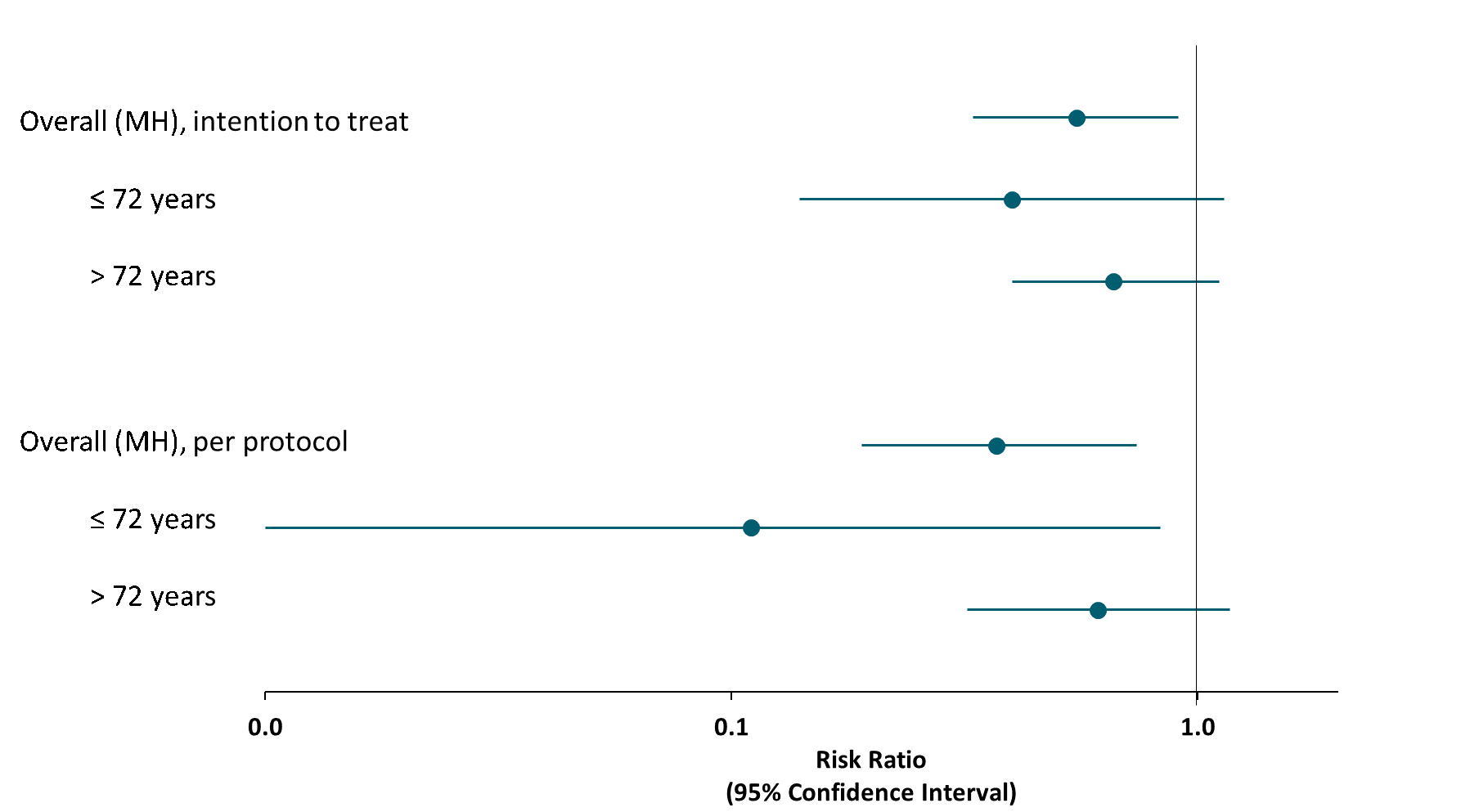COVID-19 Science Update released: June 26, 2020 Edition 25

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgiumexternal icon. Steensels et al. JAMA. (June 15, 2020).
Key findings:
- 6.4% of hospital staff had an antibody (IgG) response to SARS-CoV-2.
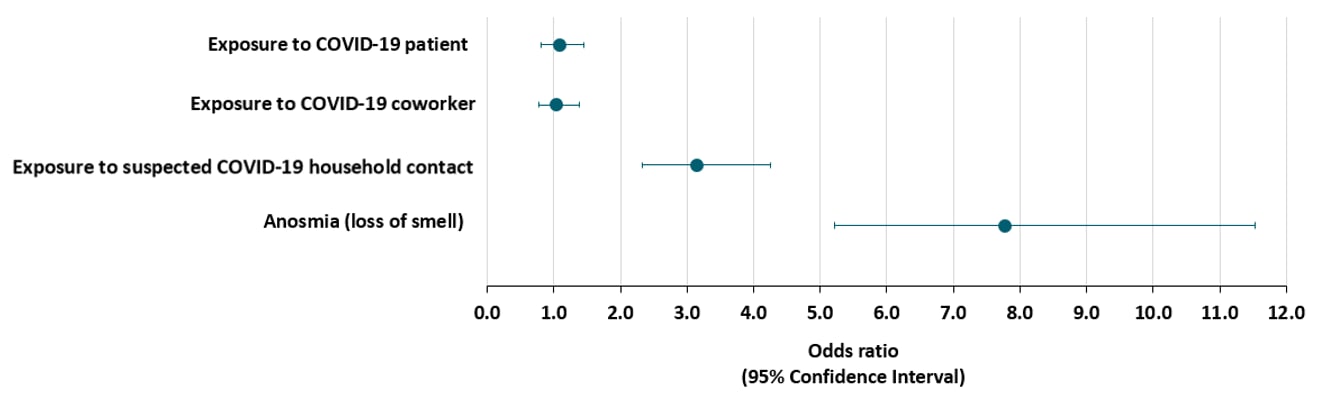
- Exposure to a household contact with suspected COVID-19 was associated with presence of antibodies but exposure to a patient or coworker with diagnosed COVID-19 was not (Figure).
