COVID-19 Science Update released: June 18, 2021 Edition 94

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update align with the CDC Science Agenda for COVID-19.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Seroconversion rates following COVID-19 vaccination amongst patients with cancer.external icon Thakkar et al. Cancer Cell (June 5, 2021).
Key findings:
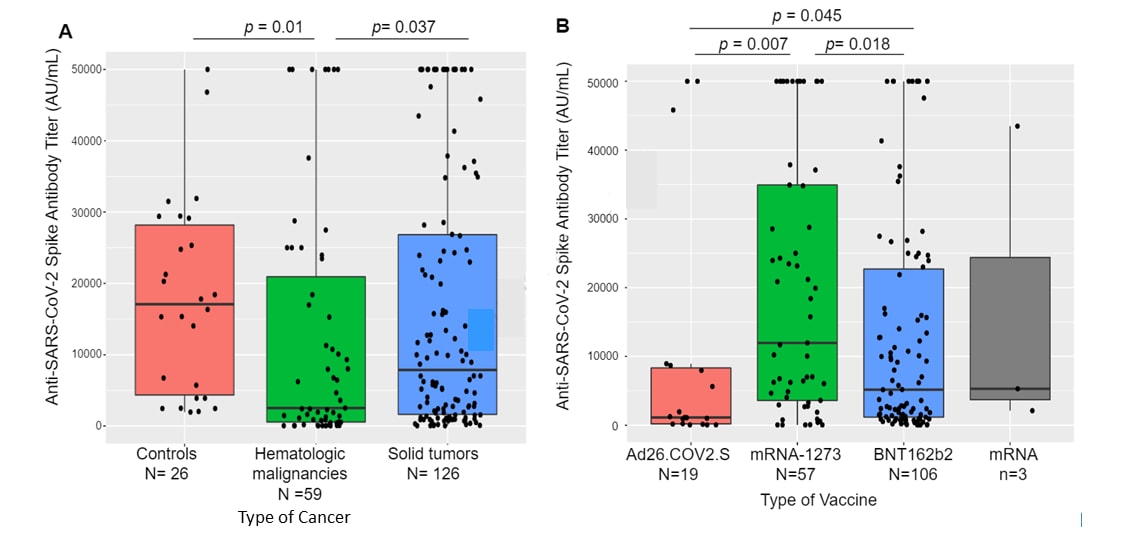
- 187/200 (94%) of cancer patients developed SARS-CoV-2 anti-spike protein antibodies, post-vaccination.
- Seroconversion rates for persons with solid tumors (98%) were significantly higher than those with hematologic cancers (85%), and especially those on highly immunosuppressive therapies (~70%).
- In a sub-group of 185 patients, anti-SARS-CoV-2 spike antibody titers were significantly lower for:
- Persons with hematological cancers versus solid tumors (Figure, left panel).
- Persons vaccinated with the Janssen (Johnson & Johnson) Ad26.COV2.S vaccine compared with an mRNA vaccine (Figure, right panel).
Methods: Cross-sectional study examining SARS-CoV-2 anti-spike protein antibody responses in 200 cancer patients in New York City >7 days following complete vaccination with Pfizer/BioNTech BNT162b2 (n = 115), Moderna mRNA1273 (n = 62), Janssen (Johnson & Johnson) Ad26.COV2.S (n = 20), or unspecified mRNA vaccine (n = 3). Antibody responses were compared to vaccinated controls without a cancer diagnosis (n = 26) and between cancer subtypes and treatments. Limitations: Underrepresentation of less common malignancies and overrepresentation of persons on active therapy.
Implications: Although most cancer patients vaccinated against COVID-19 seroconvert, persons with hematologic cancers and those on highly immunosuppressive therapies may have reduced antibody response and might benefit from an additional vaccine dose and continued non-pharmaceutical interventions such as masking and social distancing.
Note: Adapted from Thakkar et al. Box plots showing median (horizontal line) and the 25th and 75th quartiles of anti-spike protein antibody titers. A. Comparison of antibody titers of controls and persons with hematologic cancers or solid tumors. B. Comparison of antibody titers of persons vaccinated with Janssen (Johnson & Johnson) Ad26.COV2.S, Moderna mRNA-1273, Pfizer/BioNTech BNT162b2 or an unspecified mRNA vaccine. Reprinted from Cancer Cell, online June 5, 2021, Thakkar et al., Seroconversion rates following COVID-19 vaccination amongst patients with cancer. Copyright 2021, with permission from Elsevier.
Antibody response to the Janssen COVID-19 vaccine in solid organ transplant recipients.external icon Boyarsky et al. Transplantation (June 7, 2021).
