COVID-19 Science Update released: March 12, 2021 Edition 80

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update align with the CDC Science Agenda for COVID-19.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
Since the implementation of mask mandates, concerns over the safety and possible adverse outcomes associated with mask use have been voiced. However, the following two studies (one among adults and one among children, including those <24 months), did not find indication of decreased oxygen or increased carbon dioxide blood level, nor clinical signs of respiratory distress among participants.
PEER-REVIEWED
A. Assessment of respiratory function in infants and young children wearing face masks during the COVID-19 pandemic.external icon Lubrano et al. JAMA Network Open (March 2, 2021).
Key finding:
- Use of surgical masks among healthy children <24 months of age was not associated with clinical signs of respiratory distress or oxygen desaturation during normal play or a walking test.
Methods: Cohort study of the use of surgical masks among healthy children aged 4-144 months (n = 47) in Rome, Italy between May and June, 2020. Partial pressure of end-tidal carbon dioxide, oxygen saturation, pulse rate, and respiratory rate were measured every 15 minutes during 30-minute sessions with and without a mask. Limitations: Small sample size; masks worn for maximum of 30 minutes; not generalizable to children with health conditions.
B. The effects of wearing facemasks on oxygenation and ventilation at rest and during physical activityexternal icon. Shein et al. PloS One (February 24, 2021).
Key finding:
- No participants developed hypoxemia (decreased oxygen level) or hypercarbia (increased carbon dioxide level) while wearing either a cloth or surgical mask during rest or physical activity.
Methods: Mask testing among a cohort of recruited hospital employees aged 29-45 years (n = 50) at the University Hospitals of Cleveland between August and October 2020. Measurements of heart rate, oxygen saturation, and carbon dioxide tension were taken after each of three 10-minute study phases: sitting and walking briskly without a mask, with a cloth mask, and with a surgical mask. Paired comparisons were performed using the Wilcoxon signed rank test. Limitations: Lacks generalizability – all young (29-45 years), without significant co-morbidities and female (68%) participants; small sample size.
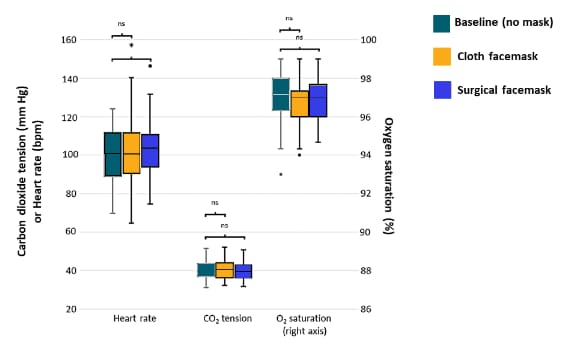
Figure:
Note: Adapted from Shein et al. Measurements taken during physical activity. Boxes represent the 25th-75th percentiles of each variable. The horizontal line in each box represents the median. The whiskers represent the local maximum and local minimum values. Values that are >1.5 times the interquartile range from either end of the box are considered outliers and denoted with a small circle. Non-significant differences are denoted “ns” and statistically significant differences are denoted with an asterisk. Licensed under CC BY 4.0.
Implications for both studies (Shein et al. & Lubrano et al.): In the adult population, the use of cloth or surgical face masks will not impede breathing while at rest or during physical activity. A study by Mapelli et alexternal icon, provides additional evidence in middle-aged healthy adults that face masks are safe even during maximal exercise. For children <24 months, 3-layer disposable surgical masks appear to be safe when used under adult supervision. Studies that evaluate longer periods of mask wearing in this population and among children with comorbidities are needed.
PREPRINTS (NOT PEER-REVIEWED)
Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination.external icon Müller et al. medRxiv (March 5, 2021).
Key findings:
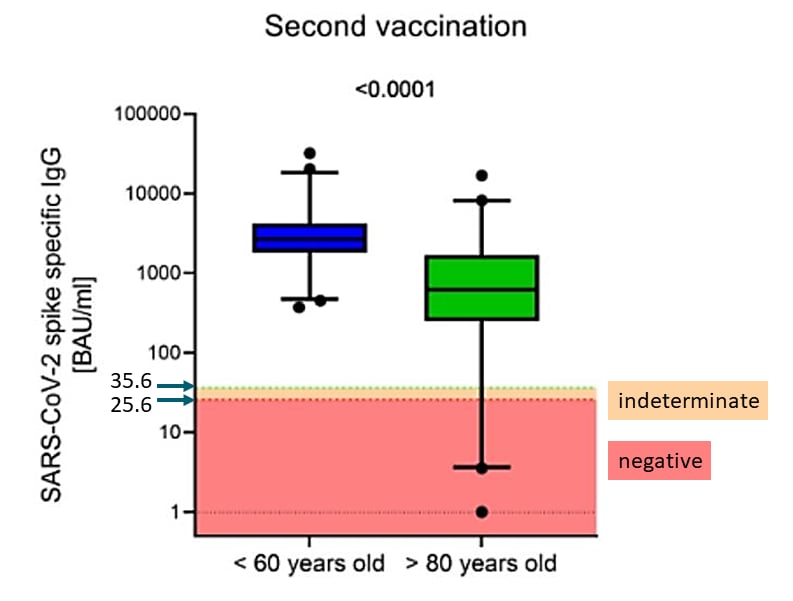
- The majority of older (>80 years) and younger (<60 years) adults produced specific IgG antibody titers against SARS-CoV-2 spike protein.