- Anosmia (loss of smell) was the only symptom associated with presence of antibodies (Figure).
Methods: 3,056 staff were screened for antibodies to SARS-CoV-2; associations of demographic, clinical and exposure characteristics with presence of anti-SARS-CoV-2 IgG were examined. Limitations: Single-center study; some recent infections could have been missed since didn’t test for IgM antibodies to SARS-CoV-2.
Implications: Among hospital staff, community and/or household exposure to infection may confer greater risk for acquiring SARS-CoV-2 infection than exposure as a healthcare worker.
Figure:
Adapted from Steensels et al. Odds ratio and 95% confidence intervals on the association between clinical and exposure characteristics with IgG antibodies to SARS-CoV-2. Reproduced with permission from JAMA. doi:10.1001/jama.2020.11160. Copyright©2020 American Medical Association. All rights reserved.
Prevalence of COVID-19 infection and outcomes among symptomatic healthcare workers in Seattle, Washington.external icon Mani et al. Clinical Infectious Diseases. (June 16, 2020).
Key findings:
- 5% of symptomatic hospital staff tested positive for SARS-CoV-2 RNA.
- Frontline and non-frontline staff had similar prevalences (5.2% and 5.5%, respectively).
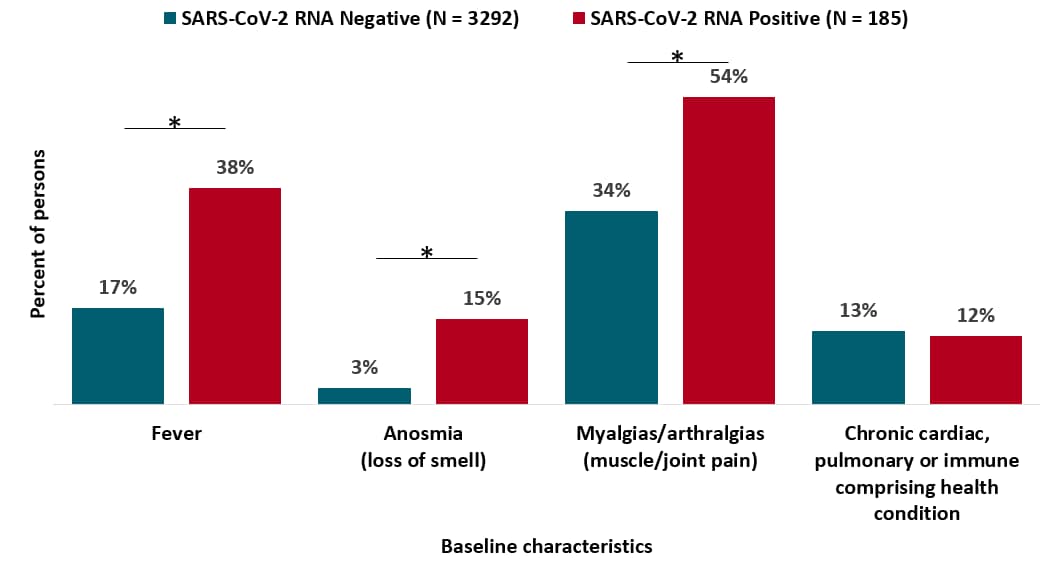
- Fever, myalgias/arthralgias, and anosmia were more frequently reported among persons with positive test results (Figure).
- Prevalences of chronic health conditions (including immunosuppression) were similar among persons with positive and negative test results (Figure).
- 6/174 (3%) of those contacted during the 14-day follow-up period after diagnosis reported hospitalization due to COVID-19.
Methods: 3,477 symptomatic hospital staff were tested for SARS-CoV-2 RNA. SARS-CoV-2 prevalence and baseline characteristics were assessed for frontline and non-frontline staff. Limitations: Prevalence likely underestimated since only symptomatic staff tested; anosmia was added as a symptom later in the study.
Implications: Similar SARS-CoV-2 prevalences were seen in frontline and non-frontline healthcare workers suggesting that factors other than face-to-face patient contact may contribute to acquisition of SARS-CoV-2 infection among hospital employees.
Figure:
Adapted from Mani et al. Baseline characteristics for persons testing negative and positive for SARS-CoV-2 in blue and red, respectively; * indicates p value < 0.001. Available via Oxford University Press Public Health Emergency Collection through PubMed Central.
