Rotavirus
Margaret M. Cortese, MD and Penina Haber, MPH
The 14th edition of the “Pink Book” was published August 2021. Vaccine-specific recommendations may be outdated. Refer to the Advisory Committee on Immunization Practices Vaccine Recommendations and Guidelines for the most updated vaccine-specific recommendations.
Rotavirus
- First identified as cause of diarrhea in 1973
- Most common cause of severe gastroenteritis in infants and children*
- Nearly universal infection by age 5 years*
- Responsible for up to 500,000 diarrheal deaths each year worldwide*
- *Prevaccine era
Printer friendly version [12 pages]
Diarrheal disease has been recognized in humans since antiquity. Until the early 1970s, a bacterial, viral, or parasitic etiology of diarrheal disease in children could be detected in fewer than 30% of cases. In 1973, Ruth Bishop and colleagues observed a virus particle in the intestinal tissue of children with diarrhea by using electron micrography. This virus was subsequently called “rotavirus” because of its similarity in appearance to a wheel (rota is Latin for wheel). By 1980, rotavirus was recognized as the most common cause of severe gastroenteritis in infants and young children in the United States. In the prevaccine era, the majority of children were infected by age 5 years, and rotavirus was responsible for up to 500,000 deaths among children annually worldwide. A vaccine to prevent rotavirus gastroenteritis was first licensed in the United States in 1998 but was withdrawn in 1999 because of its association with intussusception, a type of bowel blockage when the bowel folds into itself like a telescope. Second-generation vaccines were licensed in the United States in 2006 and 2008.
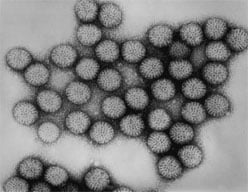
Rotavirus
- Reovirus (RNA)
- VP7 and VP4 proteins define virus serotype and induce neutralizing antibody
- G1 and G12 strains account for most infections
- Very stable and may remain viable for weeks or months if not disinfected
Rotavirus
Rotavirus is a double-stranded RNA virus of the family Reoviridae. The virus is composed of three concentric shells that enclose 11 gene segments. The outermost shell contains two important proteins: VP7, or G-protein, and VP4, or P-protein. VP7 and VP4 induce neutralizing antibodies that are believed to be involved in immune protection. From 1996 through 2005, five genotypes of rotavirus (G1P[8], G2P[4], G3P[8], G4P[8] and G9P[8]) accounted for 90% of strains isolated from children younger than age 5 years in the United States. Of these, genotype G1P[8] accounted for more than 75% of strains. In the recent past, G12P[8] has become the most common genotype identified in the United States.
Rotavirus is very stable and may remain viable in the environment for weeks or months if disinfection does not occur.
Rotavirus Pathogenesis
- Entry through mouth
- Replication in epithelium of small intestine
- In severe infections-rotavirus antigen can be detectable in serum
- Infection leads to isotonic diarrhea
Pathogenesis
The virus enters the body through the mouth. Viral replication occurs in the villous epithelium of the small intestine. Up to two-thirds of children with severe rotavirus gastroenteritis show the presence of rotavirus antigen in serum (antigenemia) and children can have rotavirus RNA detected in serum.
Infection may result in decreased intestinal absorption of sodium, glucose, and water, and decreased levels of intestinal lactase, alkaline phosphatase, and sucrase activity, and may lead to isotonic diarrhea.
The immune correlates of protection from rotavirus are not fully understood. Serum and mucosal antibodies against VP7 and VP4 are probably important for protection from disease. Cell-mediated immunity probably plays a role in protection and in recovery from infection.
Recovery from a first rotavirus infection usually does not lead to permanent immunity. Reinfection can occur at any age. A cohort study in Mexico found that after a single natural infection, 38% of children were protected against any subsequent rotavirus infection, 77% were protected against rotavirus diarrhea, and 87% were protected against severe diarrhea. Subsequent infections conferred progressively greater protection and were generally less severe than the first.
Rotavirus Clinical Features
- Short incubation period (usually less than 48 hours)
- May be asymptomatic or result in severe dehydrating diarrhea with fever and vomiting
- First infection after age 3 months generally most severe
- Gastrointestinal symptoms generally resolve in 3 to 7 days
Clinical Features
The incubation period for rotavirus diarrhea is short, usually less than 48 hours. The clinical manifestations of infection vary and depend on whether it is the first infection or reinfection. Infection may be asymptomatic, may cause self-limited watery diarrhea, or may result in severe dehydrating diarrhea with fever and vomiting. Up to one-third of infected children may have a temperature greater than 39°C (102°F). The first infection after 3 months of age is generally the most severe. The gastrointestinal symptoms generally resolve in 3 to 7 days.