- Titers were lower in those >80 years; although the titer increase after the 2nd dose was greater in adults >80 years, the absolute mean titer remained lower than in adults <60 years (Figure 1).
- There was no correlation between IgG titers and presence of symptoms.
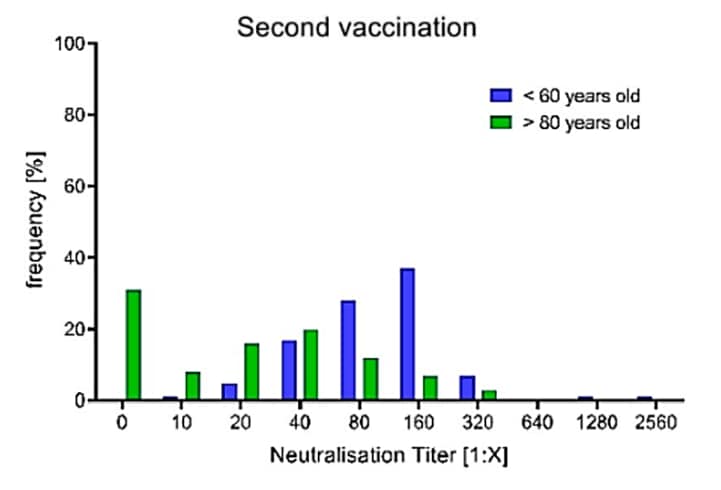
- After the 2nd dose, 31.3% of older and 2.2% of younger adults had no detectable neutralizing antibodies (Figure 2).
Methods: Cohort study of 176 German adults <60 (n = 91) and >80 (n = 85) years old who received BNT162b2 vaccine. Measured SARS-CoV-2 spike-specific IgG and SARS-CoV-2 neutralizing antibody titers 17-19 days after 1st dose and 17 days after 2nd dose. Summed self-reported number of post-vaccination symptoms (fever, chills, pain at injection site, head/limb pain, fatigue, nausea). Limitations: Data on 60-80 year-olds not included.
Implications: It is unclear whether absent neutralizing antibody responses to vaccination against SARS-CoV-2 in some older adults will translate to reduced protection from infection and disease. Observational data from Scotlandexternal icon suggest that one dose of the BNT162b2 or ChAdOx1 vaccine protects older adults against hospitalization in the near term. Further study is needed to confirm the long-term effectiveness of SARS-CoV-2 vaccines in older adults and to determine the duration of protection, specifically for adults over 80 in order to assess need for and ideal schedule for revaccination.
Figure 1
Figure 2
Note: Adapted from Müller et al. Figure 1. Antibody titers 17-19 days after 2nd vaccination in adults <60 vs >80 years old. Boxes span the interquartile range, the line within each box shows the median, and whiskers show the 2.5th and 97.5th percentiles. Results <25.6 binding antibody units/milliliter (BAU/ml) are considered negative, ≥25.6 and ≤35.6 BAU/ml indeterminate, and >35.6 BAU/ml positive. Antibody titers below the detection limit were set to 1.0. Figure 2. Frequencies of adults <60 and >80 years old with specific neutralizing antibody titers after the second vaccination dose. Licensed under CC-BY-NC-ND 4.0
PREPRINTS (NOT PEER-REVIEWED)
Rapid SARS-CoV-2 variants spread detected in France using specific RT-PCR testing.external icon Haim-Boukobza et al. medRxiv (February 23, 2021).
Key findings:
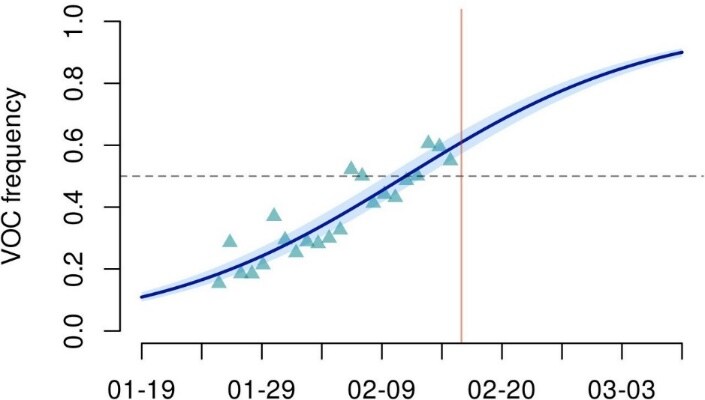
- The proportion of variants in SARS-CoV-2 samples increased between January 26 and February 16, 2021 (Figure).
