COVID-19 Science Update released: May 5, 2020 Edition 10

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
A. Reduced rate of hospital admissions for ACS during COVID-19 outbreak in northern Italyexternal icon. De Filippo et al. NEJM (April 28, 2020).
Key findings:
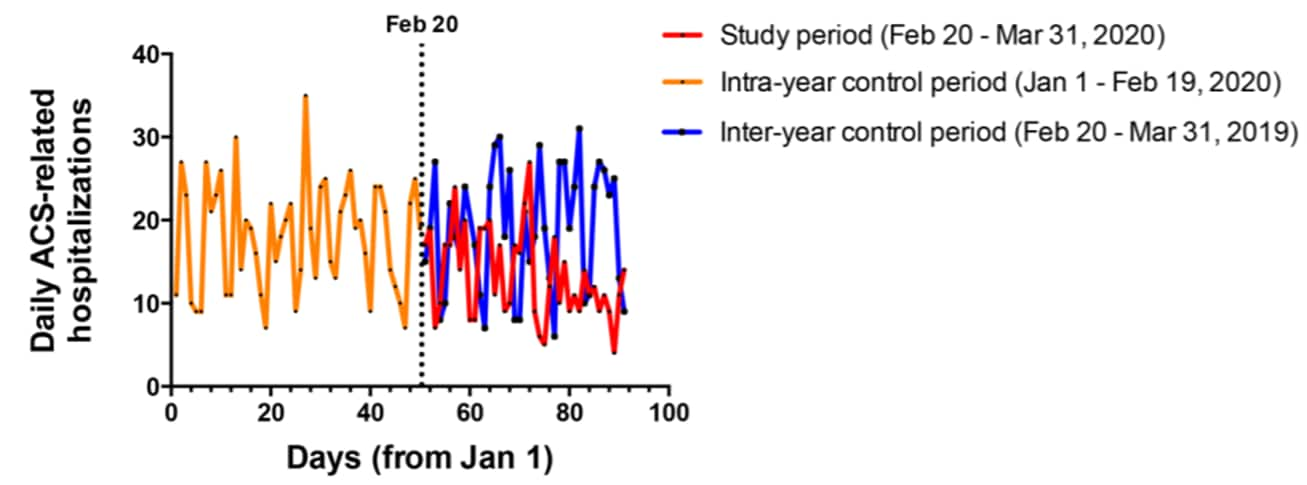
- Admissions for acute coronary syndrome (ACS) were significantly lower during the six weeks after the first confirmed case of COVID-19 in Italy on February 20, 2020 (13.3 per day) compared with the same period in 2019 (18.9 per day) and the first 7 weeks of 2020 (18.8 per day) (Figure 1).
- Admissions were lowest after the national lockdown was issued on March 8, 2020 (12.1 per day).
Methods: Retrospective analysis of admission rates for ACS at 15 hospitals in Northern Italy during February 20 to March 31, 2020 and two control periods (intra-year: first 7 weeks of 2020; and inter-year: same period in 2019). Incidence rate ratios comparing the study period with each of the control periods were calculated using Poisson regression. Limitations: Ecological study; no data on ACS among patients admitted with COVID-19 or deaths from ACS.
Figure 1
Note: Adapted from De Filippo et al. Daily acute coronary syndrome (ACS) hospital admissions between February 20 and March 31, 2020, January 1 – February 19, 2020, and February 20 – March 31, 2019. The dashed vertical line represents February 20, 2020, the date of the first confirmed COVID-19 case in Italy. From NEJM. 383:88-89. DOI: 10.1056/NEJMc2009166. Copyright ©2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
B. Out-of-hospital cardiac arrest during the COVID-19 outbreak in Italyexternal icon. Baldi et al. NEJM (April 29, 2020).