Syringe services program (SSP) operational changes during the COVID-19 global outbreak.external icon Bartholomew et al. International Journal of Drug Policy (June 12, 2020).
Key findings:
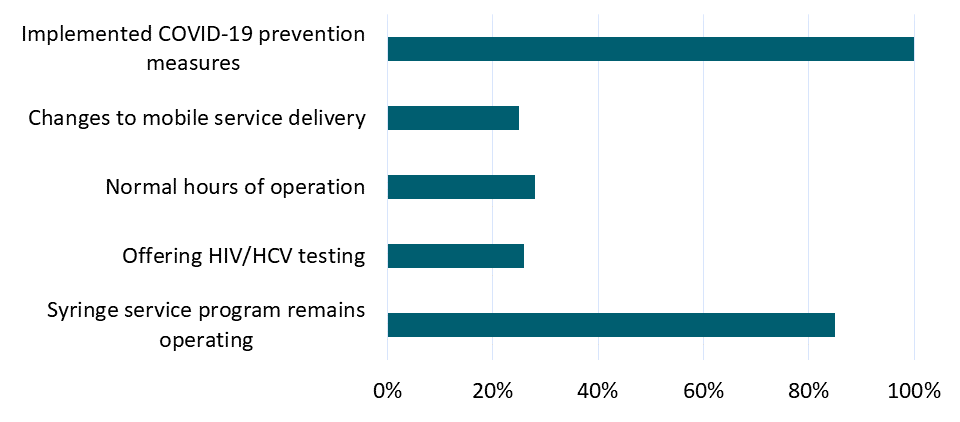
- 85% (55/65) of syringe service program sites surveyed have remained open during the COVID-19 pandemic and 15% have discontinued all syringe service program operations.
- Of those still offering syringe service programs, 29% (16/55) switched to mobile delivery of new injection equipment.
- Only 26% of programs have continued HIV/HCV testing on site (Figure).
Methods: Cross sectional telephone survey of 65 syringe service program sites from 33 U.S. states including 14 sites in New York City, March 19-26, 2020. If sites could not be reached by phone, data were collected from publicly available sources, such as websites and social media. Limitations: Convenience sample; not all sites were interviewed.
Implications: Most syringe service programs have continued to operate during the COVID-19 pandemic, but many have discontinued HIV/HVC testing services. Reduced access to syringes and HIV/HCV testing may increase the risk of HIV/HCV transmission.
Figure:
Note: Adapted from Bartholomew et al. Percentage of programs providing syringe services, offering HIV/HCV testing, keeping normal hours of operation, changing to mobile service delivery and implementing COVID-19 prevention measures. This article was published in International Journal of Drug Policy, Bartholomew et al., Syringe services program (SSP) operational changes during the COVID-19 global outbreak, Page 102821, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
A cross-sectional community-based observational study of asymptomatic SARS-CoV-2 prevalence in the greater Indianapolis areaexternal icon. Meyers et al. Journal of Medical Virology (June 16, 2020).
Key findings:
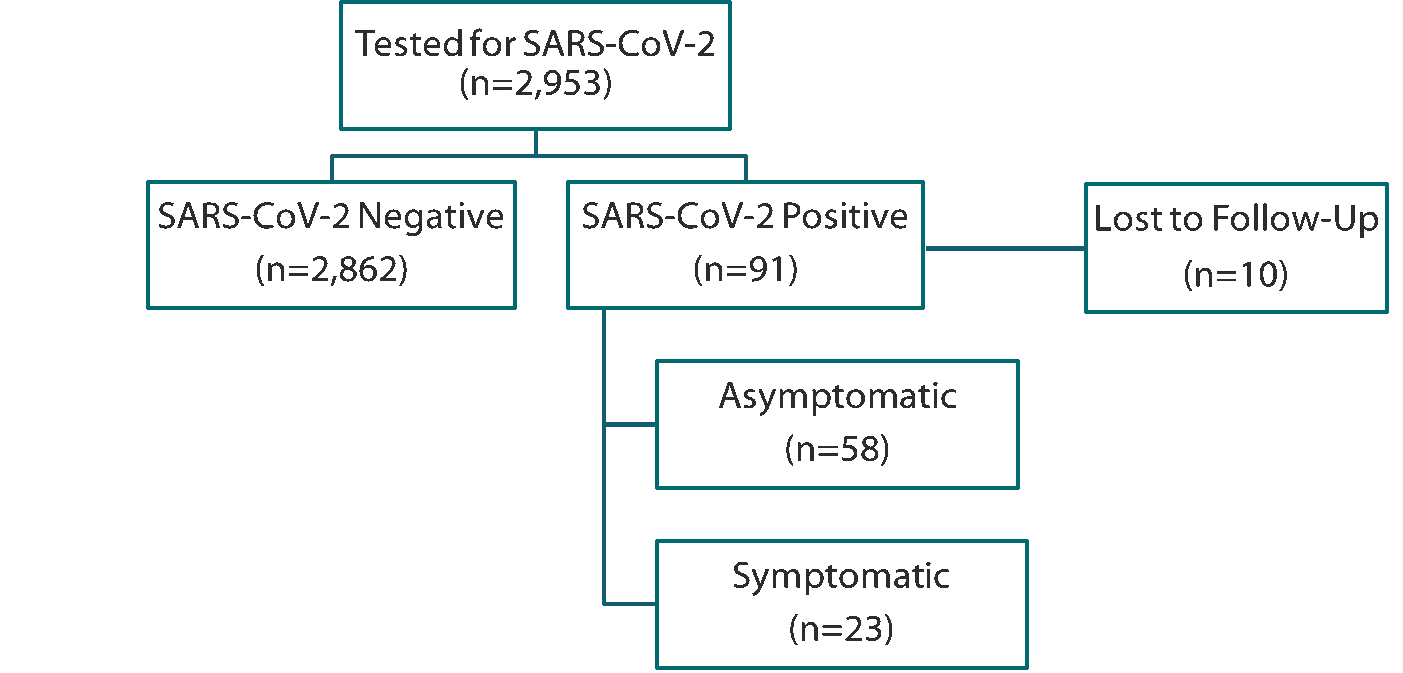
- 3% (91/2,953) of adults who reported no new or worsening fever, cough, and shortness of breath in the last 7 days tested positive for SARS-CoV-2 (Figure).