Key findings:
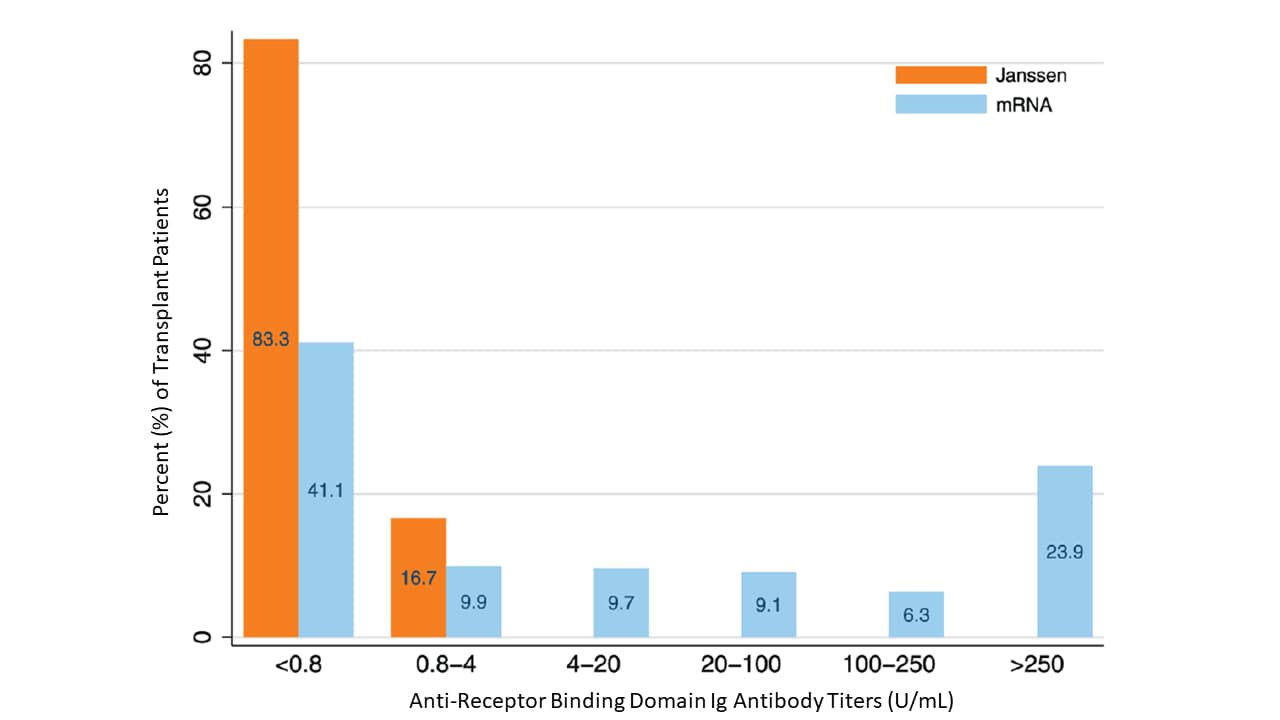
- Antibodies to SARS-CoV-2 receptor binding domain (RBD) were detectable in 17% of transplant recipients vaccinated with Janssen (Johnson & Johnson) Ad26.COV2.S and in 59% (p = 0.005) of those who completed an mRNA vaccine series.
- 83.3% of patients receiving Janssen (Johnson & Johnson) Ad26.COV2.S and 41.1% of those receiving an mRNA vaccine had anti-RBD antibody titers <0.8 Ig U/mL. (Figure).
Methods: SARS-CoV-2 RBD-specific antibody responses in solid organ transplant recipients, without prior COVID-19, who were fully vaccinated with Janssen (Johnson & Johnson) Ad26.COV2.S (n = 12) or an mRNA vaccine (n = 725), between December 2020 and March 2021 were compared approximately 1 month after the last vaccine dose. Limitations: Small sample size of Janssen (Johnson & Johnson) Ad26.COV2.S vaccine group.
Implications: The Janssen (Johnson & Johnson) Ad26.COV2.S vaccine may result in lower humoral immunity than mRNA vaccines in transplant patients, which might make SARS-CoV-2 mRNA vaccines a better option for these individuals.
Figure:
Note: Adapted from Boyarsky et al. SARS-CoV-2 anti-RBD antibody titers among solid organ transplant recipients fully vaccinated with Janssen (Johnson & Johnson) Ad26.COV2.S vaccine or an mRNA vaccine. Used by permission of Wolters Kluwer Health, Inc. and the Transplantation Society: Boyarsky et al., Antibody response to the Janssen COVID-19 vaccine in solid organ transplant recipients, Transplantationexternal icon.
Community-level evidence for SARS-CoV-2 vaccine protection of unvaccinated individuals.external iconMilman et al. Nature Medicine (June 10, 2021).
Key findings:
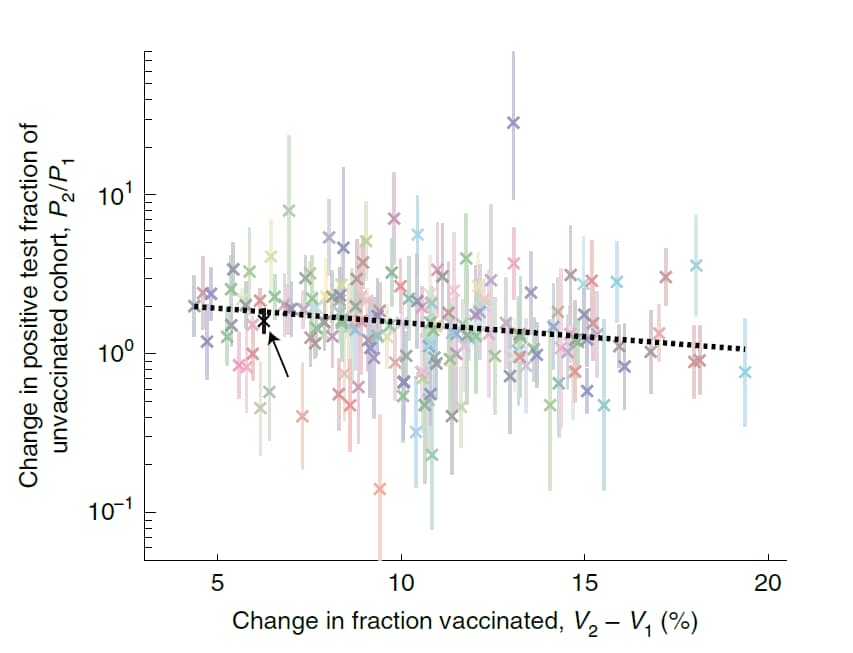
- High community vaccination rates against SARS-CoV-2 were associated with lower infection rates among unvaccinated persons aged <16 years within the same community (Figure).
