NIOSH Conformity Assessment Letter to Manufacturers
Subject: Interim guidance regarding applications for NIOSH Approval of Filtering Facepiece Respirators in accordance with the Food and Drug Administration (FDA) Final Order published May 17, 2018, and FDA/NIOSH MOU 225-18-006, dated November 2017 (included as a reference in this notice).
Revision Supersedes the November 2018 version
NIOSH CA 2018-1010R1.0
Revised August 2020
Summary
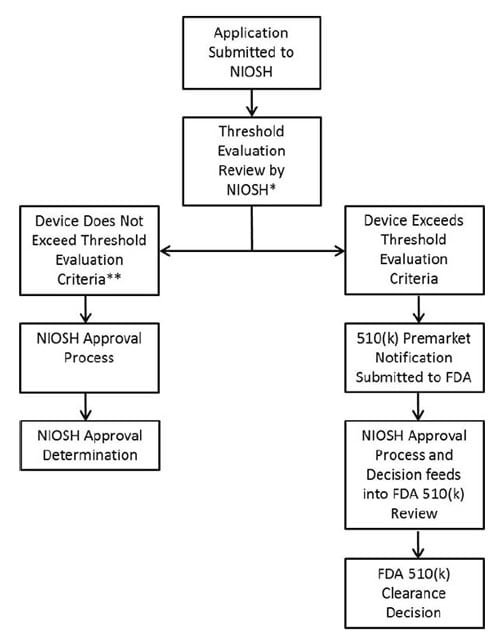
Beginning August 24, 2020, the National Institute for Occupational Safety and Health (NIOSH) will accept applications to implement the coordinated regulatory process to exempt a subset of single-use disposable N95 filtering facepiece respirators (FFRs) from Food and Drug Administration (FDA) premarket notification requirements. The N95 FFRs and data provided by the applicant must demonstrate:
- all applicable NIOSH N95 FFR requirements found in 42 CFR 84
- flammability, fluid resistance (penetration by synthetic blood), and biocompatibility, previously reviewed by the FDA, and evaluated by NIOSH in accordance with the Memorandum of Understanding (MOU) between the FDA/Center for Devices & Radiological Health and the Centers For Disease Control & Prevention/NIOSH/National Personal Protective Technology Laboratory when FDA Threshold Criteria is not exceeded
- flammability, fluid resistance, and biocompatibility data provided to NIOSH must be generated by a laboratory meeting FDA’s Good Laboratory Practices requirement (21 CFR Part 58)
- no significant deviation from respirator designs previously cleared by the FDA under product code MSH, such as the addition of exhalation valves, novel head suspensions, sterility claims, antimicrobial treatments, drug delivery systems, or nano scale technologies
- consideration of FDA recommendations for labeling medical products to inform users that the product is not made with natural rubber latex.
Any device approved under this guidance will be labeled with the respiratory protection of “N95”, referred to as a Surgical N95, and will also be documented as meeting the FDA specified flammability, fluid resistance, and biocompatibility requirements and can be used in healthcare settings. Monthly NIOSH Certified Equipment List updates will include searchable information about approved Surgical N95 respirators.
- Surgical N95 respirator approval labels must include caution and limitation “S” defined as: Special or critical User Instructions and/or specific use limitations apply. Refer to User Instructions before donning.
- The User Instructions and packaging must state the following under the “S – Special or Critical User Instructions” heading: This respirator has been approved as a NIOSH N95 filtering facepiece respirator, for use in healthcare settings, as a Surgical N95 Respirator conforming to recognized standards for biocompatibility, flammability, and fluid resistance.
- The approval label is no longer required to have the caution and limitation “P” – NIOSH does not evaluate respirators for use as surgical masks.
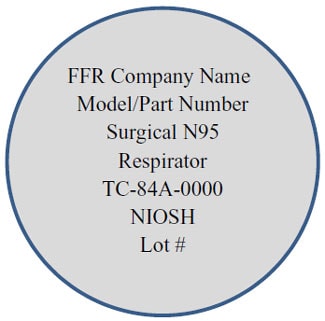
- The abbreviated label (what is printed on every FFR) will include additional wording to inform users: “Surgical N95 Respirator”. See the example below.
Four specific situations are noted:
- No immediate action under this guidance is required for an existing device that has previously obtained NIOSH approval and FDA 510(k) clearance (i.e., Surgical N95 FFRs). The existing approval will remain effective as long as the approval holder continues to 1) pay the annual NIOSH maintenance fee, 2) register and list your company with the FDA, and 3) meet NIOSH and FDA post market requirements. The approval holder will be expected to revise the User Instructions and packaging to include caution and limitation “S” and remove “P”, as defined above, the next time that the approval is submitted for an extension of approval. And in some cases, the approval holder must update the abbreviated label to align with this guidance.