- 25% (23/81) of those with complete follow-up developed symptoms within 14 days of testing positive.
- The most frequently reported symptoms were headaches, fatigue and/or muscle aches, and shortness of breath.
Methods: Cross-sectional community testing in the Indianapolis metro area, between April 7 and May 16, 2020 of adults ≥18 years with no previous positive test for SARS-CoV-2 and who reported no fever and no new onset or worsening cough or shortness of breath in the past 7 days. SARS-CoV-2 infection was diagnosed by RT-PCR using NP swabs. Participants were re-interviewed and re-tested at 14 days. Limitations: Reporting of mild and moderate symptoms at baseline may be subject to recall bias; participants who tested positive at baseline may be more likely to report symptoms at follow-up; results may lack generalizability due to convenience sampling.
Implications: Broader population screening and testing beyond symptomatic individuals will help identify individuals infected with SARS-CoV-2.
Figure:
Note: Adapted from Meyers et al. Number of study participants tested for SARS-CoV-2, positive, and reporting symptoms. Licensed under CC-BY-NC-ND 4.0.
Assessing the impact of a rapidly scaled virtual urgent care in New York City during the COVID-19 pandemicexternal icon. Koziatek et al. Journal of Emergency Medicine (June 12, 2020).
Key findings:
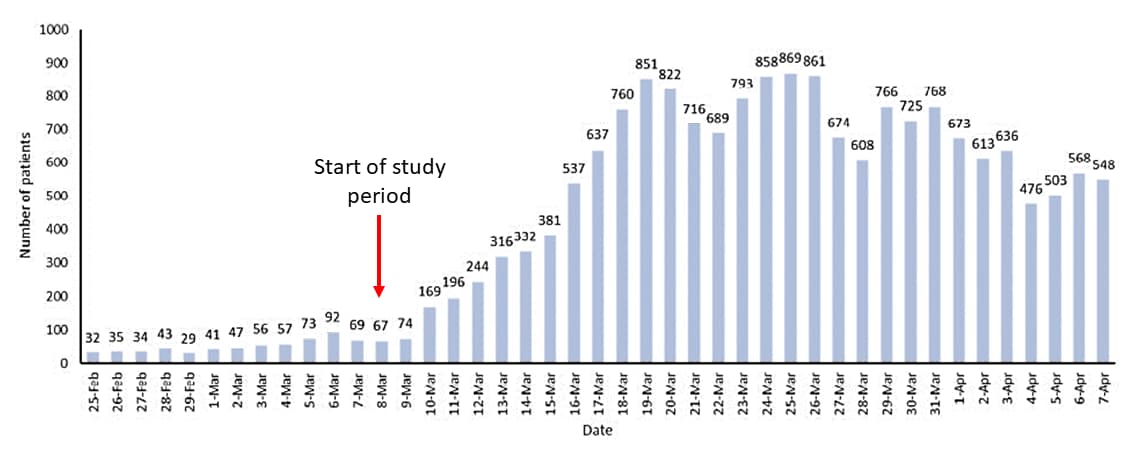
- 17,730 patients were seen via virtual urgent care (VUC) where patients interact with a healthcare provider via video teleconference.
- Daily VUC visits rapidly increased and peaked at 869 by mid-March (Figure 1).