- On average, for each 20-percentage point increase in the vaccination rate the positive test fraction for unvaccinated persons decreased on average 2-fold.
Methods: COVID-19 vaccination records (n = 1.37 million, Pfizer/BioNTech BNT162b2) and SARS-CoV-2 PCR test results (n = 2,137,063) from 177 Israeli communities between December 2020 and March 2021 were used to determine the positive test fraction among unvaccinated persons <16 years of age. Limitations: Study does not completely account for naturally acquired immunity, asymptomatic infection, or individual behavior.
Implications: SARS-CoV-2 vaccination provides cross-protection to unvaccinated individuals in the community.
Figure:
Note: Adapted from Milman et al. The change in the positive test fraction among unvaccinated persons <16 years of age between time intervals as a function of the change in the vaccinated fraction in corresponding vaccination intervals for each of the 177 communities. P1 = positive test fraction for 1st 3-week testing interval; P2 = positive test fraction for 2nd 3-week testing interval. V1 = mean cumulative fraction of individuals vaccinated with first vaccine dose for 1st vaccination interval; V2 = mean cumulative fraction of individuals vaccinated for 2nd vaccination interval. Each 3-week testing interval was matched with a corresponding 3-week vaccination interval that was time-shifted by 28 days. Data are the mean ± 1 standard error of mean. The dotted line shows linear fit (P = 0.0003). Example community is indicated by black x. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature. Nature Medicine, Community-level evidence for SARS-CoV-2 vaccine protection of unvaccinated individuals. Milman et al. COPYRIGHT 2021.
PREPRINTS (NOT PEER-REVIEWED)
Neutralizing antibodies elicited by the Ad26.COV2.S COVID-19 vaccine show reduced activity against 501Y.V2 (B.1.351), despite protection against severe disease by this variant.external icon Moore et al. bioRxiv (June 11, 2021).
Key findings:
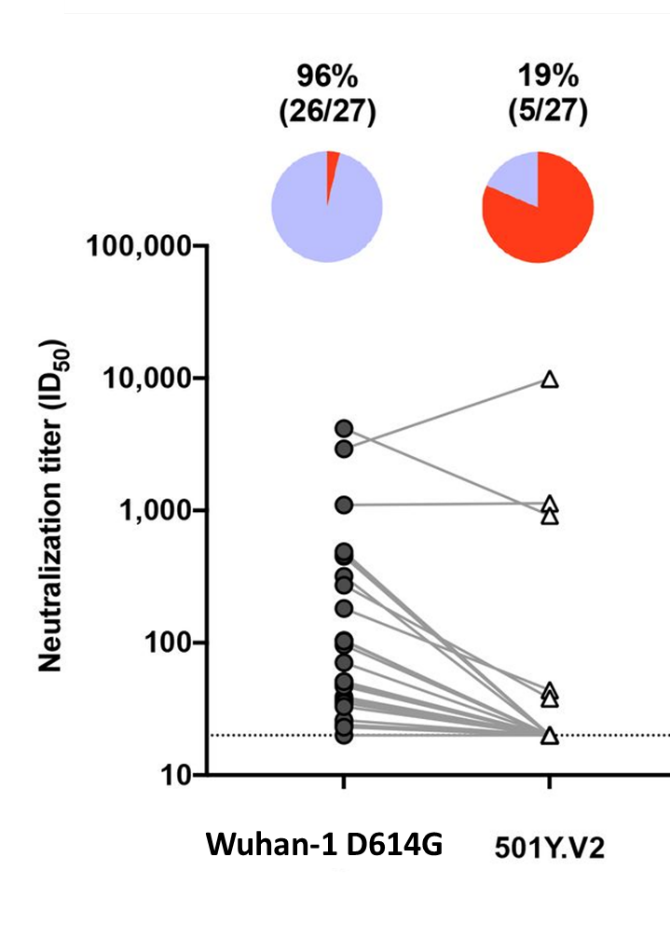
- Although binding to 501Y.V2 (B.1.351) was reduced by 1.8-fold compared to Wuhan-1 D614G, the Janssen (Johnson & Johnson) Ad26.COV2.S vaccine elicited cross-reactive binding antibodies between variants.
- 26/27 (96%) serum samples neutralized the Wuhan-1 D614G variant, but only 5 (19%) neutralized 501Y.V2 (B.1.351) (Figure).
Methods: 118 serum samples obtained in South Africa, including 88 vaccinee (Janssen (Johnson & Johnson) Ad26.COV2.S) and 30 placebo participants, were tested for neutralization capacity at Day 1 (pre-vaccination) and Day 29 (28 days post-vaccination). Neutralization of the original Wuhan-1 D614G and 501Y.V2 (B.1.351) variants were tested from a subset of 27 participants on Day 29. Limitations: Small sample size in subset.
Implications: Given Sadoff et alexternal icon. found Ad26COV2.S protected against severe COVID-19 including where there was a high prevalence of B.1.351, these data suggest that low levels of neutralizing antibodies and/or cellular immunity might contribute to protection against this variant.
Figure:
Note: Adapted from Moore et al. Neutralization titers of the original variant and 501Y.V2 (B.1.351). In the pie charts, purple indicates the proportion of samples with neutralization activity and red the proportion of samples with no detectable neutralization activity. The threshold of detection for the neutralization assay is ID50>20. Licensed under CC-BY-NC 4.0.
