.
- If testing were delayed by ≥3 days after symptom development, even the most effective contact tracing strategies would not sufficiently reduce the spread of the virus and result in R0 (reproduction number) >1 (Figure).
Methods: Mathematical modelling to evaluate the impact of testing delays (time between symptom development and receipt of test result) and contact tracing delays and coverage (including use of mobile app technology) on reducing the SARS-CoV-2 transmission by generating R0 (the number of individuals infected by a single infected person) for various scenarios. Limitations: Modelling did not consider age structure of population.
Implications: Reducing delays in testing individuals for SARS-CoV-2 is critical for a successful contact tracing strategy in containing the virus. The use of mobile app technology could enhance the speed and effectiveness of contact tracing.
Figure:
Note: Adapted from Kretzschmar et al. Comparison of conventional and mobile app contact tracing strategies by testing delay (in days) on the SARS-CoV-2 R0, which must be below 1 (red dashed line) to reduce spread of the virus. The isolation only strategy is shown in green shading. The testing and tracing coverage for conventional contract tracing (yellow line) was assumed to be 80%, while mobile app testing and contact tracing coverage was assumed to be 60% (light blue line), 80% (blue line), or 100% (purple line). With a delay in testing of 3 days (red circle), none of the contacting tracing strategies are effective in reducing R0 below 1 (red arrow). Licensed under CC-BY-NC-ND 4.0.
PEER-REVIEWED
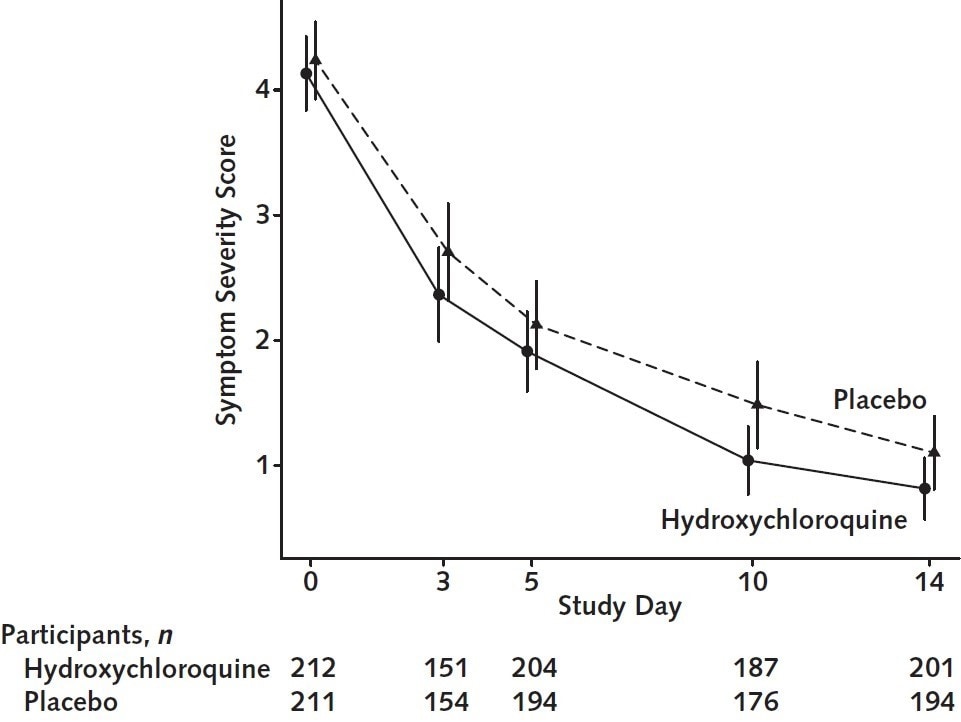
Hydroxychloroquine in nonhospitalized adults with early COVID-19: A randomized trialexternal icon. Skipper et al. Annals of Internal Medicine (July 16, 2020).