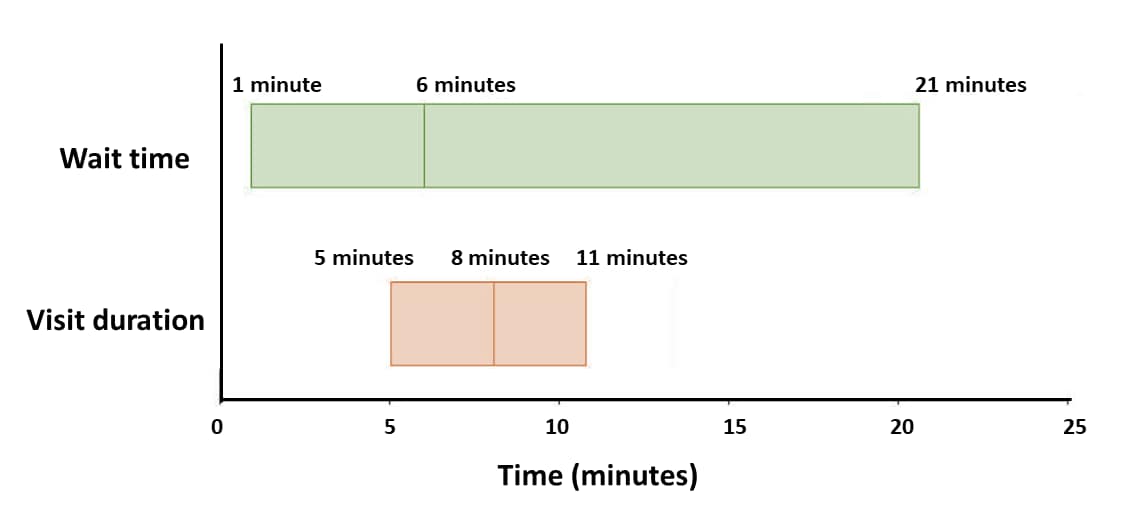
- Median wait time and visit duration for VUC was 6 and 8 minutes, respectively (Figure 2).
- If VUC was unavailable, 81% of patients responding to the post-visit survey reported they would have sought care in-person from the emergency department, urgent care or primary care provider.
Methods: Description of the rapid scaling of a VUC to evaluate and treat patients in New York City during March 8-April 7, 2020. 15% (2668/17,730) participated in a post-visit online survey. Limitations: Quality of care was not assessed; results not generalizable outside of New York City in a pandemic situation; low post-visit survey response rate.
Implications: Rapid scale up of telemedicine VUC is feasible and could help alleviate crowded emergency departments and urgent care centers.
Figure 1
Note: Adapted from Koziatek et al. Daily patient volumes in VUC during the study period. The twelve days preceding the study period are also included for comparison.
Figure 2
Note: Adapted from Koziatek et al. Median wait time and median visit duration, with interquartile ranges, for VUC visits during the one-month study period. This article was published in Journal of Emergency Medicine, Koziatek et al., Assessing the impact of a rapidly scaled virtual urgent care in New York City during the COVID-19 pandemic, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
PREPRINTS (NOT PEER-REVIEWED)
Evaluation of SARS-CoV-2 in breastmilk from 18 infected womenexternal icon. Chambers et al. medRxiv (June 16, 2020).
Key findings:
- Of 64 breastmilk samples collected from 18 women with SARS-CoV-2 infection, 1 sample had detectable SARS-CoV-2 RNA.
- The positive sample was collected the day of symptom onset, but samples collected from the same patient 2 days prior to symptom onset and 12 and 41 days after were negative.
- No samples yielded replication-competent SARS-CoV-2.
Methods: Serial, cross-sectional cohort study of 64 breastmilk samples from 18 lactating women with self-reported SARS-CoV-2 infection March 27-May 6, 2020. Samples were analyzed for SARS-CoV-2 RNA by quantitative RT-PCR and also cultured for replication-competent virus (26 samples from 9 women). Limitations: Small sample size; SARS-CoV-2 test result and COVID-19 symptoms were self-reported.
Implications: SARS-CoV-2 RNA was detected in only one breast milk sample and the viral culture for that sample was negative. These preliminary data suggest that breastmilk may not be source of infection for the infant.
PEER-REVIEWED
Prevalence and outcomes of acute ischemic stroke among patients ≤ 50 years of age with laboratory confirmed COVID-19 infectionexternal icon. Annie et al. American Journal of Cardiology (June 14, 2020).
Key findings:
- 0.7% of COVID-19 patients aged ≤ 50 years had an acute stroke.
- These patients had a higher prevalence of hypertension, diabetes, heart failure, nicotine dependence, obesity, chronic obstructive pulmonary diseases, prior stroke, and kidney dysfunction.
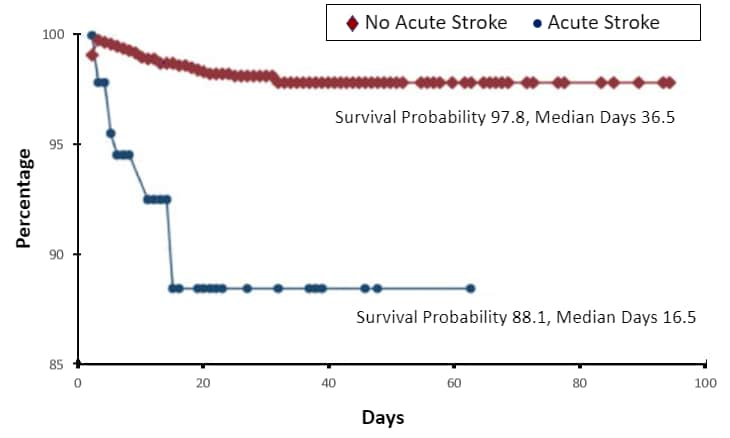
- There were significantly more deaths in patients with stroke than those without, 15.6% of vs. 0.6%, respectively (Figure).
