COVID-19 Science Update released: May 8, 2020 Edition 11

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Bostonexternal icon. Baggett et al. JAMA (April 27, 2020).
Key findings:
- 0% (147/408) of homeless shelter residents had positive RT-PCR test results for SARS-CoV-2.
- Among the 147 residents with positive RT-PCR test results, 87.8% were asymptomatic; cough was the most common symptom reported (7.5%) (Figure).
Methods: Descriptive study of SARS-CoV-2 infection and symptoms in a large homeless shelter in Boston, April 2020. NP swabs were tested by RT-PCR. Limitations: Single shelter; some symptomatic persons were removed from analysis by prior symptom screening or self-referral to outside care.
Implications: Asymptomatic persons in homeless shelters should also be tested because symptom screening alone may miss a substantial portion of infections.
Figure:
Note: Adapted from Baggett et al. Prevalence of key symptoms among 147 homeless shelter residents who had positive RT-PCR test results for SARS-CoV-2 infection. Reproduced with permission from JAMA. doi:10.1001/jama.2020.6887. Copyright©2020 American Medical Association. All rights reserved.
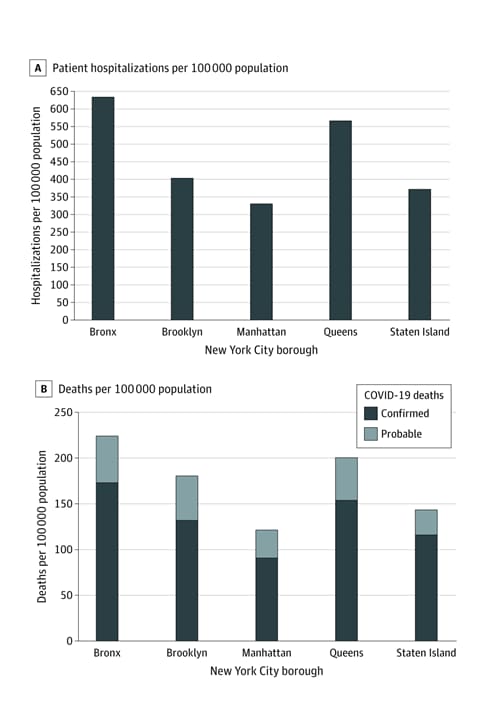
Variation in COVID-19 hospitalizations and deaths across New York City boroughsexternal icon. Wadhera et al. JAMA (April 29, 2020).
Key findings:
- Differences in COVID-19 hospitalization and death rates were observed across the 5 New York City (NYC) boroughs
- Highest rates in the Bronx, which has the highest proportion of racial/ethnic minorities and poverty, and the lowest levels of educational attainment.
- Lowest rates in Manhattan, which is the most affluent borough and is predominately white.
- Other factors, including underlying comorbid illnesses, occupational exposures, and socioeconomic determinants, may explain observed differences.
Methods: An ecological study of COVID-19 hospitalization and death rates among five NYC boroughs. Information on population characteristics and hospital bed capacity was obtained from the 2018 American Community Survey and the American Hospital Association 2016 file, respectively. Limitations: Ecological design.
Implications: In the 5 NYC boroughs, increased rates of COVID-19 hospitalization and death have occurred among populations with lower incomes, lower levels of educational attainment, and a higher proportion of racial/ethnic minorities. Further research is warranted to ascertain whether similar differences exist elsewhere in the US.
Figure:
Note: Adapted from Wadhera et al. Rates of COVID-19 hospitalizations (A) and deaths (B), by New York City borough. Reproduced with permission from JAMA. doi:10.1001/jama.2020.7197. Copyright©2020 American Medical Association. All rights reserved.
Remdesivir has been shown in vitro (i.e., in laboratory settings) to inhibit the growth of SARS-CoV-2. However, studies in humans have been limited. There are ongoing randomized clinical trials (RCT) examining the efficacy of remdesivir in treating patients with COVID-19. Results from many of these studies are not yet available. Some emerging results from existing studies are summarized below.
Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trialexternal icon. Wang et al. Lancet (April 29, 2020; Correctionexternal icon on May 30, 2020).
Key findings:
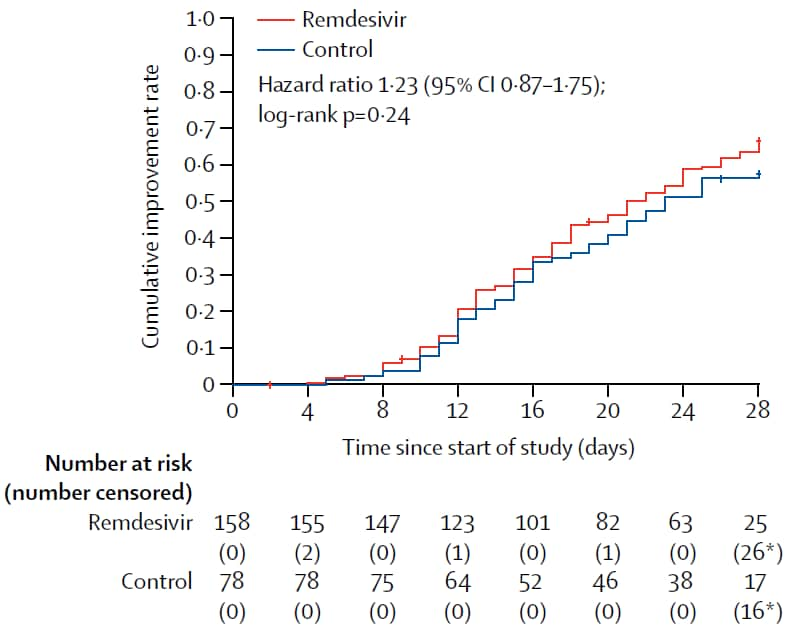
- Use of remdesivir was not associated with clinical improvement (hazard ratio (HR): 1.23, 95% CI 0.87-1.75) (Figure 1).
- Of those who received treatment <10 days after illness onset, time to clinical improvement was faster among those using remdesivir; however, the difference between groups was not statistically significant (HR 1.52, 95% CI 0.95-2.43).
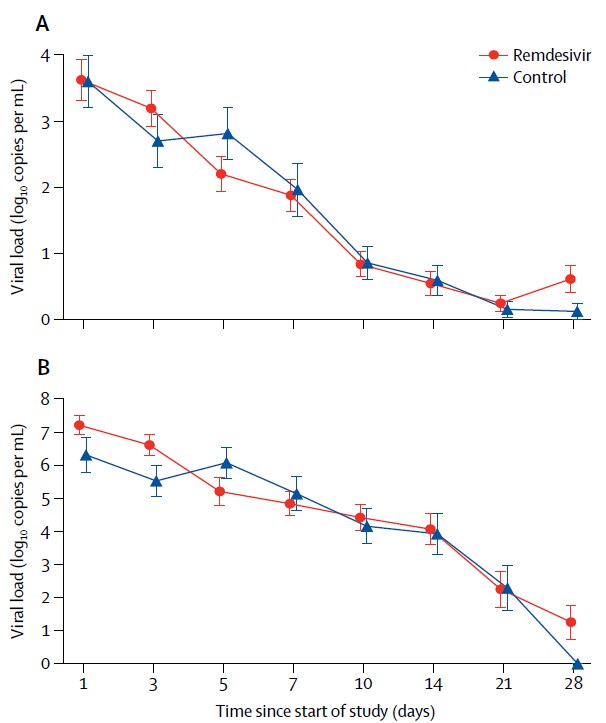
- There were no differences in viral load at baseline, or over time, between those using remdesivir and those not using remdesivir (Figure 2).
- Adverse events were reported among 66% of patients using remdesivir and 64% of patients not using remdesivir.
- Enrollment was stopped before reaching the target sample size (n = 453).