Key findings:
- Symptom severity during the 14 days after symptom onset did not differ between persons treated with hydroxychloroquine (HCQ) vs. a placebo (p = 0.117) (Figure).
- At 14 days, 24% HCQ-treated participants had ongoing symptoms compared with 30% receiving placebo (p = 0.21).
- A significantly greater proportion HCQ-treated participants experienced adverse effects (43%) than did persons treated with placebo (22%) (p <0.001).
- No differences in hospitalizations and deaths were found between study groups.
Methods: Randomized, double-blind, placebo-controlled trial in 423 non-hospitalized participants with either laboratory-confirmed COVID-19 or COVID-19–compatible symptoms within 4 days of illness onset and an epidemiologic link to a contact with laboratory-confirmed COVID-19, March 22-May 20, 2020 in the United States and Canada. Symptom severity was assessed on a 10-point visual analogue scale where 0 indicated no symptoms and 10 indicated severe symptoms. Limitations: Underrepresented of African American persons; subjective symptom severity scale; not generalizable; due to US testing shortages, only 58% of participants received SARS-CoV-2 testing.
Implications: This study of outpatient treatment with HCQ provides additional evidence that this medication is not effective for treatment of COVID-19 and supports guidance that it should not be used at this time other than in the context of a clinical trial.
Figure:
Note: Adapted from Skipper et al. Overall symptom severity of hydroxychloroquine (solid line) vs placebo (dashed line) over 14 days. Available via American College of Physicians Public Health Emergency Collection through PubMed Central.
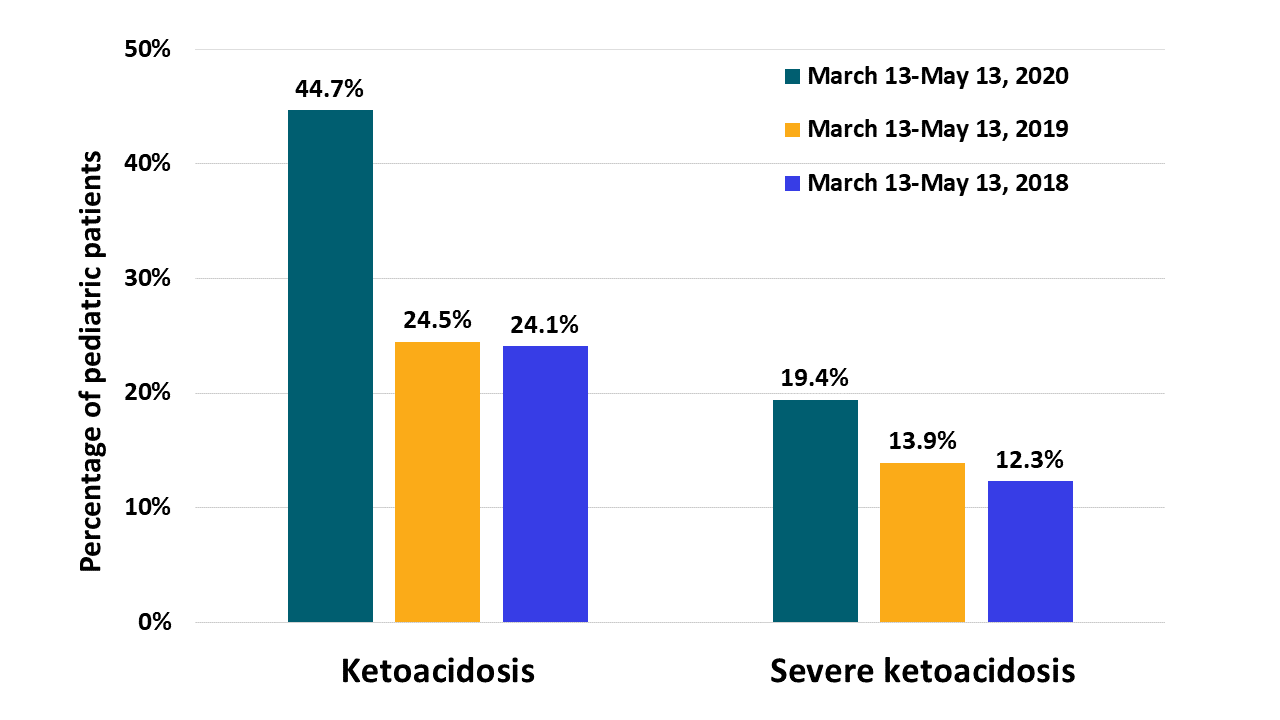
Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germanyexternal icon. Kamrath et al. JAMA (July 20, 2020).