- If a manufacturer of an existing NIOSH-approved N95 FFR respirator has not previously sought FDA 510(k) clearance and now seeks to label a device with the additional protections (flammability, fluid resistance, and biocompatibility), the manufacturer must follow this guidance to achieve a NEW NIOSH approval for the Surgical N95 respirator. The approval holder is encouraged to issue a new model/part number for the Surgical N95.
- If a manufacturer of an existing NIOSH-approved N95 FFR respirator has not previously sought FDA 510(k) clearance and does not seek to label this device with the additional protections (flammability, fluid resistance, and biocompatibility), the existing NIOSH approval will remain effective.
- If a manufacturer of a NEW N95 FFR seeks NIOSH approval for a Surgical N95, the manufacturer must follow this guidance.
NEW NIOSH APPLICANTS:
If an applicant has never previously submitted any type of respiratory protective device for NIOSH approval, the applicant must first apply to NIOSH for a three-character Manufacturer’s Code by completing the Prospective Approval Holder Form and returning it to the NIOSH NPPTL Records Room. Applicants can obtain this form by contacting the NIOSH NPPTL Records Room at recordsroom@cdc.gov. After obtaining the Manufacturer’s Code, the manufacturer seeking approval for a Surgical N95 must follow this guidance.
NEW APPLICANTS AND EXISTING NIOSH APPROVAL HOLDERS APPLYING for SURGICAL N95 APPROVAL:
The applicant will use the NIOSH Standard Application Procedure (SAP) to complete the Standard Application Form. When completing the reason for application section (C.9), the applicant will indicate they are using the consolidated process and seeking approval within the terms of the FDA Final Order (83 FR 22846) and the MOU.
- The approval label provided as part of the NIOSH Surgical N95 application must include the caution and limitation “S” defined as: Special or critical User Instructions and/or specific use limitations apply. Refer to User Instructions before donning. The User Instructions and packaging (including all private label packaging) must state the following: This respirator has been approved as a NIOSH N95 filtering facepiece respirator, for use in healthcare settings, as a Surgical N95 Respirator conforming to recognized standards for biocompatibility, flammability, and fluid resistance.
- The abbreviated label (what is printed on every FFR) will include additional wording to inform users: “Surgical N95 Respirator”. See the example below.

Applicants are required to provide documentation to NIOSH to indicate conformance to the FDA thresholds for flammability, fluid resistance, and biocompatibility, as described in the MOU. All data must be received in electronic formats as indicated in the NIOSH SAP. While NIOSH completes testing of hardware in accordance with 42 CFR 84 Subpart K, NIOSH is not conducting testing to verify the flammability, fluid resistance, or biocompatibility performance of the respirator as part of the consolidated Surgical N95 approval process.
During the NIOSH document review process, NIOSH will review flammability, fluid resistance, and biocompatibility test data and results provided by the applicant and in accordance with the Threshold Evaluation Criteria defined in the MOU.
In accordance with the MOU, Surgical N95 Approval Holders are required to comply with the general controls required under the Federal Food, Drug, and Cosmetic Act (see section 513(a)(1) of the FD&C Act) and implementing regulations, including annual registration and listing obligations, quality system regulations, post market requirements, and other requirements as established by the FD&C Act and implementing regulations (e.g., requirements set forth in 21 CFR Parts 803 and 820) and applicable special controls under 21 CFR 878.4040. Nothing in this document changes or affects applicable FDA regulatory requirements or authority.
Manufacturers making claims exceeding the threshold evaluation criteria defined in the MOU must consider the timelines as described in the MOU. (MOU Appendix, Section 2).
Note: Tuberculosis protection claims in accordance with CDC Guidance are allowed.
The NIOSH Surgical N95 Approval Holder must use NIOSH application procedures and notify NIOSH of any changes made to the NIOSH-Approved Surgical N95. Additional testing and data may be required before changes are approved by NIOSH.
REFERENCES
FDA Final Order
FDA Postmarket Requirements (Devices)
Approval of Respiratory Protective Devices, 42 C.F.R, Part 84
MOU 225-18-006 (also included below)
Reference: MEMORANDUM OF UNDERSTANDING (MOU) 225-18-006), November 2017
BETWEEN THE FOOD & DRUG ADMINISTRATION/CENTER FOR DEVICES & RADIOLOGICAL HEALTH ANDTHE CENTERS FOR DISEASE CONTROL & PREVENTION/NATIONAL INSTITUTE FOR OCCUPATIONAL SAFETY & HEALTH/NATIONAL PERSONAL PROTECTIVE TECHNOLOGY LABORATORY
Appendix: NIOSH and FDA Review Processes for N95s
| Revision (R) | Date | Reason for Revision |
| 1.0 | 14 August 2020 | NIOSH revised this notice to remove references to “N95-F” filtering facepiece respirators. On June 29,2020 FDA and NIOSH agreed to use existing terminology “Surgical N95” to define these FFRs approved before and after the 2018 MOU. |