- Variants were more likely in the general population: OR 1.25 (95% CI 1.13-1.39) compared to samples from hospitalized patients.
Methods: Analyzed SARS-CoV-2-positive samples collected between January 26 and February 16, 2021 from 35,208 individuals aged 5-80 years in France using PCR with probes targeting ∆69-70 deletion and N501Y mutation (both in the Spike gene). Classified samples with both changes (associated with lineage B.1.1.7) or with N501Y mutation only (associated with lineages B.1.153 and P.1) as variants. Used a generalized linear model (GLM) to analyze the binary strain variable (‘wild-type’ or ‘variant’). Limitations: Retrospective sampling; new variants of concern might be missed.
Implications: SARS-CoV-2 variants of concern are spreading rapidly, highlighting the need for timely mitigation (e.g., vaccination, distancing, masking) and surveillance. France has been using variant-specific RT-PCRs on all positive SARS-CoV-2 RT-PCR samples since February 5, 2021. Variant-specific RT-PCRs are less expensive than full genome sequencing and might be more rapidly and widely deployed.
Figure:
Note: Adapted from Haim-Boukobza et al. Estimated frequency of variants of concern (VOC) over time in France. Triangles indicate generalized linear model (GLM)-fitted values, line is the logistic growth model estimation, and vertical bar indicates analysis date. Licensed under CC-BY-NC 4.0
PEER-REVIEWED
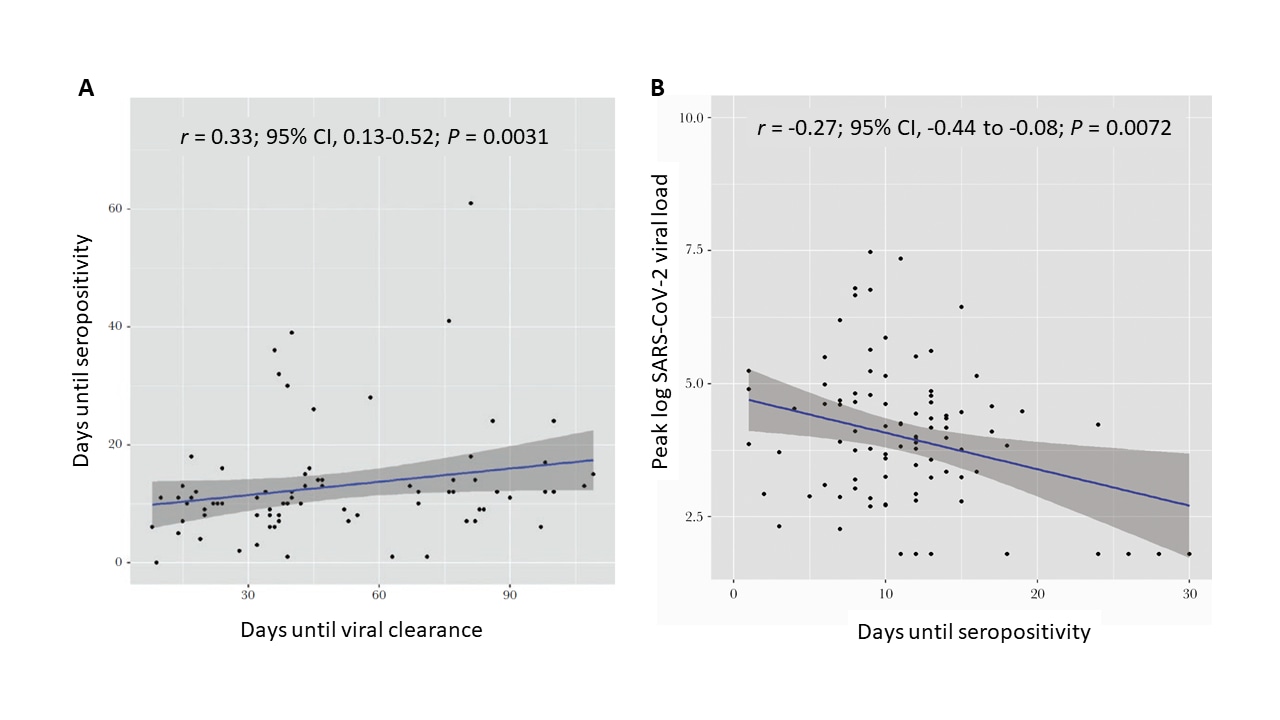
SARS-CoV-2 seroconversion and viral clearance in patients hospitalized with COVID-19: viral load predicts antibody response.external icon Masia et al. Open Forum Infectious Diseases (January 5, 2021).
Key findings:
- Peak viral load was inversely associated with time to seropositivity (Figure A). Time to seropositivity was positively associated with time to viral clearance (Figure B).