PEER-REVIEWED
Associations of vaccination and of prior infection with positive PCR test results for SARS-CoV-2 in airline passengers arriving in Qatar.external icon Bertollini et al. JAMA (June 9, 2021).
Key findings:
- Both vaccination and prior infection reduced the risk for SARS-CoV-2 PCR test positivity among airline passengers.
- 0.82% (95% CI 0.66%-1.01%) of vaccinated individuals and 1.01% (95% CI 0.80%-1.26%) of those with a prior infection tested positive.
- RR was 0.22 (95% CI 0.17-0.28) for those vaccinated and 0.26 (95% CI 0.21-0.34) for individuals with prior infection.
- 3.74%–3.81% of passengers without prior infection or vaccination tested positive.
- 0.82% (95% CI 0.66%-1.01%) of vaccinated individuals and 1.01% (95% CI 0.80%-1.26%) of those with a prior infection tested positive.
- Variants of concern found among arriving passengers were either B.1.351 (44.4%), B.1.1.7 (27.8%), or B.1.617 (11.1%).
Methods: All passengers arriving at Qatar’s Hamad International Airport between February 18 and April 26, 2021 (n = 261,849, 75.1% male) were tested for SARS-CoV-2 by PCR. PCR results were matched to national vaccination and health records and resulted in 17,786 persons with complete data (10,092 with record of vaccination or prior infection, 7,694 with no record of vaccination or prior infection). PCR positivity was compared after one-to-one matching by age, sex, nationality (>40 nationalities), and testing date. Sequencing was performed on 72 PCR positive specimens. Limitations: Prior infection history determined by records of positive PCR test results, so mild or asymptomatic cases largely missed; 99.7% of vaccinated individuals had been vaccinated with Pfizer/BioNTech BNT162b2; findings not generalizable to other airports, regions, or domestic travel.
Implications: Natural and vaccine-induced immunity were similar; however, screening passengers regardless of vaccine status or status of prior infection identified PCR positive cases.
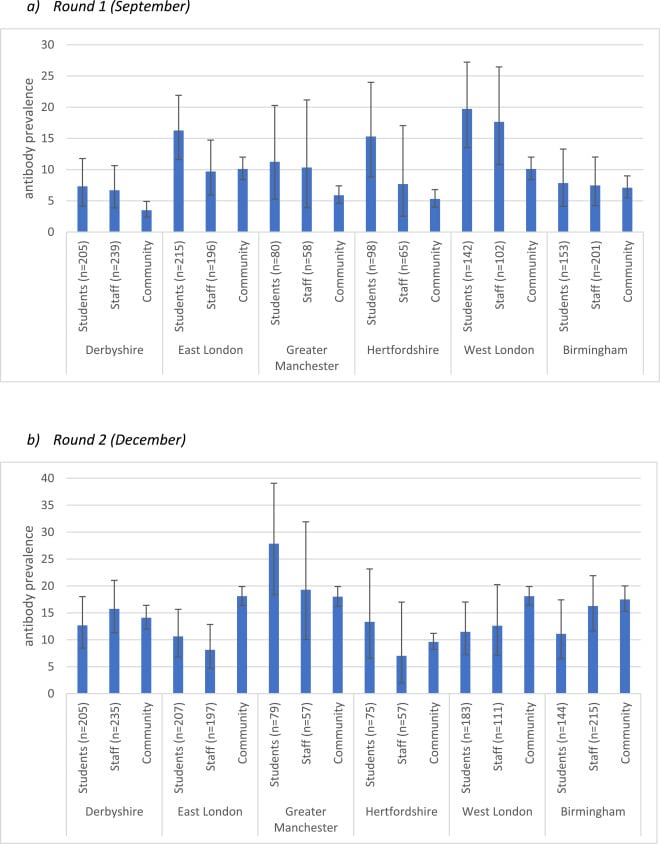
SARS-CoV-2 infection, antibody positivity and seroconversion rates in staff and students following full reopening of secondary schools in England: A prospective cohort study, September-December 2020.external icon Ladhani et al. EClinicalMedicine (June 9, 2021).
Key findings:
- SARS-CoV-2 seroprevalence was higher for students (12.8%) than for staff (9.2%) at the beginning of the term (September 2020) (Figure).
- Overall PCR positivity was 0.38% (7/1,824; 95% CI 0.1-0.79).
- Seroprevalence was similar for students (13.1%) and staff (13.3%) by end of term in December 2020 (Figure) and was comparable to community rates.
- Overall PCR positivity was 0.93 (17/1834; 95% CI 0.54-1.48).
- B.1.1.7 was not found in September, but the variant comprised 47% of sequenced strains by December.
Methods: SARS-CoV-2 surveillance study in staff and students following full reopening of 18 British secondary schools. PCR and SARS-CoV-2 antibody testing was done in round 1 (September 2020; 948 students and 877 staff) and round 2 (December 2020; 950 students and 852 staff). In round 2, 239 students and 128 staff did not return, and 341 students and 143 staff were newly enrolled. Four (57.1%) and 15 (88.2%) positive samples from rounds 1 and 2, respectively, were sequenced. Limitations: Selection bias to regions with study staff; not known if confirmed infections occurred within or outside of school; did not measure antibodies to spike proteins.
Implications: SARS-CoV-2 infection, seropositivity, and seroconversion rates were similar among students and staff in secondary schools and comparable to community rates by the end of the term. Ongoing surveillance will be important for monitoring the impact of new variants in educational settings.
