COVID-19 Science Update released: June 19, 2020 Edition 23

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spainexternal icon. Martínez-Perez et al. JAMA. (June 8, 2020; Correction external iconon July 21, 2020).
Key findings:
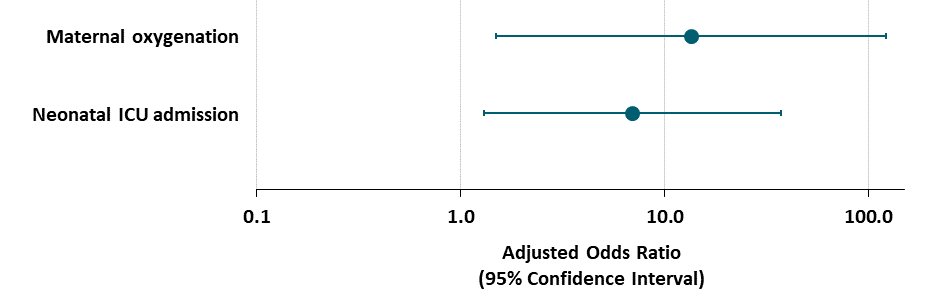
- Among pregnant women who were asymptomatic or had mild COVID-19 symptoms, cesarean delivery was associated with an increased maternal need for oxygen supplementation after delivery compared with women who delivered vaginally (Figure).
- Cesarean birth was also associated with higher neonatal ICU admission (Figure).
Methods: Cross sectional study of 82 pregnant women who tested positive for SARS-CoV-2 RNA at delivery during March 12 and April 6, 2020 (asymptomatic/mild symptoms: n = 78; severe symptoms: n = 4). For women with asymptomatic/mild symptoms, association between delivery mode (vaginal vs. cesarean) and maternal and neonatal outcomes was estimated with multivariable logistic regression, adjusting for maternal age, body mass index, comorbidities, maternal need for oxygen supplementation at admission, abnormal chest x-ray findings at admission, nulliparity, smoking, and prematurity. Limitations: Small sample size; wide confidence intervals; residual and unmeasured confounding may bias results; women with cesarean deliveries had obstetrical indications and 8 had cesarean section due to COVID-19 symptoms.
Implications: Cesarean delivery may be associated with poorer maternal and neonatal outcomes for women with asymptomatic or mild COVID-19.
Figure:
Note: Adapted from Martinez-Perez et al. Odds ratios and 95% CIs of need for maternal oxygenation and NICU admissions after cesarean delivery versus vaginal delivery among women with asymptomatic/mild COVD-19, adjusted for maternal age, body mass index, maternal comorbidities, need for oxygen supplementation at admission, chest X-ray findings at admission, nulliparity, smoking, and prematurity. Reproduced with permission from JAMA. doi:10.1001/jama.2020.10125. Copyright©2020 American Medical Association. All rights reserved.
Correction issued by authors external iconon July 21, 2020.
The second key finding, the implications section and the created figure above are no longer in keeping with the data presented in the paper.
The authors explained that there were errors in the data and had erroneously reported statistical significance for the outcome of admission to the neonatal intensive care unit. The authors conclude: “Limitations include a lack of sufficient information on newborns to determine vertical transmission. The lack of association between cesarean delivery and risk of NICU admission may have been related to the lack of statistical power. Also, the 95% CIs around the odds ratios for cesarean birth and clinical deterioration were wide and the estimates fragile.”
PREPRINTS (NOT PEER-REVIEWED)
ICON (Ivermectin in COvid Nineteen) study: Use of ivermectin is associated with lower mortality in hospitalized patients with COVID-19external icon. Rajter et al. medRxiv. (June 10, 2020). Publishedexternal icon in Chest (October 13, 2020).