Key findings:
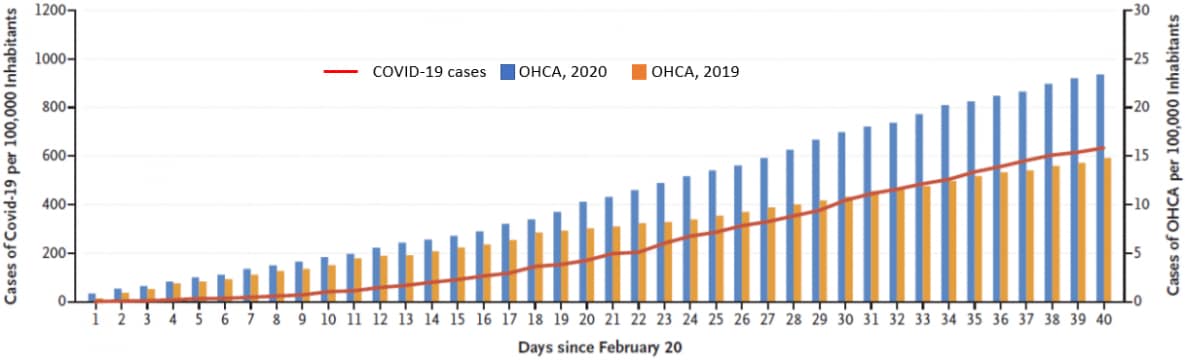
- Out-of-hospital cardiac arrests (OHCA) increased by 58%, and deaths from OHCA increase by 11%, during the six weeks after the first confirmed case of COVID-19 in Italy on February 20, 2020 compared with the same period in 2019 (Figure 2).
- The increase in OHCA followed the time course of the COVID-19 outbreak (Figure 2).
- 28% of OHCA cases had suspected symptoms of COVID-19 or confirmed SARS-CoV-2 infection.
Methods: Retrospective analysis of OHCA data from four provinces in Northern Italy between February 20 and March 31, 2020, compared with the same period in 2019. Suspected symptoms of COVID-19 were fever lasting ≥3 days before OHCA with cough, dyspnea, or both cough and dyspnea. Limitations: Ecological study.
Figure 2
Note: Adapted from Baldi et al. Cumulative incidences of diagnosed COVID-19 and cases of out-of-hospital cardiac arrest (OHCA) in four provinces in Northern Italy, between February 20 and March 31, 2019 and 2020. The red line shows the cumulative number of cases of COVID-19 per 100,000 inhabitants in 2020. From NEJM. 383:496-498. DOI: 10.1056/NEJMc2010418. Copyright ©2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Implications of both studies (De Filippo et al. & Baldi et al.): Fewer people may be seeking needed care for acute coronary heart disease due to the COVID-19 pandemic.
PEER-REVIEWED
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) pneumoniaexternal icon. Hu et al. Obstetrics & Gynecology (April 24, 2020).
Key findings:
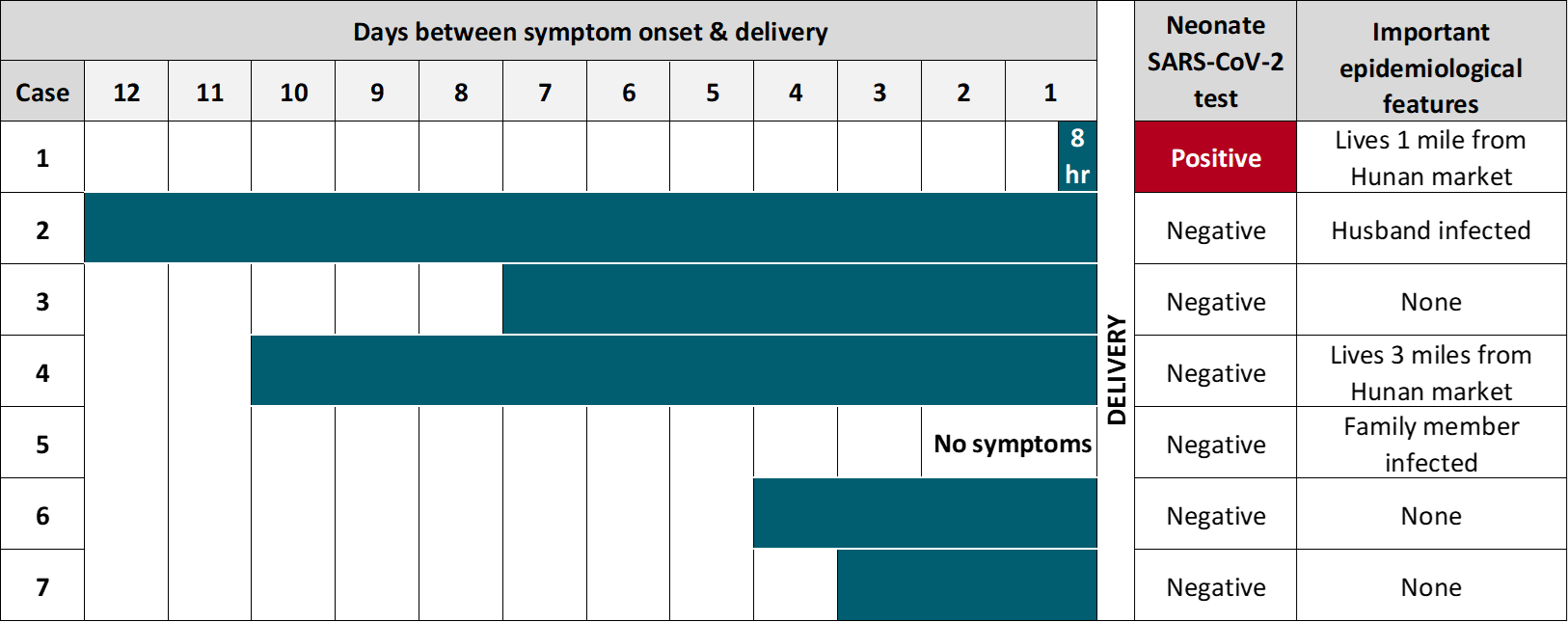
- One neonate delivered by pre-labor caesarean section was positive for SARS-CoV-2 at 36 hours of life despite isolation from the infected mother (Figure).
- Subsequent tests on this neonate for SARS-CoV-2 were negative.
- Amniotic fluid samples obtained at delivery were negative for SARS-CoV-2.
- The mother of the SARS-CoV-2-positive neonate had less time between symptom onset and delivery (8 hours) than other women in the study (range: 3 to 12 days) (Figure).