Methods: Double-blinded RCT examining the efficacy of remdesivir in treating COVID-19. Patients aged ≥18 years presenting to one of ten different hospitals in Hubei, China with COVID-19 pneumonia, and ≤94% oxygen saturation or a ratio of arterial oxygen partial pressure to fractional inspired oxygen of ≤300 mm Hg, were enrolled ≤12 days after illness onset. Patients were randomized to intravenous remdesivir (200 mg the first day, 100 mg the next 9 days) or a placebo in a 2:1 ratio (treatment arm: n = 158; placebo arm: n = 78); an equal proportion of patients needing oxygen support were randomized to the treatment and placebo arms. All patients could take other experimental treatments, including lopinavir-ritonavir, interferon, and corticosteroids. Patients were followed for 28 days and monitored for time to improvement of clinical symptoms. Serially collected respiratory samples were tested for viral RNA. Limitations: Study may have been underpowered; imbalances in clinical presentation between groups at enrollment; delays in initiating remdesivir may have impacted findings.
Implications: Although use of remdesivir was not associated with improved clinical symptoms, the study did not reach the target sample size; additional randomized controlled trials are needed to assess the efficacy of remdesivir as a treatment for COVID-19.
Figure 1
Figure 2
Note: From Wang et al. Figure 1. Kaplan-Meier plot showing time to clinical improvement by treatment arm; log-rank test showed there were no differences in clinical improvement by treatment group (p = 0.24). Figure 2. There were no differences in viral load measurements taken from the upper respiratory tract (A) and lower respiratory tract (B) over time by treatment arm. Available via Elsevier COVID-19 Resource Centre through PubMed Central.
PEER-REVIEWED
In a recent editorial, Norrie (Remdesivir for COVID-19: Challenges of underpowered studies, Lancetexternal icon) acknowledged the limitations of Wang et al, including that it did not have a sufficient sample size to detect differences between groups. He encourages the scientific community to compile evidence from other studies to make an informed decision about the efficacy and safety of remdesivir in treating COVID-19 instead of basing clinical treatment and management guidance on a single study.
NEWS RELEASE (NOT PEER-REVIEWED)
NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19: According to a recent news release, preliminary data from a larger clinical trial, the Adaptive COVID-19 Treatment Trialexternal icon, demonstrated that recovery times among 1,063 hospitalized COVID-19 patients with mostly severe disease were 31% shorter (11 days vs 15 days; p <0.001). Mortality was lower among those who received remdesivir than those who did not (8.0% vs 11.6%), but the difference was not statistically significant (p = 0.059).
PEER-REVIEWED
Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitalsexternal icon. Liu et al. Nature (April 27, 2020).
Key findings:
- In patient areas, the concentration of aerosolized SARS-CoV-2 RNA was higher in unventilated spaces than ventilated spaces; it was high in a small unventilated patient bathroom (1 m3).
- In some areas accessed by medical staff, concentrations of aerosolized SARS-CoV2 RNA were higher, especially where staff removed personal protective equipment in hospital #2.
- RNA-evidence of SARS-CoV-2 became undetectable after thorough sanitation procedures were implemented.
- Concentrations of SARS-CoV-2 particles were low in most public areas, except for 2 high-traffic areas: outside a department store and outside hospital #1.
Methods: During February and March 2020, authors took air samples from 30 sites in public areas and 2 hospitals treating COVID-19 patients. Hospital #1 was designated to treat more severe COVID-19 patients, and hospital #2 was a “makeshift” healthcare setting repurposed from a large multipurpose facility. Samples were taken from areas: (1) where patients were treated, (2) only accessed by medical staff who were exposed to COVID-19 patients, and (3) open to the public, such as inside the hospital pharmacy and outside the hospital. Authors determined aerosolized concentration of SARS-CoV-2 RNA using droplet digital PCR-based detection. Limitations: Concentration of viral RNA may not represent infectivity; no cultures were performed, so it is unclear whether RNA detected was from active virus; the relationship between viral particle size and infectivity is unknown; authors could not establish whether aerosolized SARS-CoV-2 was resuspended from surrounding fomites, other patients or staff, or remained in the air for long periods of time.