Key findings:
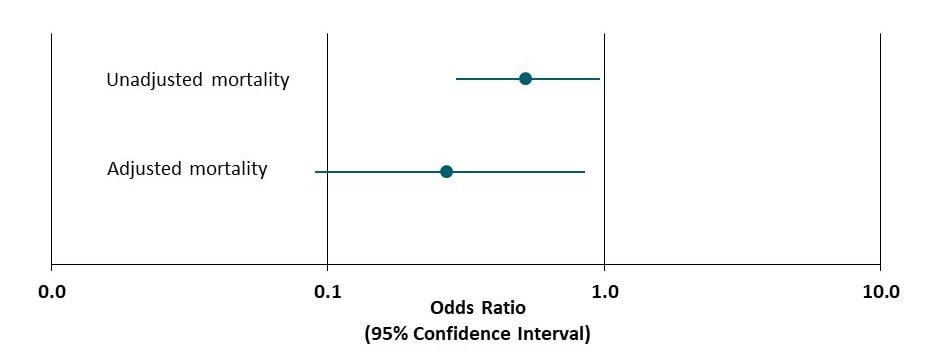
- Among patients hospitalized with COVID-19, those treated with ivermectin were less likely to die than those treated with usual care (mortality: ivermectin 15%; usual care 25%; p=0.03) (Figure).
- Mortality was lower among patients with severe pulmonary disease (mortality: ivermectin 39%; usual care 81%; p=0.001).
Methods: Observational cohort study of 280 adults hospitalized with COVID-19 between March 15 to May 11, 2020. Multivariable logistic regression was used to estimate association between ivermectin and mortality, compared to usual care (no treatment or treatment with azithromycin and/or hydroxychloroquine), adjusted for patient demographics, comorbidities, and concomitant treatments. Limitations: Convenience sample; residual and unmeasured confounding may bias results; timing, dose, frequency, and indication for ivermectin and usual care treatments poorly described and varied by patient; wide confidence intervals; biological mechanism to explain association unclear.
Implications: Preliminary data suggests ivermectin may reduce mortality among hospitalized patients with COVID-19. Rigorous observational studies and randomized clinical trials are needed.
Figure:
Note: Adapted from Ratjer et al. Unadjusted and adjusted odds ratios and 95% CIs for mortality among all patients treated with ivermectin compared to usual care. Licensed under CC-BY-NC-ND 4.0.
PREPRINTS (NOT PEER-REVIEWED)
Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinantsexternal icon. van Kampen et al. medRxiv (June 9, 2020). Published in Nature Communicationsexternal icon (January 11, 2021).
Key findings:
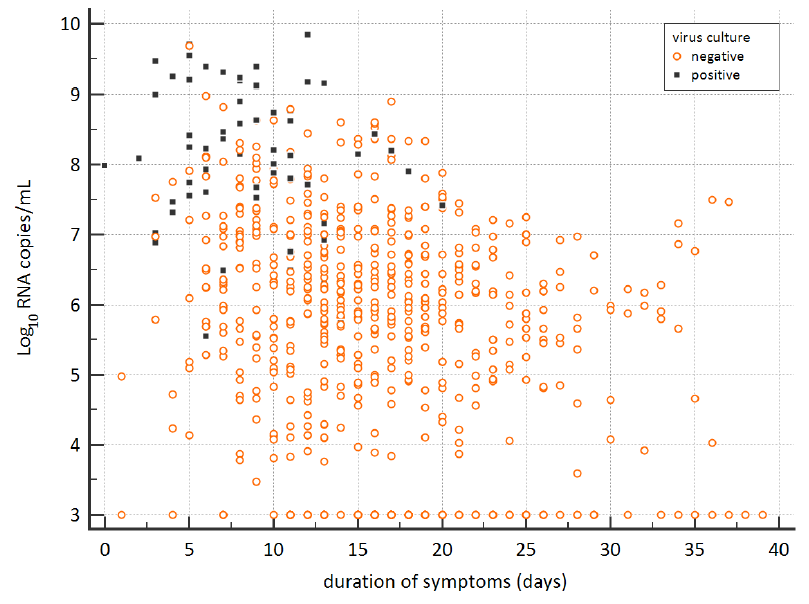
- Respiratory samples from 23 severely ill COVID-19 patients were SARS-CoV-2 culture-positive.