Methods: Observational study of 7 neonates born during January 20 and February 20, 2020 to women infected with SARS-CoV-2 in late-stage pregnancy. Women were tested for SARS-CoV-2 24 to 36 hours after birth. 6 out of 7 women had caesarean section. All neonates were immediately isolated from their mothers. Limitations: Convenience sample; could not distinguish neonate infection from transient maternal contamination.
Implications: Transplacental transmission of SARS-CoV-2 may be possible, but further research is needed that can distinguish between transplacental transmission and transient maternal contamination.
Figure:
Note: Adapted from Hu et al. Clinical and epidemiological features of pregnant women infected with SARS-CoV-2, and neonates. Available via Wolters Kluwer Public Health Emergency Collection through PubMed Central.
Severe COVID-19 during pregnancy and possible vertical transmissionexternal icon. Alzamora et al. American Journal of Perinatology (April 18, 2020).
Key findings:
- A pregnant woman with COVID-19 required invasive mechanical ventilation (IMV) with Caesarian section at 33 weeks.
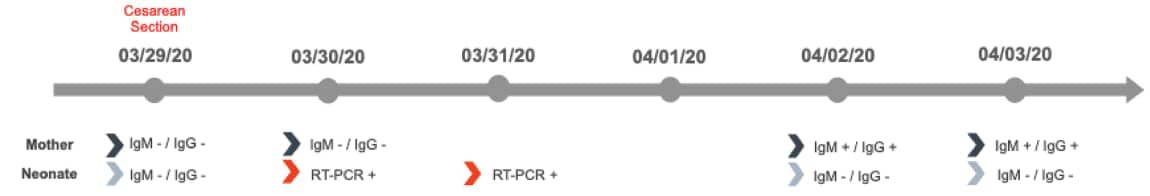
- SARS-CoV-2 IgM and IgG negative 1 day postpartum, but positive 4 days postpartum (Figure).
- Neonate was SARS-CoV-2 positive by RT-PCR from NP swab at 16 and 64 hours of life (Figure).
- Chest X-ray normal, IMV required for 12 hours.
- SARS-CoV-2 Ig-M and Ig-G negative on days 1, 4, and 5.
Methods: Case study of a 41-year-old woman with diabetes and obesity presenting with severe COVID-19 during pregnancy. Mechanical ventilation was required from day 5 of symptom onset. After Cesarean delivery, neonate was immediately isolated without delayed cord clamp, skin-to-skin contact, or breast feeding. Neonatal RT-PCR on days 1 and 3 and serial serologic testing of mother and neonate. Limitations: Could not distinguish neonate infection from transient contamination. No testing of other pregnancy-related specimens (e.g., amniotic fluid, umbilical cord blood, or pregnancy tissues), maternal vaginal fluid, or stool.