Implications: Viral RNA was detected in patient areas, medical staff areas, and public areas, but concentrations were relatively low and viral infectivity was not established. This study does not conclusively demonstrate that airborne transmission can occur, but suggests that transmission risk may be limited through ventilation and sanitation of frequently used areas (e.g., toilets), social distancing, and use of masks in public.
Feasibility of controlling COVID-19 outbreaks by isolation of cases and contactsexternal icon. Hellewell et al. Lancet Global Health (February 28, 2020).
Key findings:
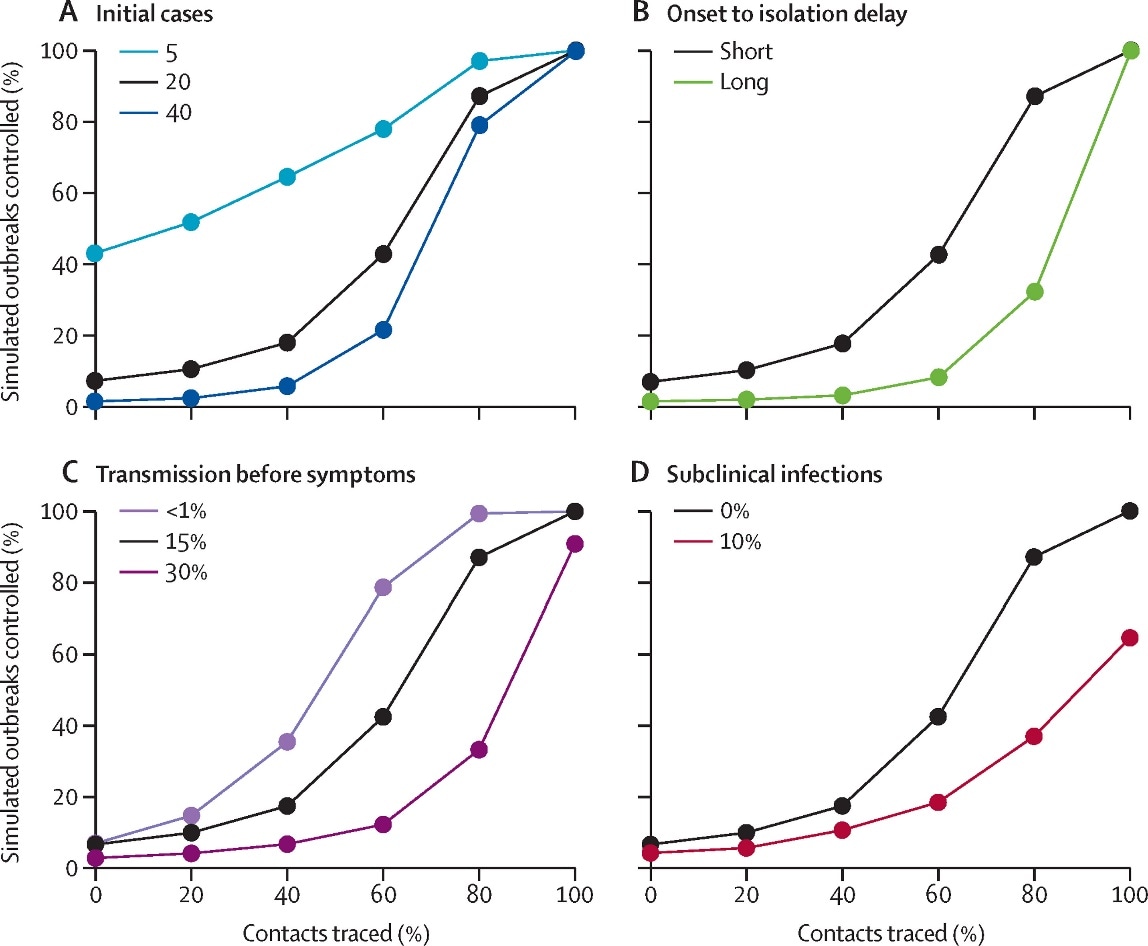
- Using mathematical modeling, the probability of controlling outbreaks decreased when there was a high number of initial cases, higher basic reproduction number (R0), and more transmissions before symptom onset. (Figure)
- The delay from symptom onset to isolation played a major role in controlling the outbreaks.