- Live virus shedding (indicated by culture-positive samples) sometimes occurred more than 10 days after symptom onset (Figure).
- 23% also had moderate to severe immunocompromise.
- High levels of neutralizing antibodies (serum titer > 1:20) greatly decreased odds of live viral shedding (adjusted odds ratio (aOR) and 95% CI: 0.01 [0.002-0.08]).
- High viral load (>107 copies/ml) was associated with live viral shedding (aOR and 95% CI: 14.7 [3.7-58.1]).
- The likelihood of isolating virus was < 5% which was not observed at 6.63 log10 copies/mL viral transport medium (VTM, swabs were placed in ~ 4 mL of VTM).
Methods: Duration and predictors of infectious SARS-CoV-2 shedding were examined in 129 severely ill COVID-19 patients by conducting RT-PCR and virus culture on respiratory samples and measuring neutralizing antibodies. Limitations: Only patients with severe or critical disease; samples were not prospectively collected at specified timepoints but reflect routine virologic monitoring of patients.
Implications: COVID-19 patients with severe disease or who are immunocompromised may be infectious 20 days or more after presenting with symptoms. There is also a consistent viral burden below which patients are no longer likely infectious.
Figure:
Note: Adapted from van Kampen et al. Viral load and culture results over the span of symptom duration. Black squares and orange circles indicate virus culture-positive and -negative samples, respectively. Used by permission from author.
Although the respiratory tract is most often affected, coronaviruses can impact the central nervous system. Neurological manifestations from hospitalizations and autopsies of people with COVID-19 are described.
PEER-REVIEWED
A. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Benameur et al. Emerging Infectious Diseases (June 2, 2020).
Key findings:
- 3 patients with severe COVID-19 and neurologic symptoms developed brain swelling and altered mental status (encephalitis and encephalopathy).
- Brain imaging showed changes not readily attributable to vascular causes.
- Brain and spinal fluid showed:
- High anti-IgM levels (antibodies).
- High interleukin 6, 8, and 10 levels.
- No evidence of SARS-CoV-2 RNA.
Methods: Case series of 3 patients with SARS-CoV-2 infection measured by RT-PCR. Limitations: One patient had sickle cell disease which might influence findings.
B. Early evidence of pronounced brain involvement in fatal COVID-19 outcomesexternal icon. von Weyhern et al. Lancet (June 4, 2020).
Key findings:
- Among 6 patients who died from COVID-19, brain and spinal cord membrane swelling (meningitis and panencephalitis) and brainstem cell damage were observed at autopsy.
- The cause of death for the 3 patients ≥ 65 years old was cardiorespiratory failure.
- The cause of death for the 3 patients < 65 years old was intracranial hemorrhage or pulmonary embolism.
Methods: Autopsies of 6 patients who died from COVID-19 in April. Limitations: Unclear how patients were chosen for autopsy.
C. Neuropathological features of COVID-19external icon. Solomon et al. NEJM. (June 12, 2020)
Key findings:
- Among 18 patients who died from COVID-19, none showed signs of encephalitis or other specific brain changes.