- High viral loads were associated with an earlier antibody response. There was a positive association between time to antibody response and time to viral clearance.
- 25% (n=33) of participants did not seroconvert. These participants were older, had a high frequency of comorbidities, the lowest viral loads, shorter time to viral clearance, and were more likely to have SARS-CoV-2 RNA only detected in fecal samples.
Methods: Prospective study among Spanish patients (n = 132) hospitalized with PCR-confirmed COVID-19 between March 10, 2020 and May 19, 2020. Serial nasopharyngeal and oropharyngeal swabs were taken >14 days since initiation of symptoms to test for SARS-CoV-2 RNA and antibodies. Limitations: All patients received treatments that could affect viral dynamics and/or immune response.
Implications: Viral replication determines the magnitude of the antibody response, which contributes to viral clearance. Persons infected with SARS-CoV-2 who do not seroconvert have a different virologic and clinical response to infection. Understanding both the immune response among those who seroconvert and lack of immune response among non-seroconverters is important in the development of vaccine strategies for previously infected persons.
Figure:
Note: Adapted from Masia et al. A. Correlation between peak log SARS CoV-2 viral load (blue line) and time to seropositivity. B. Correlation between time to seropositivity and time to viral clearance. Licensed under CC-BY-NC-ND 4.0
Persistent SARS-CoV-2 RNA shedding without evidence of infectiousness: A cohort study of individuals with COVID-19external icon. Owusu et al. Journal of Infectious Diseases (February 27, 2021).
Key findings:
- No replication-competent virus was recovered in any cultures of specimens collected 10-36 days after symptom onset.
- The likelihood of resolution of viral RNA shedding 10 days after symptom onset was 3%.
- Compared to adults aged 18-49 years, shedding resolved sooner among youth, <18 years, (adjusted hazards ratio [aHR]: 3.01; 95% CI: 1.6—5.6) and later among those ≥50 years (aHR: 0.50; 95% CI: 0.3—0.9).
Methods: Observational cohort study of RT-PCR confirmed COVID-19 patients (n = 109) from March–May 2020, in Utah and Wisconsin. Serial nasopharyngeal (NP) specimens were analyzed to determine SARS-CoV-2 viral shedding and associated characteristics. Viral culture was attempted in a subset (n = 35) of specimens collected 10-36 days after symptom onset.
Implications: Individuals with mild to moderate COVID-19 are unlikely to be infectious ≥10 days after symptom onset even if they test positive for SARS-CoV-2. Findings support current guidance on home isolation for persons infected with SARS-CoV-2.
PEER-REVIEWED
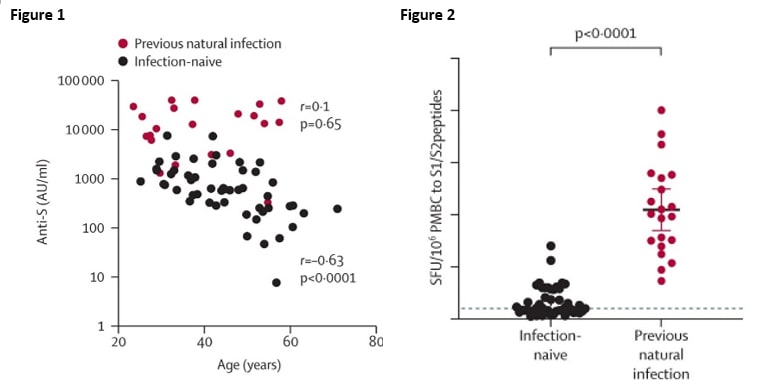
Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine.external icon Prendecki et al. Lancet (February 25, 2021).
Key findings:
- After one dose of vaccine, antibodies against SARS-CoV-2 spike protein were significantly higher in individuals with previous natural infection than in infection-naive individuals (16353 arbitrary units [AU] per mL vs 615·1 AU/mL, p <0.0001)
- There was a significant correlation between anti-spike Ab levels after the first dose of vaccination and age among infection-naïve individuals but not among those previously infected Figure 1).
- Infection-naïve persons generated weaker T cell responses and had lower neutralizing antibodies titers than persons with natural SARS-COV-2infection (Figure 2).
Methods: A cohort study of 72 healthcare workers in the UK vaccinated with BNT162b2 (Pfizer/BioNTech) between December 23 and 31, 2020 were included. Twenty-one persons were classified as having previous exposure and 51 were defined as infection-naïve based on the presence of SARS-CoV-2 antibodies. Post vaccination antibody and T-cell responses were assessed at days 21 – 25. Limitations: Small sample size with some samples not paired for direct comparison; non-representative sample.
Implications: Data suggest that it could be useful to delay administration of the second dose of SARS-CoV-2 BNT162b2 vaccine to previously infected and recovered persons especially in times of low vaccine supply. The age specific immune response also buttresses recommendations for prioritization of older individuals and use of facemasks post-vaccination.