Methods: Evaluation of all-cause mortality in an observational cohort of 9,358 patients aged ≤ 50 years with COVID-19 infection at 37 global health care settings, January 20-April 24, 2020 with data from TriNetx Research Network. Difference in all-cause mortality between those with and without stroke was assessed. Limitations: Timing of presentation with stroke in relation to testing for SARS CoV-2 unclear; data primarily from large health care settings; no control group without COVID-19 infection.
Implications: Stroke occurs in COVID-19 patients ≤ 50 years and there is lower probability of survival; these results may guide clinical management of young COVID-19 patients with co-morbidities.
Figure:
Note: Adapted from Annie et al. Percentage of COVID-19 cases alive stratified by patients with acute stroke and no stroke. This article was published in American Journal of Cardiology, Vol 130, Annie et al., Prevalence and outcomes of acute ischemic stroke among patients ≤50 years of age with laboratory confirmed COVID-19 infection, Page 169-170, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
PREPRINTS (NOT PEER-REVIEWED)
Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCRexternal icon. Lu et al. medRxiv. (June 17, 2020). Published external iconin EBioMedicine (August 24, 2020).
Key findings:
- 87 (14%) COVID-19 patients re-tested positive for SARS-CoV-2 RNA within 14 days after discharge.
- Infectious virus could not be cultured from 36 RT-PCR-positive samples from 33 patients who re-tested positive.
- SARS-CoV-2 neutralizing antibody titers in re-test positive patients were comparable to those of patients with negative RT-PCR results after discharge and with those of hospitalized COVID-19 cases.
Methods: 619 COVID-19 patients who had been discharged based on 2 consecutive negative SARS-CoV-2 RNA RT-PCR tests results were re-tested at least twice during the 14-day period after discharge regardless of symptoms. Samples from a subset of patients who re-tested positive were analyzed for infectious virus and for neutralizing antibodies. Neutralizing antibody titers were obtained from discharged patients who re-tested positive, discharged patients who were SARS-CoV-2 negative 14 days after discharge and a separate group of hospitalized COVID-19 patients not yet discharged. Limitations: Small sample size.
Implications: Persons who re-test positive for SARS-CoV-2 RNA after consecutive negative PCR test results are not infectious and had a neutralizing antibody response comparable to that observed in other persons with COVID-19.
PEER-REVIEWED
Renal dysfunction in hospitalised children with COVID-19external icon. Stewart et al. Lancet Child & Adolescent Health. (June 15, 2020).
Key findings:
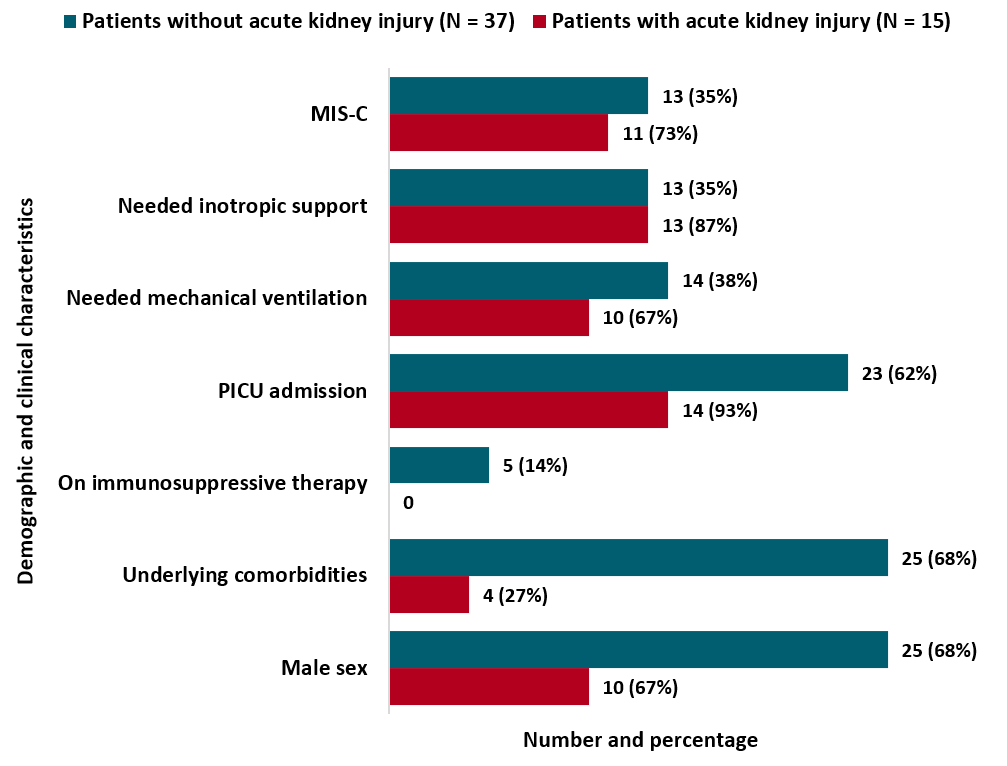
- 15 (29%) COVID-19 patients had acute kidney injury.
- 14 (93%) were admitted to pediatric ICU (Figure).
- 11 (73%) had multisystem inflammatory syndrome in children (Figure).
- 5 (33%) had enlarged kidneys.
- Serum creatinine values normalized, and renal function improved in most patients; none required kidney biopsy or continuous renal replacement therapy.