Figure:
Note: Adapted from Ladhani et al. Shown are SARS-CoV-2 antibody seroprevalence (y-axis) for students, staff, and surrounding community in 6 regions (x-axis) at the start and end of the autumn term. (a) Round 1 (September): specimens collected from 17 Sept to 14 Oct 2020. (b) Round 2 (December): specimens collected from 10 Dec 2020 to 6 Jan 2021. Error bars represent 95% CIs. Licensed under CC-BY-NC-ND.
PEER-REVIEWED
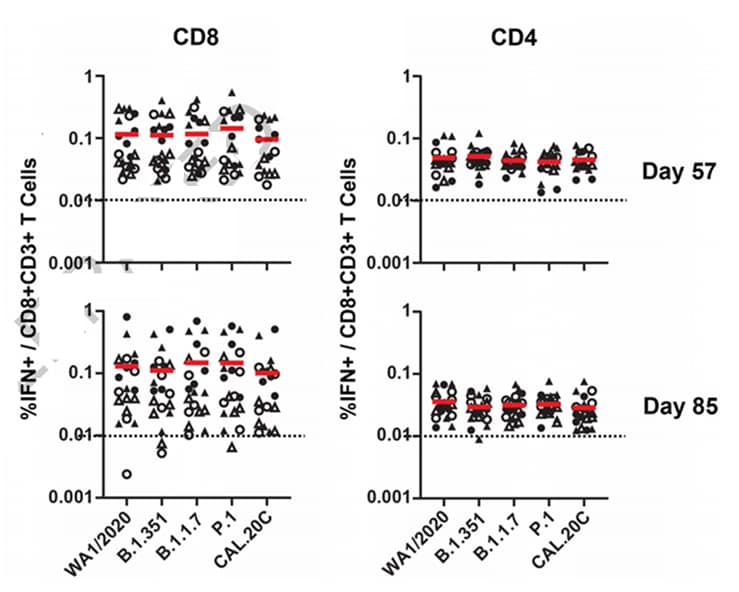
Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans.external icon Alter et al. Nature (June 9, 2021).
Key findings:
- Neutralizing antibodies produced in response to a single dose of Janssen (Johnson & Johnson) Ad26.COV2.S vaccine were 5.0- and 3.3-fold lower for B.1.351 and P.1, respectively, than for WA1/2020.
- CD8 and CD4 T cell responses were comparable for WA1/2020, B.1.1.7, B.1.351, P.1, and CAL.20C when assessed 57 and 85 days post vaccination (Figure).
Methods: A randomized, double-blind, placebo-controlled phase 1/2 trial (n = 20) to assess antibody and cellular responses against the SARS-CoV-2 (WA1/2020 strain, B.1.351, B.1.1.7, P.1 and CAL.20C variants) after Janssen (Johnson & Johnson) Ad26.COV2.S vaccination. Antibody responses were assessed 57 and 71 days post vaccination and cellular immune responses were assessed 57 and 85 days post vaccination. Limitations: Small sample size.
Implications: Neutralizing antibody responses elicited by Janssen (Johnson & Johnson) Ad26.COV2.S were reduced against the B.1.351 and P.1 variants, but other functional antibody and T-cell responses were largely preserved against these variants.
Figure:
Note: Adapted from Alter et al. Cellular immune responses to SARS-CoV-2 variants. S-specific pooled peptide IFN-γ CD4+ and CD8+ T cell responses by intracellular cytokine staining (ICS) assays (y-axis) against WA1/2020, B.1.351, B.1.1.7, P.1, and CAL.20C (x-axis). Red bars are medians. Dotted lines are lower limits of quantitation. Half received 5×1010 vp (triangles), half, 1×1011 vp (circles). Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature. Nature, Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Alter et al. COPYRIGHT 2021.
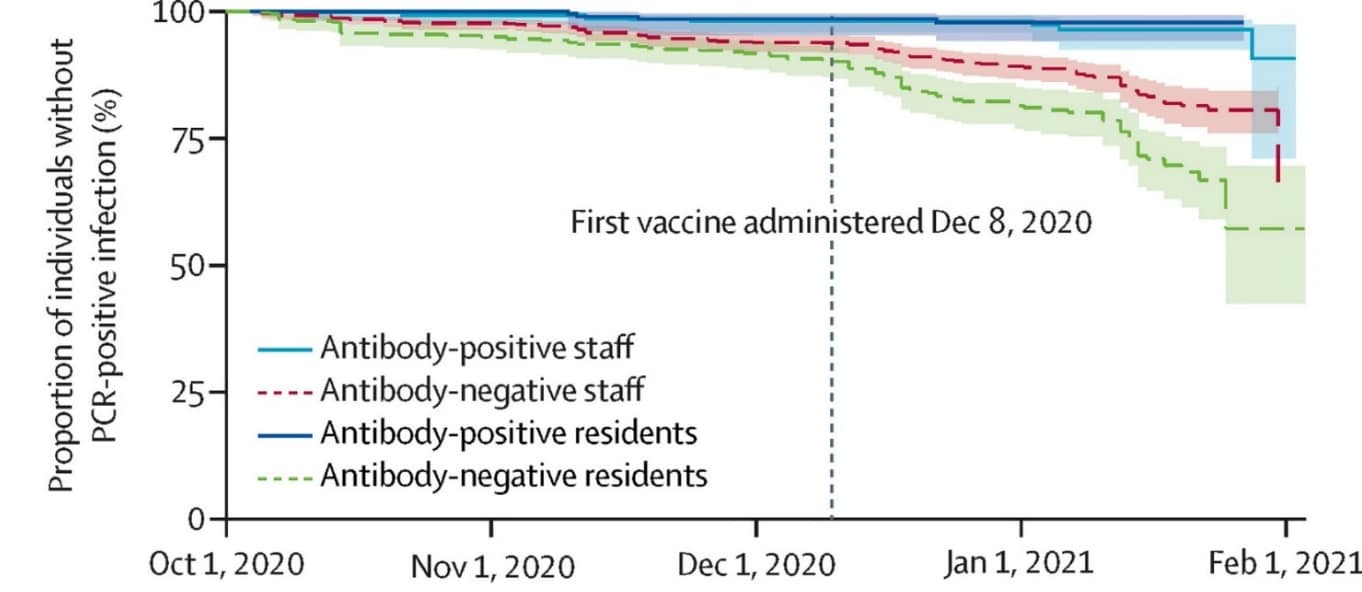
Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): a prospective cohort study.external icon Krutikov et al. The Lancet Healthy Longevity (June 1, 2021).