Note: Adapted from Prendecki et al. Figure 1. Correlation of anti-spike Ab levels post first-dose vaccination with age. In infection-naïve persons, Ab levels generally fell as age increased. No correlation was observed for those previously infected. Figure 2. Comparison of neutralizing antibody titers in infection-naïve and previously infected individuals with paired serum at day 0 and day 21 post first dose of vaccination. The dotted line represents the mean plus standard deviation for spike peptide pool. Reprinted from The Lancet, Feb 25: S0140-6736(21)00502-X, Predecki et al, Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine, Epub ahead of print, Copyright 2021, with permission from Elsevier.
PREPRINTS (NOT PEER-REVIEWED)
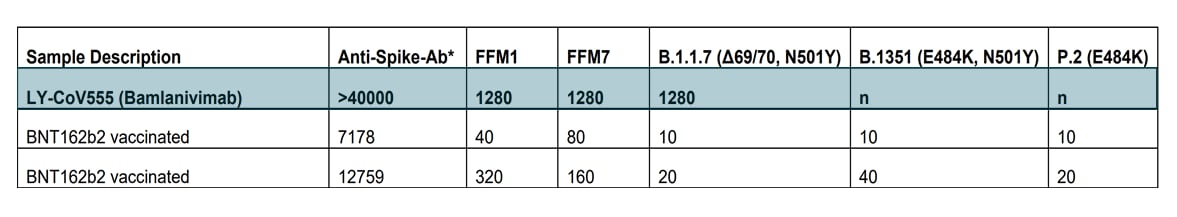
Bamlanivimab does not neutralize two SARS-CoV-2 variants carrying E484K in vitro.external icon Widera et al. medRxiv (February 26, 2021).
Key findings:
- The monoclonal antibody (mAb) bamlanivimab was effective at neutralizing SARS-CoV-2 variants FFM1, FFM7, and B.1.1.7; whereas it was completely ineffective against variants B.1.351 and P.2, both of which contain the E484K amino acid substitution (Figure).
- BNT162b2 (Pfizer-BioNTech) vaccine induced sera was mildly effective at neutralizing the E484K containing variants.
Methods: BNT162b2 vaccine-elicited sera and convalescent sera were analyzed for ability to neutralize SARS-CoV-2 variants. Monoclonal antibody solution was serially diluted and incubated with SARS-CoV-2 isolates FFM1, FFM7, B.1.1.7., B.1.351, or P.2, and tested for neutralizing activity. Limitations: Small sample size.
Implications: Data show that bamlanivimab may not be active against SARS-CoV-2 variants with the E484K substitution. Diamond et al (below), also found diminished neutralizing activity against E484K variants suggesting that screening for E484K may be necessary before initiating mAb treatment . Data bolster previous studies showing effectiveness of the BNT162b2 vaccine against the non- E484K containing variants.
Figure:
Note: Adapted from Widera et al. Neutralization titers against SARS-CoV-2 variants. Values indicate SARS-CoV-2 microneutralization titer (NT50) with two replicates per cell line. Results are given as AU/mL (analytical measurement range: 21.0 – 40,000.0). n-no neutralization; NC-negative control sera. Permission request in process.
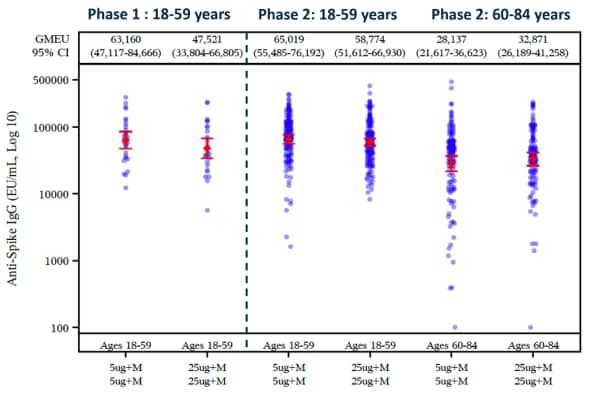
Evaluation of a SARS-CoV-2 vaccine NVX-CoV2373 in younger and older adults.external icon Formica et al. medRxiv (March 1, 2021).
Key findings:
- Both dose levels (5 μg and 25 μg) of NVX-CoV2373 were well-tolerated among younger (aged 18-59 years) and older (60-84) participants, although higher frequencies and intensity of reactogenicity were seen with the higher (25 μg) dose (Figure 1).
- Following 2nd vaccine dose, NVX-CoV2373 induced high levels of anti-spike protein IgG (Figure 2) and neutralizing antibodies in both younger and older adults, with no major differences between the primary 5-μg and the 25-μg regimens (Figure 2).