Methods: 52 pediatric COVID-19 patients were assessed for acute kidney injury, defined as serum creatinine 1.5 times greater than the upper limit of normal. Limitations: Small sample size; baseline creatinine values were unavailable for most patients.
Implications: Monitoring renal function is important in hospitalized COVID-19 pediatric patients. Risk for acute kidney injury in pediatric patients with SARS-CoV-2 infection needs to be further studied.
Figure:
Note: Adapted from Stewart et al. Demographic and clinical characteristics of COVID-19 patients with and without acute kidney injury. Abbreviations: MIS-C, multisystem inflammatory syndrome in children; PICU, pediatric intensive care unit. This article was published in Lancet Child & Adolescent Health, Vol 4, Stewart et al., Renal dysfunction in hospitalised children with COVID-19, Page E28-E29, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
PREPRINTS (NOT PEER-REVIEWED)
GLUCOCOVID: A controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumoniaexternal icon. Corral-Gudino et al. medRxiv (June 18, 2020).
Key findings:
- Methylprednisolone treatment was associated with reduced risk of death, ICU admission, and non-invasive ventilation among adult COVID-19 patients at risk of ARDS, compared with standard of care (age-weighted, per-protocol risk ratio: 0.37, 95% CI 0.19-0.74).
- Reduced risk was most pronounced among patients 72 years or younger (Figure).
Methods: Interim results from an open label, partially randomized trial in 5 Spanish hospitals, April-May 2020. Adult COVID-19 patients were treated with standard of care (n= 29) or standard of care plus methylprednisolone (n=56). Inclusion criteria included moderate to severe lung disease; patients on mechanical ventilation or in the ICU were excluded. Limitations: Small sample size (interim trial results); possible confounding due to physician preference for use of methylprednisolone; standard of care treatments may differ across providers and hospitals.
Implications: Interim trial results suggest methylprednisolone may have beneficial effects for adult patients with COVID-19 who are at risk of developing ARDS. Complete trial results are needed to confirm findings.
Figure:
Note: Adapted from Corral-Gudino et al. Per protocol overall (age-weighted) and age-stratified risk ratios of death, ICU admission, and non-invasive ventilation using methylprednisolone treatment versus standard of care. MH-Mantel-Hanzel. Used by permission from author.
Three studies described the clinical outcomes of patients with COVID-19 and cancer. Predictors of severe illness and mortality were also investigated.
PEER-REVIEWED
A. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort studyexternal icon. Kuderer et al. Lancet (May 28, 2020).
Key findings:
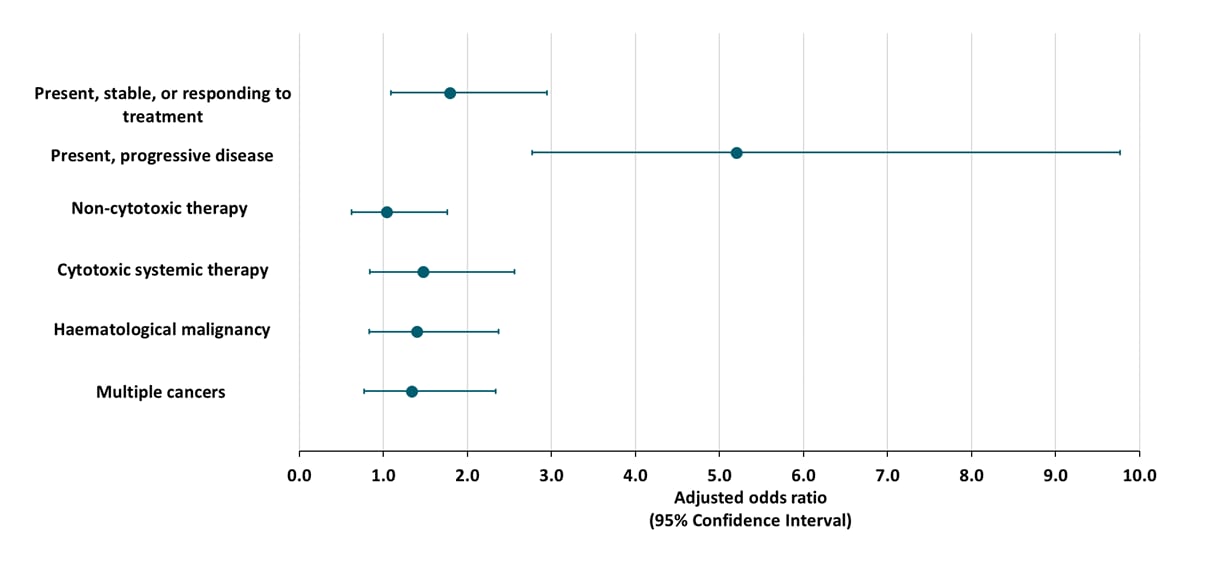
- 13% of COVID-19 patients with cancer died.
- Patients with active cancer were more likely to die than patients in remission, after adjusting for age, sex, smoking status, and obesity (Figure).
- Death was also associated with increased age, male sex, history of smoking, comorbidities, and treatment with azithromycin plus hydroxychloroquine.
- Type of cancer was not associated with death (Figure).
- 39% of patients received cancer treatment within 4 weeks of COVID-19 diagnosis.