Infants younger than age 3 months have relatively low rates of rotavirus infection, probably because of passive maternal antibody, and possibly because of breastfeeding. Rotavirus infection of adults is usually asymptomatic but may cause diarrheal illness.
The clinical features and stool characteristics of rotavirus diarrhea are nonspecific, and similar illness may be caused by other pathogens. As a result, confirmation of a diarrheal illness as rotavirus requires laboratory testing.
Rotavirus Complications
- Severe diarrhea
- Dehydration
- Electrolyte imbalance
- Metabolic acidosis
- Children who are immunocompromised may have more severe or persistent disease
Complications
Rotavirus infection in infants and young children can lead to severe diarrhea, dehydration, electrolyte imbalance, and metabolic acidosis. Treatment is supportive; feeding should be continued during the illness. Children who are immunocompromised because of congenital immunodeficiency or because of bone marrow or solid organ transplantation may experience severe or prolonged rotavirus gastroenteritis and may have evidence of resulting abnormalities in multiple organ systems, particularly the kidney and liver.
Laboratory Testing
Several commercial test kits are available for testing stool samples that detect a rotavirus antigen (VP6) common to human rotaviruses by enzyme linked immunoassay (EIA). These kits are simple to use, inexpensive, and very sensitive. With the marked reduction in rotavirus disease in children in the United States due to rotavirus vaccination, the positive predictive value of EIA is expected to be lower (and the negative predictive value to be higher) compared with that from the prevaccine era. Multi-pathogen polymerase chain reaction (PCR)-based assays for stool samples that include the ability to detect rotavirus RNA are being increasingly used in clinical laboratories. The clinical interpretation of results from these very sensitive assays may be challenging because detection of nucleic acid of rotavirus or other pathogens in stool may indicate a previous infection and not necessarily the cause of a current illness. Sequence analysis and viral culture are available in research laboratories.
Rotavirus Epidemiology
- Reservoir
- Human-GI tract and stool
- Transmission
- Fecal-oral, person-to-person and fomites
- Temporal pattern
- Fall and winter (temperate areas)
- Communicability
- 2 days before onset of diarrhea
Epidemiology
Occurrence
Rotavirus occurs throughout the world. In the prevaccine era, the proportion of severe diarrhea in children younger than age 5 years that was due to rotavirus was similar (about 35% to 40%) in developed and developing countries, suggesting that improved sanitation alone is not sufficient to prevent infection. The distribution of specific rotavirus genotypes can differ by geographic area and time period.
Reservoir
The reservoir of rotavirus is the gastrointestinal tract and stool of infected humans. Although rotavirus infection occurs in many nonhuman mammals, transmission of animal rotaviruses to humans is believed to be uncommon and probably does not lead to clinical illness. These animal strains are antigenically distinct from those causing human infection, and they rarely cause infection in humans. Although immunocompromised persons may shed rotavirus for a prolonged period, a true carrier state has not been described.
Transmission
Rotaviruses are shed in high concentration in the stool of infected persons. Transmission is by fecal-oral route, both through close person-to-person contact and by fomites (such as toys and other environmental surfaces contaminated by stool). Transmission of rotavirus through contaminated water or food appears to be uncommon.
Temporal Pattern
In temperate climates, disease is more prevalent during fall and winter. In the United States in the prevaccine era, annual epidemic peaks usually progressed from the Southwest during November and December to the Northeast by April and May. Following vaccine introduction, a biennial pattern of disease activity has emerged with less notable differences in timing by geographic region. In tropical climates, the disease is less seasonal than in temperate areas.
Communicability
Rotavirus is highly communicable, as evidenced by the nearly universal infection of children by age 5 years in the prevaccine era. Infected persons shed large quantities of virus in their stool beginning 2 days before the onset of diarrhea and for several days after the onset of symptoms. Rotavirus may be detected in the stool of immunocompromised persons for more than 30 days after infection. Spread is common within families, institutions, hospitals, and child care settings.