Methods: Phase I/II randomized, placebo-controlled, multi-site trial of the Novavax recombinant nanoparticle vaccine (NVX-CoV2373) in Australia and the United States. Adults (aged 18–84 years) were randomized to 1 of 5 groups each receiving vaccine doses 21 days apart : 2-dose primary vaccination regimens: 5-μg regimen (n = 257), 25-μg regimen (n = 258), or placebo both doses (n = 255); and 1-dose regimens5-μg followed by placebo (n = 257), 25-μg followed by placebo (n = 258). Safety and immunogenicity data were collected through day 35. Immunoglobulin (IgG) response toward the SARS-CoV-2 spike protein was assessed at day 0, day 21 and day 35.
Implications: The two-dose regimen of 5-μg NVX-CoV2373 vaccine administered 21 days apart is effective and tolerated in both younger (aged 18-59 years) and older (60-84) participants.
Figure 1
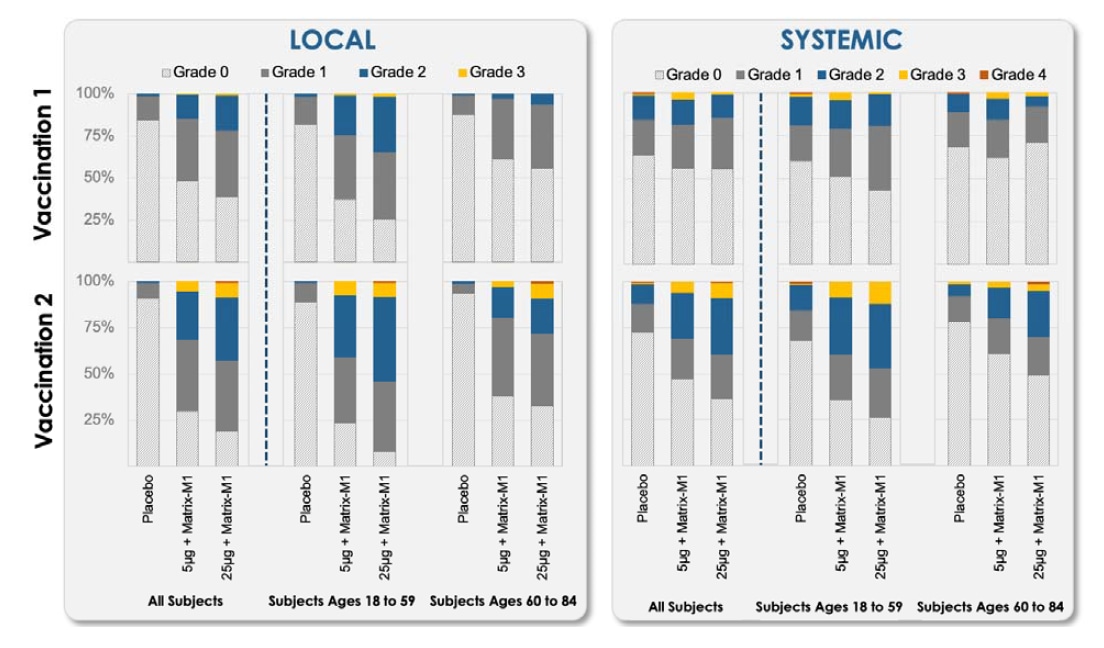
Note: Adapted from Formica et al. The percentage of participants in each of the 2-dose primary vaccine groups with local and systemic adverse events according to the maximum toxicity grade (Grade 0, Grade 1, Grade 2, Grade 3, Grade 4) by age group. Used by permission of copyright holder. © Novavax Inc. (2021).
Figure 2
Note: Adapted from Formica et al. Geometric mean anti-spike IgG enzyme-linked immunosorbent assay (ELISA) unit responses to NVX-CoV2372 protein antigens for younger and older adult age groups for the 2-dose primary vaccination treatment regimens. Used by permission of copyright holder. © Novavax Inc. (2021).
Vaccine after effects and post-vaccination infection in a real world setting: Results from the COVID symptom study appexternal icon. Menni et al. SSRN (March 4, 2021).
Key findings:
- Lower prevalence of side effects seen for BNT162b2 (Pfizer-BioNTech) (11.8%) and ChAdOx1 nCOV-19 (Oxford-AstraZeneca) (29.4%) after first dose compared with published Phase 3 results.
- Individuals with evidence of prior infection were more likely than those without known prior infection to report a systemic effect after a single dose of BNT162b2 (34.1% v 10.6%) or ChAdOx1 nCOV-19 (51.6% vs 28.6%) (Figure).
- Significant reduction in infection risk 12-21 days after the first dose compared with the unvaccinated population: BNT162b2 (- 57%), ChAdOx1 nCoV-19 (- 42%).
Methods: Observational cross-sectional study with self-reported data related to SARS-CoV-2 from the COVID Symptom Study app to assess adverse effects and infection rates from persons vaccinated December 2020 to February 2021 in the UK (n = 387,471). Limitations: Self-selected sample may not represent general population, self-reported data; data are preliminary for ChAdOx1 nCoV-19 which began distribution in January 2021; study underpowered to look at differences by race /ethnicity.