Key findings:
- SARS-CoV-2 antibodies were detected at baseline in 29% of staff and 33% of residents of long-term care facilities (LTCF).
- Compared to those antibody-negative at baseline, risk for PCR-positive infection was significantly lower among staff (adjusted hazards ratio [aHR] 0.39, 95% CI 0.19-0.82) and residents (aHR 0.15, 95% CI 0.05-0.44) who were antibody-positive.
- After vaccination roll-out, the proportion of residents and staff testing positive for SARS-CoV-2 declined more rapidly than before vaccination (Figure).
- At 10 months follow-up only 14 (2%) PCR-positive reinfections were identified among staff and residents who were antibody-positive at baseline; none required hospitalization.
Methods: Prospective cohort study of risk of SARS-CoV-2 infection by baseline antibody status among 1,429 staff and 682 residents of LTCFs in England between October 1, 2020 and February 1, 2021. HR for PCR-positive infection were estimated by baseline antibody status, stratified by LTCF, and adjusted for age and sex. Limitations: Small sample size; did not evaluate comorbidities.
Implications: Antibodies to SARS-CoV-2 were associated with reduced reinfection risk in LTCF staff and residents; natural immunity coupled with vaccination may afford long-term protection against infection in this vulnerable population.
Figure:
Note: Adapted from Krutikov et al. Cumulative probability of PCR-confirmed infection over time and by antibody status cohort: antibody-positive staff, antibody-negative staff (dashed lines), antibody-positive residents, and antibody-negative residents (dashed lines). Antibody status was based on baseline testing on October 1, 2020. Licensed under CC-BY-NC-ND.
Detection, Burden, and Impact
- Oliveira et al. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database.external icon Lancet Child & Adolescent Health (June 10, 2021). Among 11,613 children and adolescents hospitalized between January 2020 and February 2021 with laboratory-confirmed SARS-CoV-2, 886 (7.6%) died. Risk of death was highest in those < 2 years (adjusted hazards ratio (aHR) 2.36; 95% CI 1.94-2.88); 12–19 years (aHR 2.23; 95% CI 1.84-2.71); and indigenous ethnicity (aHR 3.36; 95% CI 2.15-5.24).
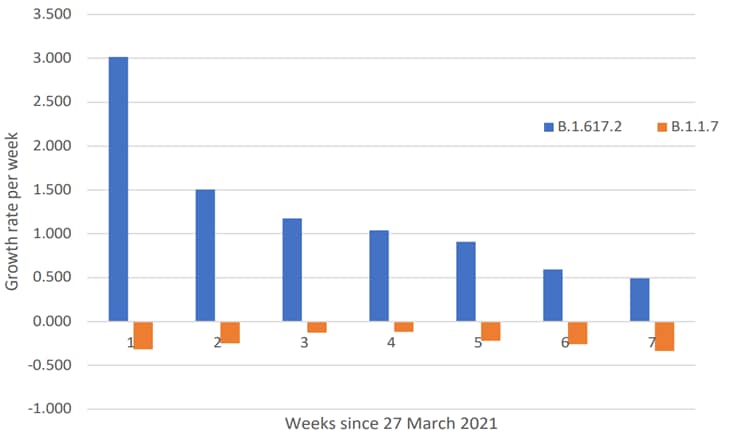
- Dagpunar et al. Interim estimates of increased transmissibility, growth rate, and reproduction number of the COVID-19 B.1.617.2 variant of concern in the United Kingdom.external icon medRxiv (Preprint; June 3, 2021). Models based on COVID-19 data in England found that reproduction numbers were higher for B.1.617.2 (1.17–1.51) compared to B.1.1.7 (0.703–0.820), and B.1.617.2 showed increased transmissibility (43–115%) over the study period. Weekly growth rates were positive (increasing) for B.1.617.2 and negative (decreasing) for B.1.1.7.
Note: Adapted from Dagpunar et al. Estimated weekly growth rates for B.1.617.2 and B.1.1.7 strains for the 7 weeks between March 27 and May 15, 2021. Licensed under CC-BY-ND 4.0.
- Hernandez et al. Molecular evidence of SARS-CoV-2 in New York before the first pandemic wave.external icon Nature Communications (June 8, 2021). A molecular analysis of 3,040 respiratory-pathogen-negative nasopharyngeal specimens collected at the beginning of 2020 indicate that SARS-CoV-2 cases had presented at New York City hospitals as early as January 25. Community transmission was well underway by the time of the first officially diagnosed case, February 29, 2020. The earlier cases were the B.1 lineage common in Europe.