Rotavirus Secular Trends in the United States
Prevaccine era:
- Estimated 2.7 million cases per year
- 95% of children infected by 5 years of age
Following the introduction of rotavirus vaccine:
- Annually averted:
- 280,000 clinic visits
- 62,000 emergency department visits
- 45,000 hospitalizations
Secular Trends in the United States
In the prevaccine era, an estimated 2.7 million rotavirus infections occurred every year in the United States and 95% of children experienced at least one rotavirus infection by age 5 years. Rotavirus infection was responsible for 410,000 physician visits, more than 200,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths annually in children younger than age 5 years. Rotavirus accounted for 30% to 50% of all hospitalizations for gastroenteritis among children younger than age 5 years; the incidence of clinical illness was highest among children age 3 to 35 months.
Rotavirus activity has been monitored through data on routine testing for rotavirus performed at a set of clinical laboratories across the country. A biennial pattern of rotavirus activity emerged after vaccine introduction, with odd numbered years having small, short seasons starting in late winter or early spring and even numbered years having extremely low levels of circulation without a defined season.
The marked reduction in rotavirus disease burden in the United States following the introduction of rotavirus vaccine in 2006 has been documented by data on hospitalizations and emergency department care for diarrhea among young children. An evaluation of claims data of commercially insured children estimated that an average annual 280,000 clinic visits, 62,000 emergency department visits, and 45,000 hospitalizations for rotavirus disease were averted among U.S. children younger than age 5 years during 2007–2011 by routine vaccination. Indirect protection to previously uninfected and unimmunized children, as well as to some adults, has been described, illustrating the important role that infants play as drivers of rotavirus infection.
Rotavirus vaccination coverage among U.S. children continues to be lower than coverage for other vaccines administered during infancy, in part due to the narrow age restrictions for the doses and particularly the age restriction for completing the series. Among children born during 2015–2016, 73.6% completed the rotavirus vaccine series by age 8 months, the maximum age for the final dose.
Rotavirus Vaccines
- RV5 (RotaTeq)
- RV1 (Rotarix)
Rotavirus Vaccines
In 1998, a rhesus-based tetravalent rotavirus vaccine (RRV-TV [Rotashield]) was licensed and recommended for use in the United States. However, RRV-TV vaccine was withdrawn from the U.S. market within 1 year of its introduction after post-marketing surveillance detected an association with intussusception. The risk of intussusception was most elevated within 3 to 14 days after receipt of the first dose, with a smaller increase in risk within 3 to 14 days after the second dose. Overall, the risk associated with the first dose of RRV-TV vaccine was estimated to be about one case per 10,000 vaccine recipients.
Two live, oral rotavirus vaccines are currently licensed for use, RV5 (RotaTeq) and RV1 (Rotarix) vaccines.
Rotavirus Vaccine Characteristics
- RV5 (RotaTeq)
- Contains five reassortant strains developed from human and bovine parent rotavirus strains
- Administered orally
- RV1 (Rotarix)
- Contains one strain of live attenuated human rotavirus (type G1PA[8])
- Administered orally
- Oral applicator contains latex rubber
Characteristics
RV5 vaccine contains approximately 2 x 106 infectious units of each of the five reassortant strains developed from human and bovine rotavirus strains. RV1 vaccine contains one strain of live, attenuated human strain 89-12 (type G1P1A[8]) rotavirus. Each dose contains at least 106 median cell culture infective units of virus. RV1 and RV5 vaccines are administered orally. RV5 and RV1 vaccines contain no adjuvant, antibiotic, or preservative. The RV1 vaccine oral applicator contains latex rubber.
Rotavirus Vaccine Schedule
- Routine vaccination of all infants without a contraindication
- 2-dose series for RV1 vaccine (at age 2 and 4 months)
- 3-dose series for RV5 vaccine (at age 2, 4, and 6 months)
- For both rotavirus vaccines
- May be started as early as age 6 weeks
- Maximum age for first dose is 14 weeks 6 days*
- Minimum interval between doses is 4 weeks
- Maximum age for any dose is 8 months 0 days*
- ACIP did not define a maximum interval between doses
- No rotavirus vaccine should be administered to infants older than 8 months 0 days*
*Off-label use
Vaccination Schedule and Use
Rotavirus vaccine is routinely recommended for infants. The vaccine should be administered orally as a series of either two doses (at age 2 and 4 months) for RV1 vaccine or three doses (at age 2, 4, and 6 months) for RV5 vaccine. The vaccination series for both vaccines may be started as early as age 6 weeks. The minimum interval between doses is 4 weeks.