- Case isolation was more effective when there was little transmission before symptom onset and the delay from symptom onset to isolation was short.
Methods: A mathematical modeling study to assess effectiveness of contract tracing and case isolation to control outbreaks of COVID-19 in scenarios that varied in the number of initial cases, the level of R0, the delay from symptom onset to isolation, the probability that contacts were traced, the proportion of transmissions that occurred before symptom onset, and the proportion of subclinical infections. Outbreak control was defined as no new infections between 12 and 16 weeks after the initial cases. Limitations: Model assumed that isolation prevented all further transmission.
Implications: In most plausible outbreak scenarios, case isolation and contact tracing alone are insufficient to control outbreaks. However, effective contact tracing and isolation can reduce the overall size of an outbreak or bring it under control over time.
Figure:
Note: From Hellewell et al. Achieving control of simulated outbreaks under different transmission scenarios. The percentage of outbreaks controlled for the baseline scenario, and varied number of initial cases (A), time from onset to isolation (B), percentage of transmission before symptoms (C), and proportion of subclinical (asymptomatic) cases (D). The baseline scenario is a basic reproduction number (R0) of 2.5, 20 initial cases, a short delay to isolation, 15% of transmission before symptom onset, and 0% subclinical infections. A simulated outbreak is defined as controlled if there are no cases between weeks 12 and 16 after the initial cases. Licensed under CC-BY-NC-ND.
Renin-angiotensin-aldosterone system inhibitors, or RAAS inhibitors, are a class of medications commonly used to reduce high blood pressure (hypertension). Medications in this class work as different steps of the RAAS, a system that affects blood vessel size and blood volume. Examples of different types of RAAS-inhibitors include angiotensin-converting-enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARB), beta-blockers, calcium channel blockers, and thiazide diuretics. Several studies have recently assessed the impact of RAAS inhibitors on COVID-19 outcomes, which are summarized below.
PEER-REVIEWED
A. Renin–angiotensin–aldosterone system inhibitors and risk of COVID-19external icon. Reynolds et al. NEJM (May 1, 2020).
Key findings: Among patients treated with any of five commonly used classes of blood pressure reducing medications (RAAS inhibitors), none of the medications were significantly associated with the likelihood of SARS-CoV-2 infection (Figure 1), or with illness severity for confirmed COVID-19 patients (Figure 2).
Methods: Retrospective cohort study among 12,594 patients tested for SARS-CoV-2 with available electronic health records (EHR) in the NYC Langone health system, March 1 – April 15, 2020. Investigators abstracted EHR to assess patient use of five common blood pressure reducing medications (ACE-inhibitor, ARB, beta-blocker, calcium channels blocker, thiazide diuretic) in the past 18-months and examined the association between use of these medications and: (1) COVID-19 occurrence, and (2) severity among persons infected with COVID-19, controlling for hypertension. Patients were matched based on their probability of using each medication. Limitations: Possibility of false negative COVID-19 test results.