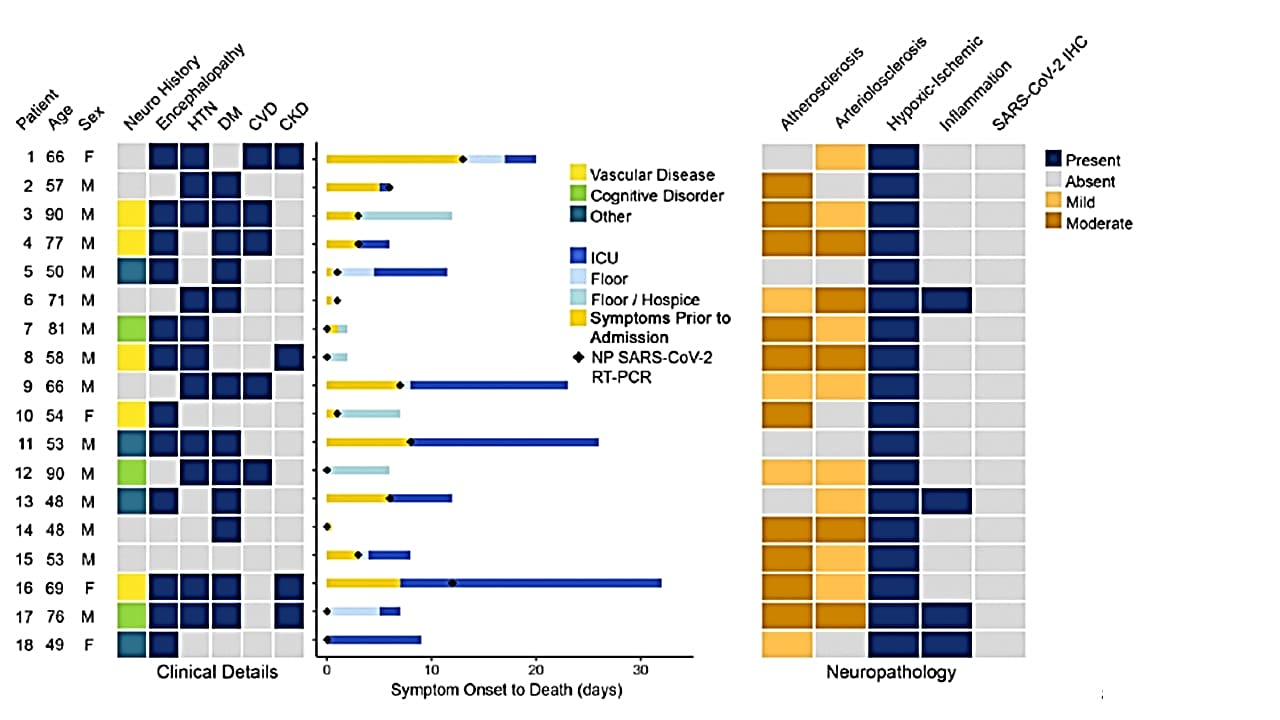
- All patients had hypoxic injury in the cerebrum and cerebellum (Figure).
- SAR-CoV-2 RNA was detected at low levels (>5 copies/ml) in brain sections from 5 patients, but levels were not consistently related to interval from symptom onset to death.
- No olfactory bulb damage was observed.
Methods: Postmortem with brain examination was conducted on 18 patients with RT-PCR-confirmed SARS-CoV-2 infection who died April 14-29, 2020. Gross inspection, microscopic examination, quantitative RT-PCR testing and immunohistochemical analysis of brain tissue were performed. Limitations: Case series; qRT-PCR positive results may have been due to in situ presence of virions in tissues or to cross-contamination of tissues from blood that contained viral RNA.