The Advisory Committee on Immunization Practices (ACIP) developed age recommendations that vary from those of the manufacturers. ACIP recommendations state that the maximum age for the first dose of each vaccine is 14 weeks 6 days. The minimum interval between doses of both rotavirus vaccines is 4 weeks. The maximum age for any dose of either rotavirus vaccine is 8 months 0 days. No rotavirus vaccine should be administered to infants older than 8 months 0 days of age. This is an off-label recommendation for both vaccines, because the labeled maximum age for RV1 vaccine is 24 weeks, and the labeled maximum age for RV5 vaccine is 32 weeks.
Rotavirus Vaccine Recommendations
- Any remaining doses should be administered on schedule
- Complete the series with the same product whenever possible
- If product used for a prior dose or doses is not available or not known, continue or complete the series with the product that is available
- If any dose in the series was RV5 (RotaTeq) or the vaccine brand used for any prior dose is not known, a total of 3 doses of rotavirus vaccine should be administered
- Infants documented to have had rotavirus gastroenteritis before receiving the full course of rotavirus vaccinations should still begin or complete the 2- or 3-dose schedule
If any dose in the series was RV5 vaccine or the vaccine brand used for any prior dose in the series is not known, a total of three doses of rotavirus vaccine should be administered.
Rotavirus vaccine should be given at the same visit as other recommended vaccines.
ACIP considers the benefits of rotavirus vaccination of preterm infants to outweigh the risks of adverse events. ACIP supports vaccination of a preterm infant according to the same schedule and precautions as a full-term infant, provided the following conditions are met: the infant’s chronological age is at least 6 weeks, the infant is clinically stable, and the vaccine is administered at the time of discharge or after discharge from the neonatal intensive care unit or nursery. The American Academy of Pediatrics will be providing updated guidance on administering vaccine to hospitalized infants in early 2021 through the Red Book.
Rotavirus Vaccine and Preterm Infants
- ACIP supports vaccination of a preterm infant if:
- Chronological age is at least 6 weeks
- Clinically stable
- Vaccine is administered at time of discharge or after discharge from neonatal intensive care unit or nursery
Breastfeeding does not appear to diminish immune response to rotavirus vaccine. Infants who are being breastfed should be vaccinated on schedule.
Infants documented to have had rotavirus gastroenteritis before receiving the full course of rotavirus vaccination should still begin or complete the 2- or 3-dose series following the age recommendations, as the initial infection may provide only partial protection against subsequent rotavirus disease.
Immunogenicity and Vaccine Efficacy
Rotavirus Vaccine Efficacy
- Any rotavirus gastroenteritis: 74%-87%
- Severe gastroenteritis: 85%-98%
- Both vaccines significantly reduced physician visits and reduced rotavirus-related hospitalization
Phase III clinical efficacy trials of RV5 vaccine were conducted in Finland and United States. The efficacy of the 3-dose series against G1-G4 rotavirus gastroenteritis of any severity was 74%, and against severe G1-G4 rotavirus gastroenteritis was 98% during the first full rotavirus season after vaccination. In a large health care utilization study evaluating children during the first 2 years of life, RV5 vaccine reduced the incidence of office visits for G1-G4 rotavirus gastroenteritis by 86%, emergency department visits for that outcome by 94%, and hospitalizations for that outcome by 96%.
Phase III clinical efficacy trials of RV1 vaccine were conducted in Latin America and Europe. In the Latin American study, the efficacy of the 2-dose series against severe rotavirus gastroenteritis to age 1 year was 85%. In the European study, the efficacy against severe rotavirus gastroenteritis was 96% through the first rotavirus season, and against any rotavirus gastroenteritis was 87%. Additionally, the efficacy against hospitalization for rotavirus gastroenteritis was 96% through the second season after vaccination.
RV5 vaccine was introduced in the United States in 2006 and RV1 vaccine was introduced in 2008; hence, most early post-introduction data from the United States were based on RV5 vaccine. Several RV5 and RV1 case-control vaccine effectiveness evaluations have been performed in the United States, usually among children younger than age 2 or 3 years. A meta-analysis using data published through 2017 found that the vaccine effectiveness for a full series against the combined outcome of emergency department visit or hospitalization for rotavirus disease was 84% for RV5 vaccine and 83% for RV1 vaccine. Vaccine effectiveness estimates tend to increase with increasing rotavirus disease severity. Both vaccines have demonstrated broad effectiveness across rotavirus genotypes.