Figure 1
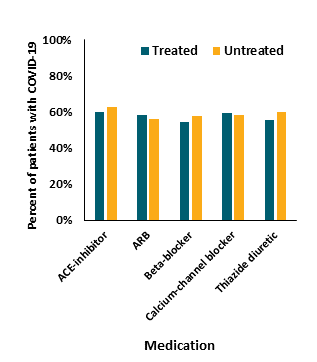
Figure 2
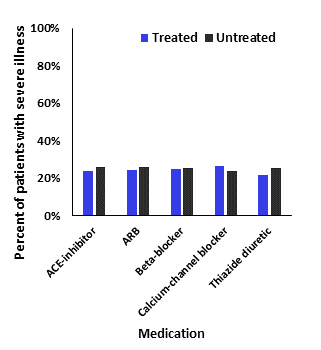
Note: Adapted from Reynolds et al. Figure 1 shows that there was little difference in testing positive for COVID-19 (percent in each medication group with COVID-19 on the y-axis) when stratifying by whether the patient was treated or untreated with the RAAS inhibitor medication. Figure 2 shows that for those with COVID-19, illness severity (percent with severe illness on the y-axis) did not differ substantially (more than a 10% difference) when stratifying by whether the patient was treated or untreated with the RAAS inhibitor medication. From NEJM. 382:2441-2448 DOI: 10.1056/NEJMoa2008975. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
B. Renin–angiotensin–aldosterone system blockers and the risk of COVID-19external icon. Mancia et al. NEJM (May 1, 2020).
Key findings:
- Use of blood pressure reducing medications (not further described) was more common in COVID-19 patients (58%) than uninfected people (50%).
- Adjusting for confounders, use of antihypertensives was not associated with risk of COVID-19 infection or severity of illness among COVID-19 patients.
Methods: Case-control study with 6,272 COVID-19 patients and 30,759 controls (matched 1:5), restricted to people 40 years of age or older in Lombardy, Italy from February 21 to March 11, 2020. Investigators abstracted 5 years of clinical history from the regional health service database to determine if the use of blood pressure reducing medications (ACE inhibitors and ARBs) increased the risk of SARS-CoV-2 infection. Limitations: Actual medication use unknown, only prescription of these medications; general population was not tested for SARS-CoV-2; few non-Caucasians included in the study.
C. Clinical impact of renin-angiotensin system inhibitors on in-hospital mortality of patients with hypertension hospitalized for COVID-19external icon. Tedeschi et al. Clinical Infectious Diseases (April 27, 2020).
Key findings:
- In-hospital mortality was 29% for all patients with COVID-19, but higher in patients with hypertension than patients with normal blood pressure (42% vs 14%, p <0.001).
-
- Patients with hypertension were older than patients without (median: 76 years vs 57 years).
- Comorbidities (Figure 1), sequential organ failure assessment (SOFA) score on admission, and older age were associated with higher in-hospital mortality for patients with hypertension; however, chronic use of blood pressure reducing medications was not.
Methods: Prospective cohort of 609 COVID-19 patients in ten Italian hospitals, of whom 311 had hypertension, between February 22 and April 3, 2020. Investigators obtained clinical data to determine if chronic use of blood pressure reducing medications impacted in-hospital mortality due to COVID-19. Limitations: Severe cases over-represented in the study population.
Figure:
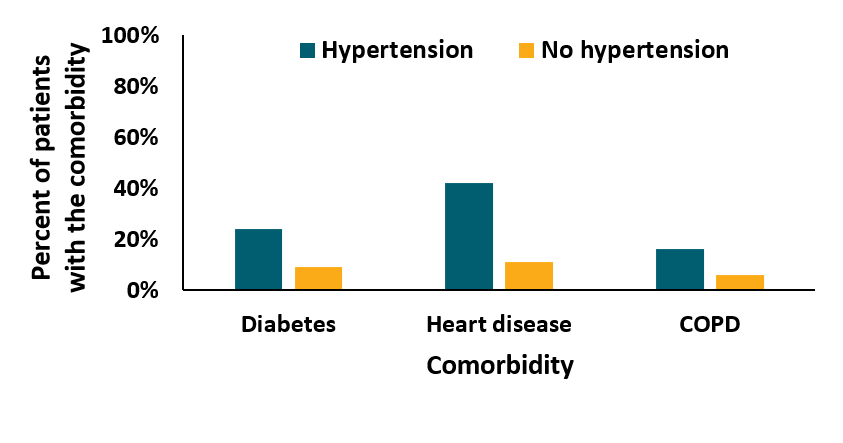
Note: Adapted from Tedeschi et al. In this figure, patients with hypertension more commonly (shown as percent of patients on the y-axis) had diabetes, heart disease, and COPD (x-axis) than patients without hypertension. Available via Oxford University Press Public Health Emergency Collection through PubMed Central.