- Parrott et al. Prevalence of SARS-CoV-2 antibodies in New York City adults, June–October, 2020: a population-based survey.external icon Journal of Infectious Diseases (June 4, 2021). In a survey of 1,074 adults conducted between June and October 2020, weighted SARS-CoV-2 antibody prevalence was 24.3% overall (95% CI 20.7%-28.3%). Latino (30.7%, 95% CI 24.1%-38.2%) and Black (30.7%, 95% CI 21.9%-41.2%) respondents had a higher weighted prevalence compared with White respondents (17.4%, 95% CI 12.5%-23.7%).
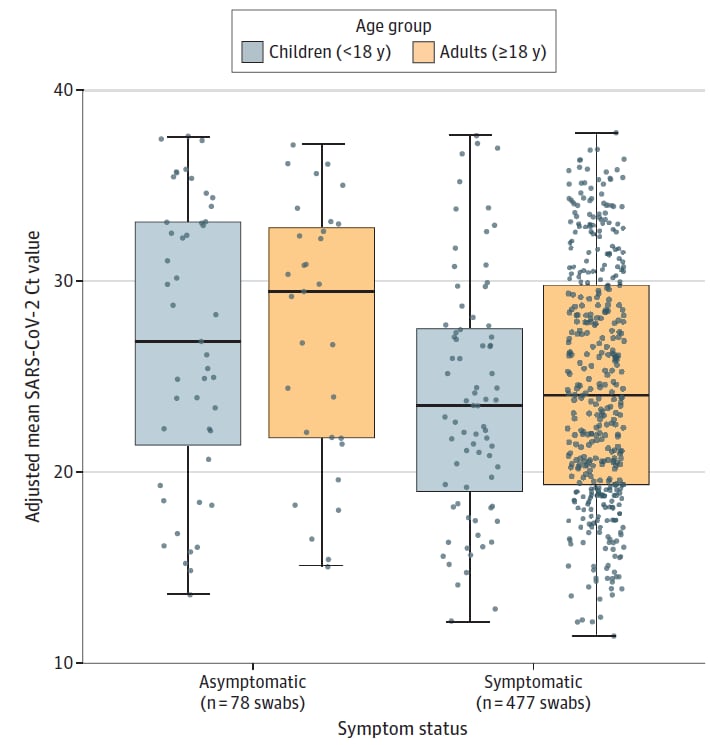
- Chung et al. Comparison of symptoms and RNA levels in children and adults with SARS-CoV-2 infection in the community setting.external icon JAMA Pediatrics (June 11, 2021). In a sample of 555 SARS-CoV-2 positive individuals in King County, WA, symptomatic individuals had lower Ct values than asymptomatic individuals (adjusted differences: children −3.0; 95% CI −5.5 to −0.6; adults −2.9; 95% CI −5.2 to −0.6). There were no differences in mean Ct values between children and adults for either symptomatic or asymptomatic individuals.
Note: Adapted from Chung, et al. Adjusted mean SARS-CoV-2 Orf1b cycle threshold (Ct) values by age group, grouped by symptom status. Boxes range from 1st to 3rd quartiles. Midlines are medians. Error bars are min and max values. Individual points are SARS-CoV-2+ individuals. 47 asymptomatic and 76 symptomatic children, 31 and 401 symptomatic asymptomatic adults. Ct values adjusted for swab type. Reproduced with permission from JAMA Pediatrics, 2021. Published online June 11, 2021. https://doi.org/10.1001/jamapediatrics.2021.2025. Copyright© 2021 American Medical Association. All rights reserved.
Prevention, Mitigation, and Intervention Strategies
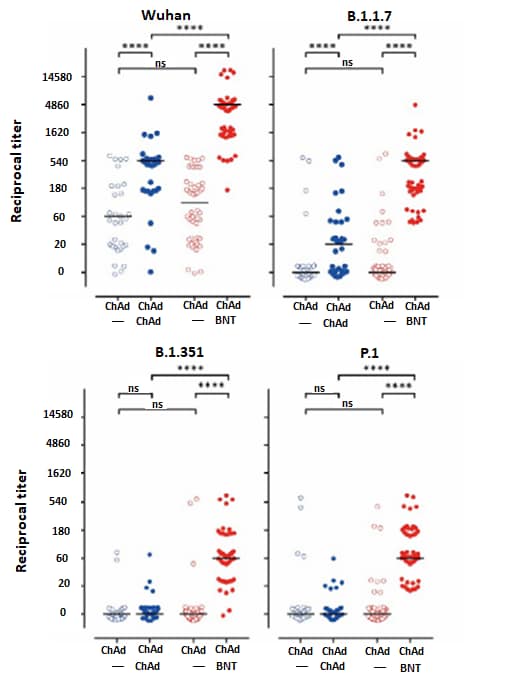
- Barros-Martins et al. Humoral and cellular immune response against SARS-CoV-2 variants following heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination.external icon medRxiv (Preprint; June 3, 2021). Published in Nature Medicine as Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccinationexternal icon (July 14, 2021). Among 129 healthcare professionals primed with Oxford/AstraZeneca ChAdOx1, both ChAdOx1 (n = 32) and Pfizer/BioNTech BNT162b2 (n = 55) boosters induced higher immunity; BNT162b2 induced higher frequencies of spike-specific CD4 and CD8 T cells and neutralizing antibodies against the B.1.1.7, B.1.351, and the P.1 variants of SARS-CoV-2.
Note: Adapted from Barros-Martin et al. Reciprocal titers of neutralizing antibodies against Wuhan, B.1.1.7, P.1, and B.1.351 SARS-CoV-2-S variants measured using surrogate virus neutralization test. Homologous ChAD/ChAD = blue symbols (2nd column); heterologous ChAD/BNT = red symbols (4th column). ChAd = Janssen (Johnson & Johnson) Ad26.COV2.S vaccine; BNT = Pfizer/BioNTech BNT162b2 vaccine. Used by permission of authors; associated manuscript under embargo until publication.