The duration of immunity from rotavirus vaccine is not precisely known, although good efficacy has been demonstrated through the first 2 to 3 years of life in the United States. In low-income countries, vaccine effectiveness has generally been lower in the second year of life compared to the first year.
Rotavirus Vaccine Contraindications and Precautions
- Contraindication
- Severe allergic reaction to a vaccine component or following a prior dose of vaccine
- Latex rubber is contained in the RV1 oral applicator, so infants with a severe allergy to latex should not receive RV1 vaccine; some experts prefer that infants with spina bifida or bladder exstrophy, who are at high risk for acquiring latex allergy, receive RV5 instead of RV1 vaccine
- History of intussusception
- Severe combined immunodeficiency (SCID)
- Precaution
- Moderate or severe acute illnesses, including gastroenteritis (defer until symptoms improve)
- Altered immunocompetence (SCID is a contraindication)
- Limited data do not indicate a different safety profile in HIV-infected versus HIV-uninfected infants
- Chronic gastrointestinal disease (data regarding the safety of rotavirus vaccine for infants with preexisting chronic gastrointestinal conditions are lacking)
Contraindications and Precautions to Vaccination
As with other vaccines, a history of a severe allergic reaction (anaphylaxis) to a vaccine component or following a prior dose is a contraindication to further doses. Moderate or severe acute illness (with or without fever) in a patient is considered a precaution to vaccination, although persons with minor illness may be vaccinated.
Latex rubber is contained in the RV1 vaccine oral applicator, so infants with a severe allergy to latex should not receive RV1 vaccine. The RV5 vaccine dosing tube is latex free. Some experts prefer that infants with spina bifida or bladder exstrophy, who are at high risk for acquiring latex allergy, receive RV5 vaccine instead of RV1 vaccine to minimize latex exposure in these children.
In response to reported cases of vaccine-acquired rotavirus infection following rotavirus vaccine administration in infants with severe combined immunodeficiency (SCID), ACIP added SCID as a contraindication to rotavirus vaccination. For children with known or suspected immune deficiency, consultation with an immunologist or infectious diseases specialist before administration of rotavirus vaccine should occur. Children who are immunocompromised because of congenital immunodeficiency, or hematopoietic stem cell or solid organ transplantation, sometimes experience severe, prolonged, and even fatal wild-type rotavirus gastroenteritis.
The limited data available at the time of the ACIP recommendations in 2009 did not indicate that rotavirus vaccines had a substantially different safety profile in human immunodeficiency virus (HIV)-infected infants that are clinically asymptomatic or mildly symptomatic compared with infants that are not HIV-infected. Additional data are available from evaluations in HIV-infected infants as well as infants who were HIV-exposed but uninfected; rotavirus vaccine was found to be well-tolerated and immunogenic.
Post-marketing studies of the currently licensed vaccines have detected an increased risk for intussusception following rotavirus vaccine administration. As a result, a history of intussusception is a contraindication to rotavirus vaccination.Infants living in households with persons who are immunocompromised can be vaccinated. The indirect protection for the immunocompromised household member provided by vaccinating the infant in the household outweighs the small risk for transmitting vaccine virus to the immunocompromised household member.
Infants living in households with pregnant women should be vaccinated according to the routine schedule. Because the majority of women of childbearing age have pre-existing immunity to rotavirus, the risk for infection by the attenuated vaccine virus is considered to be very low. It is prudent for all members of the household to employ measures to avoid contact with feces of the vaccinated infant, such as good hand washing after changing a diaper.
Rotavirus Vaccine – Conditions Not Considered to be Precautions
- Infants living in households with immunocompromised persons
- Infants living in households with pregnant women
Rotavirus vaccine should generally be deferred in infants with acute, moderate or severe gastroenteritis, or other acute illness until the condition improves. However, infants with mild acute gastroenteritis or other mild acute illness can be vaccinated, particularly if a delay in vaccination will postpone the first dose of vaccine beyond 14 weeks 6 days of age. Data regarding the safety of rotavirus vaccine for infants with preexisting chronic gastrointestinal conditions are lacking. Infants with chronic gastrointestinal diseases (e.g. Hirschsprung’s disease, short-gut syndrome, or congenital malabsorption syndromes) who are not undergoing immunosuppressive therapy should benefit from rotavirus vaccination.