Implications of 3 studies (Reynolds et al., Mancia et al., & Tedeschi et al.): In these Italian and US populations, RAAS inhibitors were not associated with an increased likelihood of having COVID-19, with more severe COVID-19 illness, or with in-hospital mortality.
Skin lesions refer to any abnormality of the skin. These include changes in color, elevation of the skin from inflammation or fluid, and any break in the skin from cuts, crusting, ulceration or tissue death. Skin lesions associated with SARS-CoV-2 infection have been reported in multiple countries. Several studies have recently assessed the presence of skin lesions in COVID-19 patients, which are summarized below.
PEER-REVIEWED
A. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 casesexternal icon. Galván Casasexternal icon et al. British Journal of Dermatology (April 29, 2020).
Key findings:
Investigators distinguished five types of distinct skin lesions:
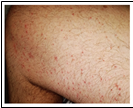
- Macules (a non-raised area of the skin which has changed color) and papules (a small [<1cm] area of raised skin with no visible fluid and varying in color) were identified in 47% of COVID-19 patients (Figure 1).
- Pseudo-chilblain (inflammation of small blood vessels in the skin causing pain, itching, redness and blisters; most commonly associated with exposure to cold air) was identified in the hands/feet in 19% of patients (Figure 2).
- Pseudo-chilblain was more common in young patients with mild or no COVID-19 symptoms, typically developing after the onset of other COVID-19 symptoms.
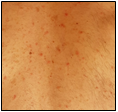
- Hives (raised and sometimes red areas of skin, commonly associated with an allergic reaction) were identified in 19% of patients (Figure 3).
- Vesicular eruptions (raised, fluid-filled blisters on the skin) were identified in 9% of patients (Figure 4).
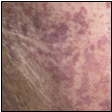
- Livedo (a mottled and purple-colored appearance near the skin surface) or necrosis (the process of skin tissue death) were identified in 6% of patients (Figure 5).
- Livedo was more common in older patients with moderate to more severe disease.
Methods: Cohort of 375 patients with suspected or confirmed COVID-19 and skin lesions of unknown origin who presented to a dermatologist. Dermatologists completed questionnaires for their patients and submitted skin lesion images, which were independently classified into skin lesion patterns. Limitations: No control group; excluded patients with severe disease; short study duration (2 weeks); no skin biopsies to confirm lesion pathology.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Note: Adapted from Galván Casas et al. All patient images shown in figures 1-5 had COVID-19. Figure 1: macules and papules; Figure 2: pseudo-chilblain; Figure 3: hives; Figure 4: vesicular eruptions, and Figure 5: livedo. Available via Wiley Public Health Emergency Collection through PubMed Central.
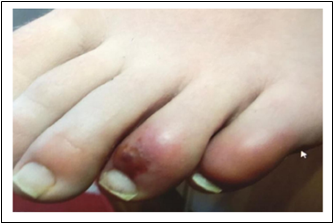
B. Chilblain-like lesions on feet and hands during the COVID-19 pandemicexternal icon. Landa et al. International Journal of Dermatology (April 24, 2020).
Key findings:
- Skin lesions similar in appearance to chilblains (Figures 1 and 2) were present on the extremities (hands and feet) of most patients, and most patients were asymptomatic for COVID-19 or had very mild symptoms. All were self-limited and resolved without intervention.
- In patients with documented symptom onset, skin lesions appeared later in their clinical course.
Methods: Cohort of six patients with multiple skin lesions of unknown origin and suspected or confirmed SARS-CoV-2 infection assessed by teleconference, Spain. Limitations: Only 3 patients were tested for SARS-CoV-2, 2 of whom tested positive; some diagnoses were confirmed remotely by teleconference; no skin biopsies performed.