Implications: In a real-world setting, side effects of BNT162b2 and ChAdOx1 nCOV-19 SARS-CoV-2 vaccinations were less common than reported in Phase III trials and were primarily mild and self-limiting.
Figure:
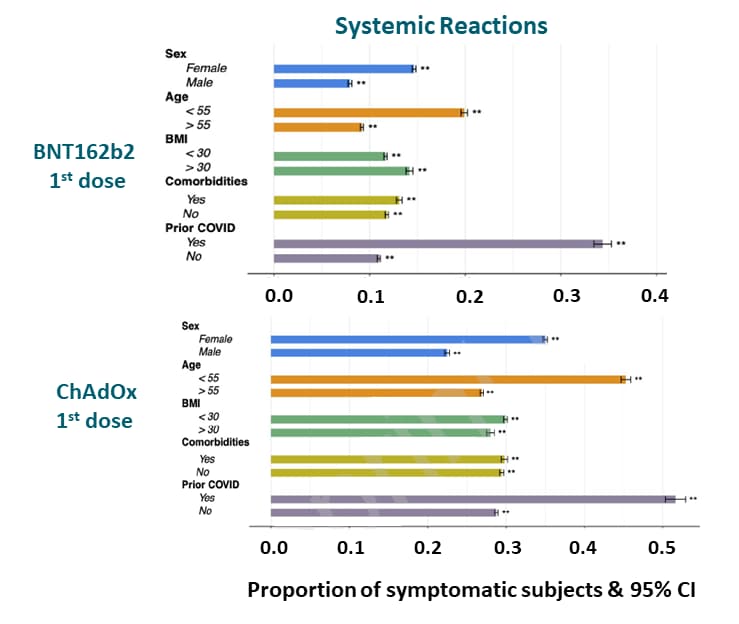
Note: Adapted from Menni et al. Proportion of subjects self-reporting symptoms stratified by sex, age, BMI, comorbidities, and prior COVID infection. Permission request in process.
Prevention, Mitigation, and Intervention Strategies
- Li et al. Do stay at home orders and cloth face coverings control COVID-19 in New York City? Results from a SIER model based on real-world dataexternal icon. Open Forum Infectious Diseases (February 2021). Public health interventions resulted in significant reductions in SARS-CoV-2 cases relative to predicted values derived from a Susceptible-Exposed-Infectious-Removed (SEIR) model: Initial R0 = 4.60 without intervention; after social distancing, Rt decreased by 68%; after mask recommendation, Rt decreased by ~60%.
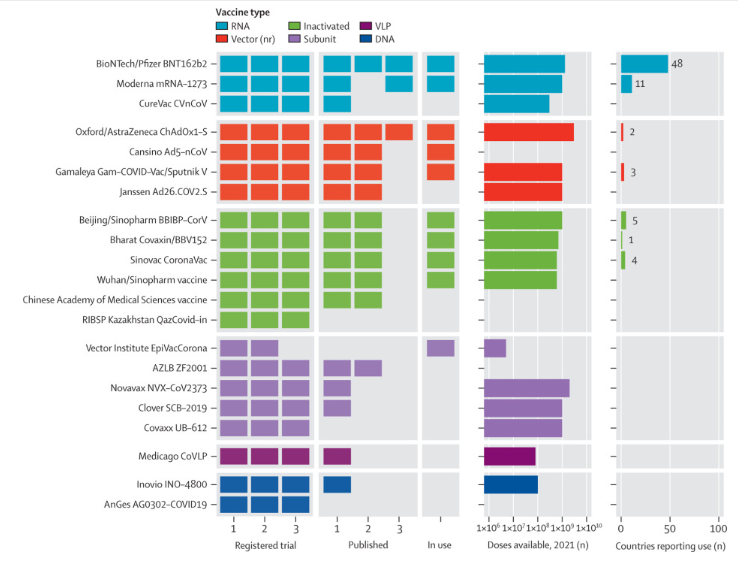
- Shrotiet al. An interactive website tracking COVID-19 vaccine development.external icon Lancet (March 2, 2021). An online, interactive vaccine tracker hosted by the Vaccine Centre (Vac) at the London School of Hygiene and Tropical Medicine (London, UK) that collates up-to-date information on all COVID-19 vaccine candidates from inception through deployment.
Figure:
Note: Licensed under CC BY-NC-ND.