Key findings:
- The percent of children and adolescents newly diagnosed with diabetes and who had ketoacidosis or severe ketoacidosis was significantly greater during the COVID-19 period than in comparator periods.
- The incidence of any ketoacidosis at diagnosis of type 1 diabetes was 44.7% during the COVID-19 period compared with 24.5% in 2019 and 24.1% in 2018 in similar periods (Figure).
- Incidence of severe ketoacidosis was 19.4% during the COVID-19 period compared with 13.9% in 2019 and 12.3% in 2018 (Figure).
Methods: A retrospective cohort study of children and adolescents diagnosed with type 1 diabetes at 216 German diabetes centers March 13-May 13, 2020. Data from similar periods in 2018 and 2019 were used as a comparison. Diabetic ketoacidosis was defined as a blood pH <7.3 and/or bicarbonate level <15 mmol/L, and severe diabetic ketoacidosis as a pH <7.1 and/or bicarbonate level <5 mmol/L. Limitations: No data on socioeconomic status and family history.
Implications: The study suggests a delay in diabetic care in children and adolescents during the COVID-19 pandemic period compared to similar periods in 2018 and 2019.
Figure:
Note: Adapted from Kamrath et al. Incidence of diabetic ketoacidosis and severe diabetic ketoacidosis among children and adolescents presenting with newly diagnosed diabetes mellitus, March13-May 13, 2020, March 13-May 13, 2019 and March 13-May 13, 2018. All rights reserved.
- Lane et a Research in the Context of a Pandemicexternal icon. NEJM. Editorial accompanying the above article and discusses the need for scientifically robust and ethically sound clinical research as the most efficient and effective way of developing treatment and prevention strategies for patients with COVID-19.
- Abbasi. Social isolation—The other COVID-19 threat in nursing homes.external icon JAMA. A perspective piece looking and issues of social isolation among residents in nursing homes that remain locked down during the novel coronavirus pandemic.
- McKee et al. Overcoming additional barriers to care for deaf and hard of hearing patients during COVID-19external icon. JAMA Otolaryngology. Highlights feasible strategies that can be used to mitigate communication barriers for healthcare workers when caring for deaf and hard of hearing patients during the COVID-19 pandemic.
- Rojo et al. Gastrointestinal perforation after treatment with tocilizumab : An unexpected consequence of COVID-19 pandemicexternal icon. American Surgeon. Case report reminding clinicians to be alert for gastrointestinal perforation as a potential complication of tocilizumab used to treat COVID-19.
- Peeling et al. Serology testing in the COVID-19 pandemic responseexternal icon. Lancet Infectious Diseases. Urges and describes appropriate use of rapid serologic testing to guide triage, self-isolation, and quarantine decisions in countries with little or no access to molecular testing.
- Shao et al. Risk assessment of airborne transmission of COVID-19 by asymptomatic individuals under different practical settingsexternal icon. arXiv. Simulated flow of exhaled aerosols during normal breathing and speaking in elevators, classrooms, and supermarkets to show that some ventilation patterns create higher aerosol concentrations in “hot spots” but that careful placement of ventilation and room occupants can reduce exposure to aerosols.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.