Infants living in households with persons who are immunocompromised can be vaccinated. The indirect protection for the immunocompromised household member provided by vaccinating the infant in the household outweighs the small risk for transmitting vaccine virus to the immunocompromised household member.
Infants living in households with pregnant women should be vaccinated according to the routine schedule. Because the majority of women of childbearing age have pre-existing immunity to rotavirus, the risk for infection by the attenuated vaccine virus is considered to be very low. It is prudent for all members of the household to employ measures to avoid contact with feces of the vaccinated infant, such as good hand washing after changing a diaper.
Rotavirus Vaccine Safety
- RV5
- Diarrhea 18.1%
- Vomiting 11.6%
- Also greater rates of otitis media, nasopharyngitis, and bronchospasm
- RV1
- Irritability 11.4%
- Cough or runny nose 3.6%
- Flatulence 2.2%
- Intussusception
- Postlicensure-evaluation of RV1 and/or RV5 identified low level risk of intussusception; 1 excess case per 20,000 to 100,000 in the U.S.
Vaccine Safety
In RV5 vaccine clinical trials, detailed information on adverse events were collected in a subset of participants for 42 days after each vaccination. In the first week after any dose, RV5 vaccine recipients had a small but statistically significant increased rate of diarrhea (18.1% in RV5, 15.3% in placebo group) and vomiting (11.6% in RV5, 9.9% in placebo). During the 42-day period following any dose, statistically significantly greater rates of diarrhea, vomiting, otitis media, nasopharyngitis, and bronchospasm occurred in RV5 recipients compared to placebo recipients. The incidence of serious adverse events (including death) within 42 days of any dose was similar among RV5 and placebo recipients. A similar rate of gastroenteritis occurring any time after a dose was reported in both RV5 and placebo recipients (0.2% in RV5, 0.3% in placebo group).
In RV1 clinical trials, participants were monitored for adverse events in the 31-day period after vaccination. During the 31-day period after vaccination, the following unsolicited adverse events occurred at a statistically higher incidence among vaccine recipients: irritability (11.4% in RV1 group, 8.7% in placebo group) and flatulence (2.2% in RV1 group, 1.3% in placebo group). The rate of serious adverse events (including death) was similar in RV1 and placebo recipients. Detailed information on adverse events were collected in a subset of participants during the first week following each dose. Rates of adverse events in this subset were similar in RV1 and placebo recipients except for grade 3 (i.e., those that prevented normal everyday activities) cough or runny nose, which occurred at a slightly but statistically higher rate in the RV1 vaccine group (3.6 %) compared to the placebo group (3.2%).
Post-marketing strain surveillance in the United States and other countries has occasionally detected rotavirus vaccine reassortant strains in stool samples of children with diarrhea. In some of these reports, the reassortant virus seemed to be the likely cause of the diarrheal illness.
Large phase III clinical trials (more than 60,000 infants each) of RV5 and RV1 vaccine evaluated the occurrence of intussusception after vaccination. No increased risk for intussusception was observed for either vaccine. However, post-licensure monitoring was planned to evaluate for a possible risk of intussusception at a lower level than that able to be evaluated in the clinical trials. Post-licensure evaluations of RV5 vaccine and/or RV1 vaccine in some middle and high income countries have identified a low-level increased risk of intussusception following vaccination. In the United States, the risk is estimated as 1 excess case of intussusception per 20,000 to 100,000 vaccinated infants. The risk appears to be primarily during the first week following the first or second dose, but may extend up to 21 days following the first dose. Parents and health care providers should be aware of the low-level increased risk of intussusception following rotavirus vaccine so that infants with possible intussusception can be promptly evaluated.
Vaccine Storage and Handling
Rotavirus vaccine should be maintained at refrigerator temperature between 2°C and 8°C (36°F and 46°F). Manufacturer package inserts contain additional information, including information on storage of the diluent in oral applicators for RV1. For complete information on best practices and recommendations, please refer to CDC’s Vaccine Storage and Handling Toolkit [3 MB, 65 pages].