Figure:
Note: Adapted from Solomon et al. Starting at the left: 1) Heat map showing age, sex, prior neurological comorbidities, neurological symptoms during hospitalization, and past medical history. 2) Bar plot showing days of symptoms prior to presentation and hospital course prior to death, with date of SARS-COV-2 NP RT-PCR indicated with black diamonds. 3) Heat map summarizing major gross and microscopic neuropathological findings and SARS-CoV-2 nucleocapsid protein immunohistochemistry results. Gross inspection showed atherosclerosis in 14 brain specimens but no acute stroke, herniation, or olfactory bulb damage; all patients showed hypoxic changes; immunohistochemical analysis did not detect SARS-CoV-2 in any of the specimens. CVD – cardiovascular disease; CKD – chronic kidney disease; ICU – intensive care unit; IHC – immunohistochemistry; HTN – hypertension; DM – diabetes mellitus; NP, nasopharyngeal swab. From NEJM. 383:989-992. DOI: 10.1056/NEJMc2019373. Copyright ©2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Implications of 3 studies (Benameur et al., Hann von Weyhern et al. & Solomon et al.): 3 case-series (from Atlanta, Boston, Munich) show a spectrum of central nervous system involvement related to COVID-19: from severe symptoms affecting cortical and brainstem functions (Benameur et al.) and pronounced CNS involvement with pan-encephalitis, meningitis and brainstem neuronal cell damage (Hann von Weyhern et al.) to not showing encephalitis or other specific brain changes attributable to the virus (Solomon et al.). Observational and randomized studies can guide treatment and prevention of COVID-19-related neurological complications.
PEER-REVIEWED
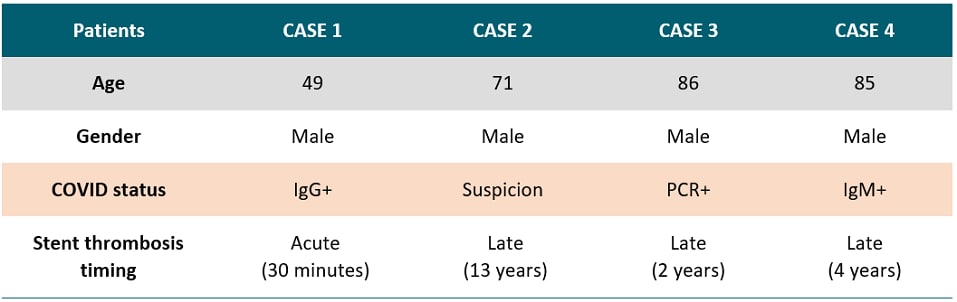
A case series of stent thrombosis during the COVID-19 pandemicexternal icon. Prieto-Lobato et al. Journal of the American College of Cardiology (JACC): Case Report (May 24, 2020)
Key findings:
- Four persons with confirmed (n=3) or suspected (n=1) COVID-19 with coronary artery stents developed stent thromboses.
- One patient developed acute thrombosis 30 minutes after stent placement.
- Three patients developed late thrombosis 2, 4 and 13 years after stent placement.
- This number of stent thromboses exceeded that observed for prior comparable periods.
Methods: Case series of stent thromboses, March-April 2020 in Spain. Limitations: Small sample size.
Implications: SARS-CoV-2 infection may lead to stent thrombosis likely due to COVID-19-associated hypercoagulability. During the COVID-19 pandemic, persons with stents should be counseled to immediately seek medical care if they develop chest pain or any symptoms like those for which the stent was placed.
Table:
Note: Adapted from Prieto-Lobato et al. Summary of four COVID-19 cases with stent thrombosis. Licensed under CC BY-NC-ND.
Inhibition of Bruton tyrosine kinase in patients with severe COVID-19external icon. Roschewski et al. Science Immunology (June 5, 2020).
A hyperinflammatory syndrome has been frequently reported among COVID-19 patients with severe disease and appears to contribute to acute respiratory distress syndrome (ARDS). Activated pulmonary macrophages are thought to play an important role in the pathogenesis of ARDS; inhibiting Bruton tyrosine kinase (BTK; the enzyme responsible for macrophage activation) is a potential mechanism to decrease the overactive inflammatory responses in these patients.
Key findings:
- Among patients with severe COVID-19 disease treated with acalabrutinib (BTK inhibitor), levels of C-reactive protein (an inflammatory marker) normalized and oxygen uptake improved in most patients (Figure).
- After treatment, COVID-19 disease did not reoccur in patients who could breathe independently.