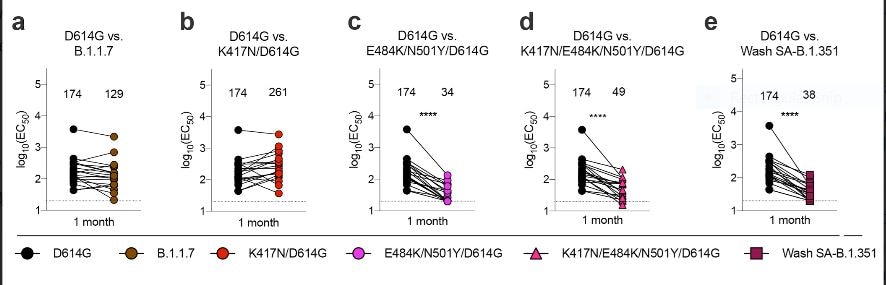
- Diamond et al. SARS-CoV-2 variants show resistance to neutralization by many monoclonal and serum-derived polyclonal antibodies.external icon Research Square (Preprint, February 10, 2021). Monoclonal antibodies, convalescent sera and human sera from Pfizer-BioNTech (BNT162b2) showed markedly diminished neutralizing activity against the Wash SA-B.1.351 (Washington strain with South African spike gene) or variants with an E484K spike mutation in a panel of SARS-CoV-2 variants.
Figure:
Note: Paired neutralization analysis of SARS-CoV-2 viral variants by convalescent human serum obtained ~1 month after mild SARS-CoV-2 infection. Licensed under CC BY 4.0.
- Manisty et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individualsexternal icon. Lancet (February 25, 2021). A case-control study including serologic data from 51 health care workers found that anti-S titers after the first dose of the Pfizer-BioNTech vaccine were comparable to peak titers after natural infection, while for persons with previous natural infection, vaccination increased anti-S titers more than 140-fold greater.
Social, Behavioral, and Communication Science
- Maaravi et al. “The Tragedy of the Commons”: How individualism and collectivism affected the spread of the COVID-19 pandemicexternal icon. Frontiers in Public Health (February 11, 2021). Findings from 3 studies [(analyses of country-level cultural and economic data (n = 69), survey data from individuals in Israel (n = 327) and the United States (n = 121)], that collectively support the hypothesis that the impact of the COVID-19 pandemic will be greater in countries that are more individualistic vs. collectivistic in orientation as described in the classic article by Hardinexternal icon.
Detection, Burden, and Impact
- Yang et al. Just 2% of SARS-CoV-2-positive individuals carry 90% of the virus circulating in communities.external icon medRxiv (Preprint, March 5, 2021). Regardless of symptoms, ~50% of positive SARS-CoV-2 individuals appeared to be non-infectious and had low viral loads. The concentration of the majority of the virus was in a small fraction of the population who serve as ‘super-spreaders’.
Natural History of SARS-CoV-2 Infection
- Feldstein et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19external icon. JAMA (February 24, 2021). A case series of 1116 US patients of which 577 were diagnosed with COVID-19 and 539 diagnosed with multisystem inflammatory syndrome in children (MIS-C), found MIS-C patients were more likely to 6-12 years, non-Hispanic black, have severe cardiovascular or mucocutaneous symptoms and more extreme inflammation.
- Voloch et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazilexternal icon. Journal of Virology (March 1, 2021). Sequencing of 180 new viral genomes from Rio de Janeiro (April to December 2020) identified a novel lineage, P2, that included the E484K mutation which was found widely across samples. The E484K mutation has been associated with escape from SARS-CoV-2 neutralizing antibodies.
Protection in Healthcare and Non-Healthcare Work Settings
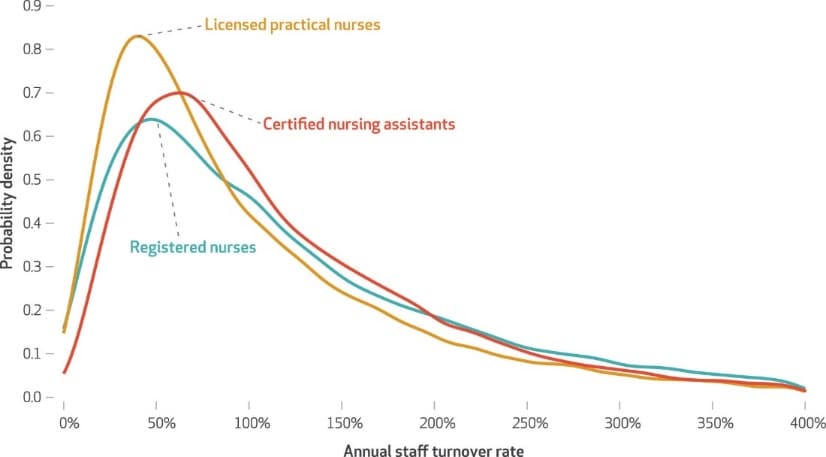
- Gandhi et al. High nursing staff turnover in nursing homes offers important quality informationexternal icon. Health Affairs (March 1, 2021). Using Centers for Medicare and Medicaid Services staffing data from 15,647 US nursing homes between October 2016 and March 2019, the authors estimated the average annual turnover rate was 128% with some facilities reaching 300%. High turnover rates have made it more difficult to implement strong infection controls during the pandemic.
Figure:
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.