Implications: A neonate born to a mother with COVID-19 was infected with SARS-CoV-2. However, the method of transmission and severity of disease is uncertain.
Figure:
Note: Adapted from Alzamora et al. Timeline illustrating RT-PCR and serologic assay results in mother and neonate. Ig – immunoglobulin. Available via Thieme Public Health Emergency Collection through PubMed Central.
PEER-REVIEWED
Large-vessel stroke as a presenting feature of COVID-19 in the youngexternal icon. Oxley et al. NEJM (April 28, 2020).
Key findings:
- In the 2 weeks between March 23 and April 7, 2020, 5 persons aged 33-49 years with COVID-19 were diagnosed with new strokes in a hospital system in New York City.
- During the previous 12 months, stroke was diagnosed in an average of 0.73 persons under age 50 every 2 weeks.
- Patients had symptoms of severe brain injury, including reduced level of consciousness, difficulty speaking, and weakness on one side of the body. Imaging showed clots in large arteries supplying blood to the brain.
- Two patients delayed seeking care because of concern about going to a hospital during the pandemic.
Methods: Case report of 5 COVID-19 patients with stroke from Mt Sinai Health System, New York City. Limitations: Retrospective case report without description of underlying mechanism.
Implications: COVID-19 cases may be at increased risk for stroke and should be encouraged to seek medical care with any warning signs.
COVID-19 and kidney transplantationexternal icon. Akalin et al. NEJM (April 24, 2020).
Key findings:
- Of 28 kidney-transplant recipients hospitalized with COVID-19, 27 had viral pneumonia, and 11 required a ventilator.
- Among these 28 patients, there was 28% mortality at 3 weeks (n = 10), compared to 1%-5% among patients with COVID-19 in the general population.
- 10 patients died, including 2 of 8 who were monitored at home.
Methods: Observational study of 36 adult kidney-transplant recipients with COVID-19 at a NYC hospital, between March 16 and April 1, 2020. Descriptive statistics. Limitations: Single institution, which limits generalizability.
Implications: Immunosuppressive treatment in kidney-transplant recipients may contribute to poor outcomes. Decreasing immunosuppressive treatment may be warranted among COVID-19 patients with renal-transplant.
ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infectionexternal icon. Liu et al. Clinical Gastroenterology and Hepatology (April 20, 2020).
Key findings:
- Pancreatic injury (elevated serum amylase and lipase) was present in 1 of 54 patients (2%) with mild COVID-19, and 12 of 64 patients (18%) with severe disease (of whom 5 [8%] died).
- Analysis of pancreatic cells from healthy people showed the presence of receptors (ACE2) that can bind SARS-CoV-2 and lead to infection of the cell. There were significantly more receptors on pancreas cells than lung cells.
Methods: (1) Case series of 121 COVID-19 patients at 2 hospitals, Wuhan, China between January 1 and February 15, 2020. Comparison of lab values with Wilcoxon sign rank test. (2) RNA analysis of cells from 4 pancreases donated by health donors for transplant. Limitations: Clinical symptoms and imaging results not reported for all patients; pancreatitis could also be explained by severe illness (known phenomenon) or other emerging sequelae of COVID-19 including inflammatory injury, endothelial injury, and/or “micro-clots”.
Implications: SARS-CoV-2 infection may cause injury to the pancreas, particularly in patients with severe disease.
Conjunctivitis can be the only presenting sign and symptom of COVID-19external icon. Scalinci et al. ID Cases (April 16, 2020).
Key findings:
- Conjunctivitis (eye redness, watering, discharge, and light sensitivity) was the only symptom among 5 persons with confirmed SARS-CoV-2 infection.
- All nasopharyngeal RT-PCR tests were strongly positive (Ct ≤22).
Methods: SARS-CoV-2 detected by RT-PCR from NP swabs. Limitation: Limited generalizability.
Implications: Conjunctivitis can be the only symptom of COVID-19.
PEER-REVIEWED
Ad hoc laboratory-based surveillance of SARS-CoV-2 by real-time RT-PCR using minipools of RNA prepared from routine respiratory samplesexternal icon. Eis-Hübinger et al. Journal of Clinical Virology (June 2020).
Key findings:
- SARS CoV-2 RT-PCR testing of 70 minipools, each containing 10 respiratory specimens collected in Germany during 2020, identified one case that was later confirmed by individual testing.