Methods: Prospective clinical study of 19 severely ill COVID-19 patients with hypoxia (low blood oxygen) and evidence of inflammation and/or lymphopenia (reduced levels of lymphocytes) who received off-label acalabrutinib for 10-14 days to reduce inflammation associated with SARS-CoV-2 infection. Limitations: Small sample size; absence of control group.
Implications: BTK inhibitors represent a promising therapeutic option to control the hyperinflammation with severe COVID-19 disease. Optimal treatment timing and specificity of the inhibitor to target innate immune cells needs to be further studied in larger controlled clinical trials to ensure treatment does not interfere with B cell activation and development of antibodies against SARS-CoV-2.
Figure:
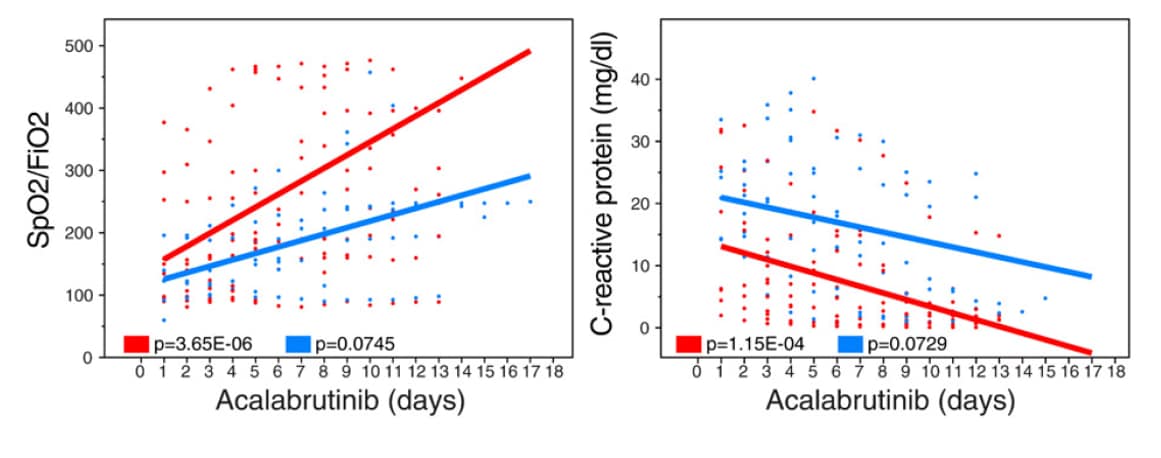
Note: Adapted from Roschewski et al. Oxygen uptake efficiency (SpO2/FiO2) and C-reactive protein levels in patients receiving supplemental oxygen or mechanical ventilation over the course of acalabrutinib treatment. Licensed under CC-BY.
PEER-REVIEWED
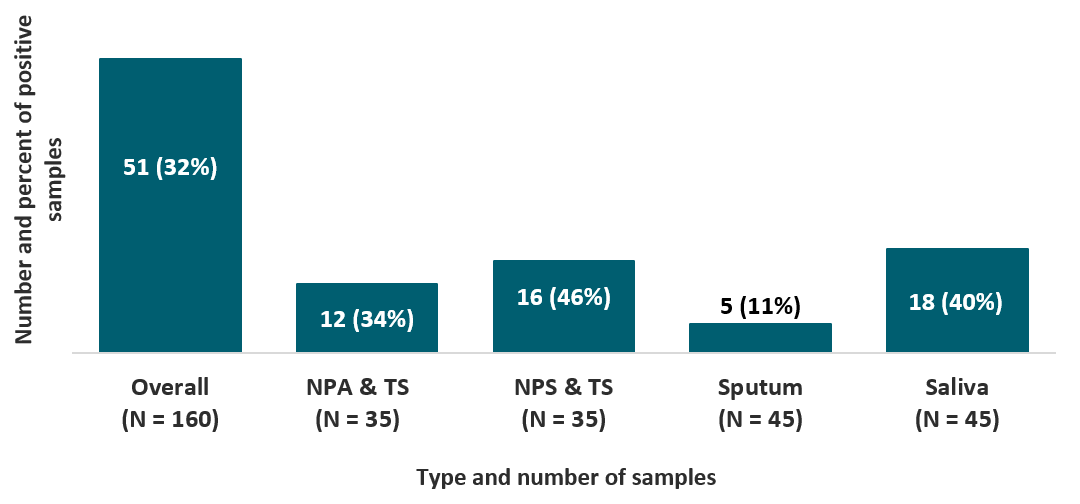
Evaluation of rapid antigen test for detection of SARS-CoV-2 virusexternal icon. Mak et al. Journal of Clinical Virology. (June 7, 2020).