Surveillance and Reporting of Rotavirus
Rotavirus gastroenteritis is not a nationally notifiable disease in the United States. Estimates of incidence and disease burden are based on special surveys, cohort studies, and hospital discharge data. Methods of surveillance for rotavirus disease at the national level include review of national hospital discharge databases for rotavirus-specific or rotavirus-compatible diagnoses, surveillance for rotavirus disease at sites that participate in the New Vaccine Surveillance Network, and reports of rotavirus detection from a sentinel system of laboratories. Special evaluations (e.g., case control and retrospective cohort methods) have been used to measure the effectiveness of rotavirus vaccine under routine use in the United States. CDC has also established a national strain surveillance system of sentinel laboratories that monitors circulating rotavirus strains. For information on guidance for state and local health department staff who are involved in surveillance activities for vaccine-preventable diseases, please consult the Manual for the Surveillance of Vaccine-Preventable Diseases.
Acknowledgements
The editors would like to acknowledge Valerie Morelli and Ginger Redmon for their contributions to this chapter.
Selected References
- American Academy of Pediatrics. Rotavirus infections. In: Kimberlin D, Brady M, Jackson M, et al., eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL: American Academy of Pediatrics; 2018:700–4.
- Baker J, Tate J, Steiner C, et al. Longer-term direct and indirect effects of infant rotavirus vaccination across all ages in the United States in 2000-2013: analysis of a large hospital discharge data set. Clin Infect Dis 2019;5;68(6):976–83.
- Bowen M, Mijatovic-Rustempasic S, Esona M, et al. Rotavirus strain trends during the postlicensure vaccine era: United States, 2008-2013. J Infect Dis 2016;214(5):732–8.
- CDC. Addition of history of intussusception as a contraindication for rotavirus vaccination. MMWR 2011;60(41):1427.
- CDC. Addition of severe combined immunodeficiency as a contraindication for administration of rotavirus vaccine. MMWR 2010;59(22):687–8.
- CDC. Prevention of rotavirus gastroenteritis among infants and children recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2009;58(No. RR-2):1–25.
- Cortes J, Curns A, Tate J, et al. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med 2011;365(12):1108–17
- Haber P, Patel M, Pan Y, et al. Intusussception after rotavirus vaccines reported to US VAERS, 2006-2012. Pediatrics 2013;131(6):1042–9.
- Hallowell B, Parashar U, Curns A, et al. Trends in the laboratory detection of rotavirus before and after implementation of routine rotavirus vaccination — United States, 2000–2018. MMWR 2019;68(24):539–43.
- Leshem E, Moritz R, Curns A, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007-2011). Pediatrics 2014;134(1):15–23.
- Patel M, Glass R, Desai R, et al. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infect Dis 2012;12(7):561–70 .
- Payne D, Englund J, Weinberg G, et al. Association of rotavirus vaccination with inpatient and emergency department visits among children seeking care for acute gastroenteritis, 2010-2016. JAMA Netw Open 2019;2(9):e1912242.
- Pindyck T, Tate J, Parashar U. A decade of experience with rotavirus vaccination in the United States – vaccine uptake, effectiveness, and impact. Expert Rev Vaccines 2018;17(7):593–606.
- Pringle K, Cardemil C, Pabst L, et al. Uptake of rotavirus vaccine among US infants at Immunization Information System Sentinel Sites. Vaccine 2016;34(50):6396–401.
- Rha B, Tate J, Weintraub E, et al. Intussusception following rotavirus vaccination: an updated reviewof the available evidence. Expert Rev Vaccines. 2014;13(11):1339–48.
- Ruiz-Palacios G, Pérez-Schael I, Velázquez F, et al; Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006;354(1):11–22.
- Staat M, Payne D, Halasa N, et al. Continued evidence of the impact of rotavirus vaccine in children less than 3 years of age from the US New Vaccine Surveillance Network- a multi-site active surveillance program, 2006-2016. Clin Infect Dis 2020;ciaa150. doi: 10.1093/cid/ciaa150. [Epub ahead of print]
- Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomized, double-blind controlled study. Lancet 2007;370(9601):1757–63.
- Vesikari T, Matson D, Dennehy P, et al. Safety and efficacy of a pentavalent human–bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006;354:23–33.
- Weintraub E, Baggs J, Duffy J, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med 2014;370(6):513–9.
- Yih W, Lieu T, Kulldorff M, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med 2014;370(6):503–12