- There were no false positive results when testing minipools.
Methods: Minipools of respiratory samples tested with SARS-CoV-2 RT-PCR in multiple German laboratories.
Implications: Minipool testing using RT-PCR could enable rapid, efficient screening for SARS-CoV-2 cases that might be missed by symptom-based case definitions.
PREPRINTS (NOT PEER-REVIEWED)
Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabsexternal icon. Wyllie et al. medRxiv (April 22, 2020). Publishedexternal icon in NEJM (September 24, 2020).
Key findings:
- Testing of self-collected saliva was at least as sensitive as NP swabs for detection of SARS-CoV-2 among hospitalized COVID-19 patients and asymptomatic health care workers.
- Virus levels were significantly higher in saliva than NP specimens.
Methods: Comparison of quantitative RT-PCR testing of paired saliva and NP specimens from hospitalized COVID-19 patients (n = 29) and asymptomatic health care workers (n = 33). Saliva specimens were self-collected and NP specimens were collected by health care providers. Statistical comparisons with Wilcoxon test. Limitations: Patients with early and mild COVID-19 and asymptomatic infection were not included.
Implications: Collection of saliva, which is non-invasive and easy to self-administer, could enable at-home, self-administered sample collection for large-scale SARS-CoV-2 testing. However, larger studies, including those with patients with mild and asymptomatic infection, are needed to confirm these findings.
Figure:
Note: Adapted from Wyllie et al. Comparison of SARS-CoV-2 levels in paired NP and saliva samples (n = 29). Dotted line indicates detection limit of the CDC N1 assay (5,610 copies/mL or Ct 38). Asterisk denotes a statistically significant difference (Wilcoxon p value <0.05). Licensed under CC-BY-ND 4.0.
- FDA. Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatmentexternal icon. On May 1, 2020 the FDA issued an emergency use authorization for the investigational antiviral drug remdesivir for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease, two days after the National Institutes of Health’s clinical trial showed promising results.
- Stefan et al. Obesity and impaired metabolic health in patients with COVID-19external icon. Nature Reviews Endocrinology. Measuring BMI, waist and hip circumferences, and levels of glucose and insulin, along with standard hospital parameters, can help better estimate risk of complications in COVID -19 patients.
- Peters et al. Transforming ORs into ICUsexternal icon. NEJM. Transformation of operating rooms and post anesthesia care units into intensive care units allowed a medical center in New York City to increase capacity for COVID-19 patients.
- Iwasaki A et al. The potential danger of suboptimal antibody responses in COVID-19external icon. Nature Reviews Immunology. There is a risk of paradoxical increased susceptibility to infection from a vaccine effect known as antibody-dependent enhancement (ADE). ADE should be fully examined as part of vaccine safety assessment.
- Glied et al. The potential effects of coronavirus on national health expendituresexternal icon. JAMA. Projected effect of COVID-19 on national health expenditure and the share of health care in the GDP.
- Kovich H. Rural matters — Coronavirus and the Navajo Nationexternal icon. NEJM. Personal account from the perspective of a family medicine doctor on how diagnosing and managing COVID19 cases in the Navajo nation is distinct from other areas.
- Gandhi et al. Asymptomatic transmission, the Achilles’ heel of current strategies to control COVID-19external icon. NEJM. Companion piece to Arons et al. study (NEJMexternal icon).Symptom-based screening alone failed to detect a high proportion of infectious, pre-symptomatic cases. Widespread screening in high-risk settings is recommended.
- When pandemics collideexternal icon. Lancet HIV. With SARS-CoV-2 infections being reported in most African countries, HIV and COVID-19 are colliding.
Pinto RM et al. COVID-19 pandemic disrupts HIV continuum of care and prevention: Implications for research and practice concerning community-based organizations and frontline providersexternal icon. AIDS and Behavior. The COVID-19 pandemic has disrupted the HIV Continuum of Care and Prevention i.e., testing, pre-exposure prophylaxis, and primary care.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.
![05-05_figure5 Comparison of SARS-CoV-2 levels in paired NP and saliva samples (n=29). Dotted line indicates detection limit of the CDC N1 assay (5610 copies/mL or Ct 38). Asterisk denotes a statistically significant difference (Wilcoxon P value [less than symbol] .05).](/library/covid19/images/05-05_figure5.png?_=99779)