Key findings:
- When compared to RT-PCR, the overall sensitivity of the BIOCREDIT COVID-19 antigen test was low (32%) and varied by type of respiratory sample (Figure).
- Sensitivity of the BIOCREDIT COVID-19 antigen test was higher for samples with high viral load (defined as mean cycle threshold value < 18.57) compared to samples with normal viral load (64% vs 10%, respectively).
Methods: Sensitivity of the BIOCREDIT COVID-19 antigen test (not FDA-approved) assessed to detect SARS-CoV-2 using various types of respiratory samples from 152 RT-PCR-confirmed patients. Limitations: Different processing for viscous samples; did not assess test performance in the field.
Implications: The BIOCREDIT COVID-19 antigen test is not sensitive enough to diagnose SARS-CoV-2.
Figure:
Note: Adapted from Mak et al. The sensitivity of BIOCREDIT COVID-19 antigen test, overall and by type of respiratory sample. Abbreviations: NPA& TS, nasopharyngeal aspirate and throat swab; NPS & TS, nasopharyngeal swab and throat swab. This article was published in Journal of Clinical Virology, Vol 129, Mak et al., Evaluation of rapid antigen test for detection of SARS-CoV-2 virus, Page 104500, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
- Sacks et al. Reconsidering risks of gun ownership and suicide in unprecedented timesexternal icon. NEJM. Editorial on the potential relationship between the recent surge in gun sales and risk of gun violence, given increased unemployment and social isolation.
- Abbasi, J. Anthony Fauci, MD, on COVID-19 vaccines, schools, and Larry Kramerexternal icon. JAMA. Discussion between Anthony Fauci and Howard Bauchner on a range of COVID-19- related topics, ranging from vaccine development to immunity passports.
- Mullard, A. COVID-19 vaccines start moving into advanced trialsexternal icon. Nature Reviews. Current landscape of leading COVID-19 vaccine candidates.
- Anonymous. Animals and coronavirus, help for African labs and a short flu seasonexternal icon. Nature. African countries priced out of the market for COVID-19 diagnostics access supplies through European and US collaborators.
- Gill J. The importance of proper death certification during the COVID-19 pandemicexternal icon. JAMA. Discussion of the importance of listing underlying medical disease and contributing comorbidities on death certificates during the COVID-19 pandemic.
- Duber et al. A novel approach to monitoring the COVID-19 pandemic using emergency department discharge diagnosesexternal icon. medRxiv. Provides insight into the use of COVID-like Illness (CLI) surveillance to predict the time between CLI emergency department (ED) visits and COVID deaths in King County, WA. CDC tracks trends of CLI in 47 states by capturing ED visit data through the National Syndromic Surveillance Program.
- Nagendra et al. The potential impact & availability of sexual health services during the COVID-19 pandemicexternal icon. Sexually Transmitted Diseases. Survey of sexual health clinicians on impact of COVID-19 on clinic services. CDC guidance for STD clinics and HIV prevention providers has focused on prioritizing patients with STD symptoms pdf iconand providing PrEP when in-person evaluation is limited, as well as encouraging health departments and community based organizations to supplement HIV prevention efforts with HIV self-testing programs.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.