Figure 1
Figure 2
Note: Adapted from Landa et al. Figure 1: Chilblain skin lesions present on five toes of a patient with suspected COVID-19 and mild COVID-19 symptoms. Figure 2: A crusting lesion on one toe of a patient with suspected COVID-19 and mild COVID-19 symptoms. Available via Wiley Public Health Emergency Collection through PubMed Central.
C. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infectionexternal icon. Diaz-Guimaraens et al. JAMA Dermatology. April 30, 2020.
Key findings:
- The patient developed red macules, papules, and petechiae (pinpoint, round spots that appear on the skin as a result of bleeding), affecting the buttocks, legs, and abdomen before the initiation of antiviral treatment.
- Lesions resolved 5 days after treatment.
Methods: Case report of a 48-year-old man with a history of high blood pressure presented to the emergency department in March 2020 with skin rash, fever, chest pain, and shortness of breath. The patient was hospitalized and treated with hydroxychloroquine, lopinavir-ritonavir, and azithromycin. Limitations: Single case.
Implications of 3 studies (Galván Casasexternal icon et al., Landa et al. & Diaz-Guimaraens et al.): A spectrum of skin lesions are associated with COVID-19 and warrant further investigation. The presence and timing of certain skin lesions may help clinicians identify early or late stage COVID-19 illness in people who are otherwise asymptomatic or have mild symptoms.
- Peavy et al. Rapid implementation of service delivery changes to mitigate COVID-19 and maintain access to methadone among persons with and at high-risk for HIV in an opioid treatment programexternal icon. AIDS and Behavior. Describes measures adopted at an Opioid Treatment Program to mitigate the spread of COVID-19 while preserving core services to patients and implementing clinical decision-making strategies aimed at maintaining patient and community safety.
- Boserup et al. Alarming trends in US domestic violence during the COVID-19 pandemicexternal icon. American Journal of Emergency Medicine. Reports of domestic violence have increased since the start of the COVID -19 pandemic in some US cities following the implementation of stay at home orders.
- Callaway E. The race for coronavirus vaccines: A graphical guideexternal icon. Nature. Provides a graphical guide on 8 types of SARS-CoV-2 vaccines.
- Wise J. COVID-19: Cancer mortality could rise at least 20% because of pandemic, study findsexternal icon. BMJ. Delays in cancer diagnosis and treatment associated with the pandemic may lead to an increase in cancer deaths.
- Baud et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infectionexternal icon. JAMA. The fetal surface of the placenta was positive for SARS-CoV-2 and the umbilical cord connective tissue was inflamed, suggesting a role of a placental infection in a second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection.
- Desai A. Twentieth-century lessons for a modern coronavirus pandemicexternal icon. JAMA. Interview with medical historian, Howard Markel, on 2007 studyexternal icon examining the effect of non-pharmaceutical interventions on the 1918–1919 influenza pandemic in 43 US cities. “Cities that implemented early, layered, and long-duration interventions had a far better mortality & morbidity rate than those that did not. And that is where the thesis for what has now been called ‘flattening the curve’ emerged.”
- Barr et al. A national medical response to crisis — The legacy of World War IIexternal icon. NEJM. At the 75th anniversary of the conclusion of WWII, authors posit that like in wartimes, the current aura of crisis, national attention, and material resources could lead to developments in medicine and surgery.
- Zagury-Orly et al. COVID-19 — A reminder to reasonexternal icon. Authors highlight the need to use reason to balance the priority to do something during a pandemic, including to avoid favoring newly acquired information, neglecting to seek reasonable alternatives, or confirmation bias.
- De Jong et al. The risks of prescribing hydroxychloroquine for treatment of COVID-19 — First, do no harmexternal icon. JAMA. Overview on hydroxychloroquine debate, highlighting online searches, increased prescriptions, emergency prescribing restrictions in 12 US states, and potential harms.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.