- Receiving cancer treatment was not associated with death (Figure).
Methods: Retrospective cohort study of 928 patients with laboratory confirmed SARS-CoV-2 infection and active cancer (N=396) or in remission (N=422) from the USA, Canada, and Spain, March 17-April 16, 2020. Multivariable logistic regression was used to estimate the odds of 30-day mortality. Limitations: Non-randomized study; short study follow-up period.
Figure:
Note: Adapted from Kuderer et al. Factors associated with 30-day all-cause mortality for cancer status, type of anticancer treatment and type of malignancy. This article was published in Lancet, Vol 395, Kuderer et al., Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study, Page 1907-1918, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
B. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study external iconLee et al. The Lancet (May 28, 2020).
Key findings:
- 28% of COVID-19 patients with active cancer died.
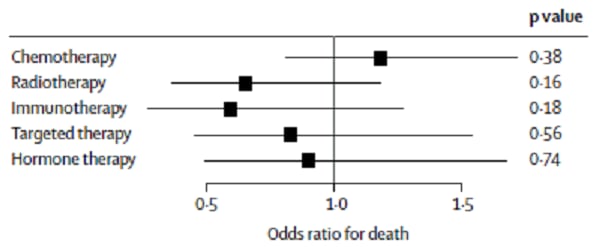
- Death was associated with increased age, male sex, and comorbidities.
- 65% received cancer treatment within 4 weeks of COVID-19 diagnosis.
- Receiving cancer treatment was not associated with death (Figure).
Methods: Retrospective cohort study from 55 cancer centers of 800 UK patients with active cancer and SARS-CoV-2 infection diagnosed by RT-PCR NP swab, March 1-April 26, 2020. Limitations: Non-randomized study; short follow-up period.
Figure:
Note: Adapted from Lee L et al. Odds ratio (black squares) and 95% confidence intervals (black bars) on the association between cancer treatments and mortality in COVID-19 patients. . This article was published in Lancet, Vol 395, Lee et al., COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study, P1919-P1926, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
C. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort studyexternal icon. Garassino et al. Lancet Oncology. (June 12, 2020).
Key findings:
- 33% of COVID-19 patients died.
- Death was associated with a history of smoking.
- 74% of patients received cancer treatment.
- Receiving chemotherapy treatment alone was not associated with death after adjusting for age, smoking status, hypertension, and chronic obstructive pulmonary disease.
Methods: Cross-section analysis of 200 COVID-19 patients with thoracic cancer within the TERAVOLT registry, eight European countries, March 26-April 12, 2020. Limitations: Small sample of patients; non-randomized study.
Implications of 3 studies (Kuderer et al., Lee et al. & Garassino et al.): Although a high percentage of cancer patients with SARS-CoV-2 die, risk of death was not associated with receipt of cancer treatment. Higher mortality is associated with same risks seen in noncancer patients (age, gender, comorbidities).
Disproportionately affected groups
- Treweek et al. COVID-19 and ethnicity: who will research results apply to?external icon Lancet. Discusses the under-representation of Black, Asian, and minority ethnic groups in COVID-19 research.
- Editorial. Medicine and medical science: Black lives must matter more.external icon Lancet. Editorial urging medical journals and the broader medical community to support Black and other racial and ethnic minority persons through the type of research published.
- Maxmen A. Why more coronavirus testing won’t automatically help the hardest hitexternal icon. Nature. Highlights the street based COVID-19 testing and outreach done by the Roots Community Health Center in Oakland, California to help economically disadvantaged communities.
Treatment
- Marovich et al. Monoclonal antibodies for prevention and treatment of COVID-19external icon. JAMA. Succinct overview of monoclonal antibodies for prevention and treatment of COVID-19.
- Ledford et al. Coronavirus breakthrough: Dexamethasone is first drug shown to save livesexternal icon. Nature. Discusses the RECOVERY trial of dexamethasone as a new treatment for COVID-19.
COVID-19 Literature and Data
- Backhaus A. Common pitfalls in the interpretation of COVID-19 data and statisticsexternal icon. Intereconomics. Discusses the main caveats regarding the interpretation of data in the context of the SARS-CoV-2 pandemic.
- Hutson M. Artificial-intelligence tools aim to tame the coronavirus literatureexternal icon. Nature. Artificial-intelligence and natural language processing to help sift through the vast amount of COVID-19 studies to identify manuscripts of interest.
- Mello et al. Ethics and governance for digital disease surveillanceexternal icon. Science. Ethical concerns raised by digital technologies and new data sources in public health surveillance during epidemics.
- Labeau S. Recommendation and protocol compliance: “Yes, I do” may not be true; the complexity of measuring provider adherence.external icon Intensive and Critical Care Nursing. Overview of methodological considerations for measuring adherence to SARS-CoV-2 pandemic guidelines.
Other Topics
- Nieuwlaat et al. COVID-19 and antimicrobial resistance: Parallel and interacting health emergenciesexternal icon. Clinical Infectious Disease. Discusses the parallel and interacting public health emergencies of COVID-19 and antimicrobial resistance.
- Clapham et al. Seroepidemiologic study designs for determining SARS-CoV-2 transmission and immunity. Emerging Infectious Diseases. Considerations for conducting serological studies.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.