- Orsi et al. Outbreak of SARS-CoV-2 lineage 20I/501Y.V1 in a nursing home underlines the crucial role of vaccination in both residents and staff.external icon Vaccines (June 2, 2021). Following an outbreak of SARS-CoV-2 in Italy, in which all residents but 1 (19/20) were fully vaccinated with Pfizer/BioNTech BNT162b2, 70% of residents tested positive by RT-PCR. 13/14 positive residents were asymptomatic. The symptomatic resident (age 86) died approximately 2 weeks after testing positive. Sequencing revealed the 20I/501Y.V1 lineage (B.1.1.7) in 6 samples including from the resident who died.
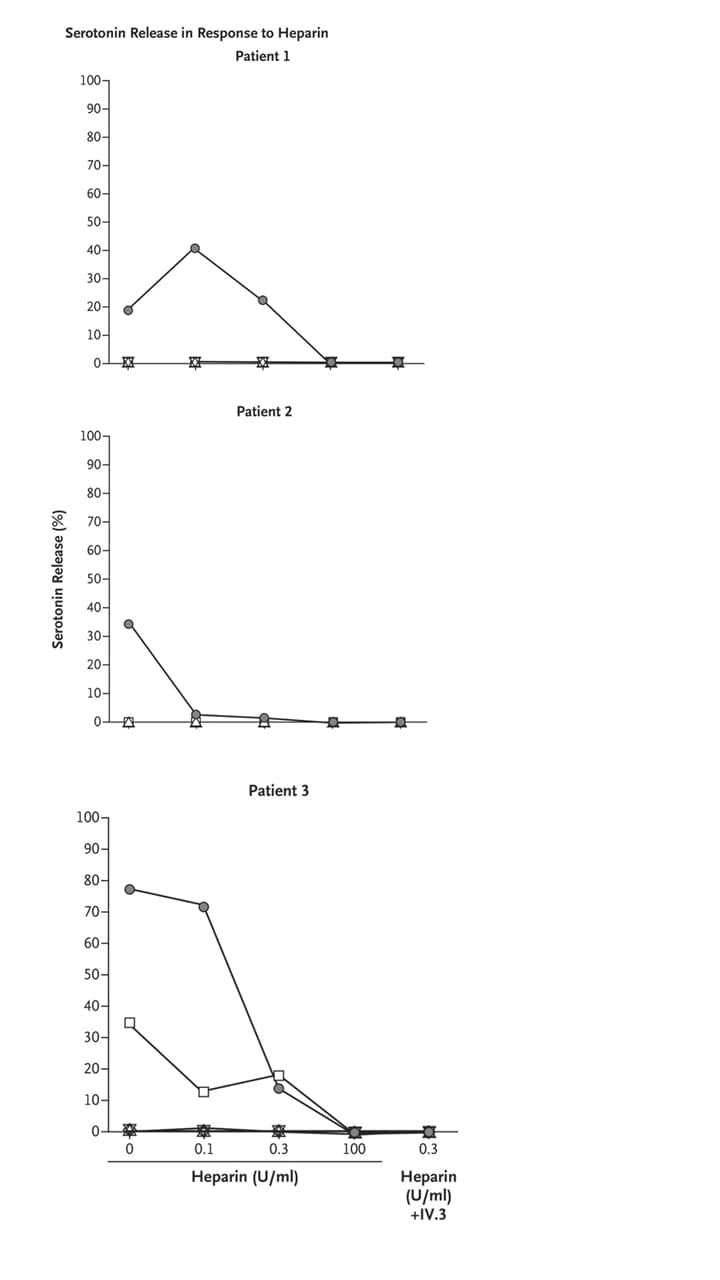
- Bourguignon et al. Adjunct immune globulin for vaccine-induced thrombotic thrombocytopenia.external icon NEJM (June 9, 2021). Three patients (aged 63, 69 and 72 years) who developed vaccine-induced immune thrombotic thrombocytopenia (VITT) 7–18 days after vaccination with ChAdOx1 nCoV-19 (Covishield) were successfully treated with high-dose intravenous immune globulin (IVIG), which reduced antibody-induced platelet activation. VITT Abs were monitored with a serotonin-release assay adapted from heparin-induced thrombocytopenia use.
Note: Adapted from Bourguignon et al. Serotonin release in response to heparin. Results of a conventional platelet activation assay for heparin-induced thrombocytopenia (a serotonin-release assay) in the 3 study patients. Platelet activation was inhibited in serum obtained from the 3 patients after treatment with IVIG. Circles = before IVIG; squares = 1st dose IVIG; Upward triangle = 2nd dose; downward triangle = 3rd dose; diamond = 4th dose. From the New England Journal of Medicine, Bourguignon et al., Adjunct immune globulin for vaccine-induced thrombotic thrombocytopenia. June 9, 2021, online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Social, Behavioral, and Communication Science
- Hu et al. Revealing public opinion towards COVID-19 vaccines with Twitter data in the United States: a spatiotemporal perspective.external icon medRxiv (Preprint; June 7, 2021). Published in the Journal of Medical Internet Researchexternal icon (September 10, 2021). An analysis of 300,000 geotagged tweets in the US between March 2020 and February 2021 reflected rising public confidence and anticipation towards COVID vaccines. Trending social media topics and announcements by political leaders could sharply influence public opinion towards vaccines, although usually temporarily.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.