Sexual Assault and Abuse and STIs – Adolescents and Adults
These guidelines are primarily limited to the identification, prophylaxis, and treatment of STIs and conditions among adolescent and adult female sexual assault survivors. However, some of the following guidelines might still apply to male sexual assault survivors. Documentation of findings, collection of nonmicrobiologic specimens for forensic purposes, and management of potential pregnancy or physical and psychological trauma are beyond the scope of these guidelines. Examinations of survivors of sexual assault should be conducted by an experienced clinician in a way that minimizes further trauma to the person. The decision to obtain genital or other specimens for STI diagnosis should be made on an individual basis. Care systems for survivors should be designed to ensure continuity, including timely review of test results, support adherence, and monitoring for adverse reactions to any prescribed therapeutic or prophylactic regimens. Laws in all 50 states limit the evidentiary use of a survivor’s previous sexual history, including evidence of previously acquired STIs, as part of an effort to undermine the credibility of the survivor’s testimony. Evidentiary privilege against revealing any aspect of the examination or treatment also is enforced in most states. Although it rarely occurs, STI diagnoses might later be accessed, and the survivor and clinician might opt to defer testing for this reason. Although collection of specimens at initial examination for laboratory STI diagnosis gives the survivor and clinician the option of deferring empiric prophylactic antimicrobial treatment, compliance with follow-up visits is typically poor (1423–1425). Among sexually active adults, identification of an STI might represent an infection acquired before the assault, and therefore might be more important for the medical management of the patient than for legal purposes.
Trichomoniasis, BV, gonorrhea, and chlamydia are the most frequently diagnosed infections among women who have been sexually assaulted. Such conditions are prevalent among the population, and detection of these infections after an assault does not necessarily imply acquisition during the assault. However, a postassault examination presents an important opportunity for identifying or preventing an STI. Chlamydial and gonococcal infections among women are of particular concern because of the possibility of ascending infection. In addition, HBV infection can be prevented through postexposure vaccination (see Hepatitis B Virus Infection). Because persons who have been sexually assaulted also are at risk for acquiring HPV infection, and the efficacy of the HPV vaccine is high (1426,1427), HPV vaccination is also recommended for females and males through age 26 years (https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/hpv.html) (11). Reproductive-aged female survivors should be evaluated for pregnancy and offered emergency contraception.
Evaluating Adolescents and Adults for STIs
Initial Examination
Decisions to perform the following tests should be made on an individual basis. An initial examination after a sexual assault might include the following:
- NAATs for C. trachomatis and N. gonorrhoeae at the sites of penetration or attempted penetration should be performed (553). These tests are preferred for diagnostic evaluation of adolescent or adult sexual assault survivors.
- Females should be offered NAAT testing for T. vaginalis from a urine or vaginal specimen. POC or wet mount with measurement of vaginal pH and KOH application for the whiff test from vaginal secretions should be performed for evidence of BV and candidiasis, especially if vaginal discharge, malodor, or itching is present.
- MSM should be offered screening for C. trachomatis and N. gonorrhoeae if they report receptive oral or anal sex during the preceding year, regardless of whether sexual contact occurred at these anatomic sites during the assault. Anoscopy should be considered in instances of reported anal penetration.
- A serum sample should be performed for HIV, HBV, and syphilis infection.
Treatment
Compliance with follow-up visits is poor among survivors of sexual assault (1423–1425). Consequently, the following routine presumptive treatments after a sexual assault are recommended:
- An empiric antimicrobial regimen for chlamydia, gonorrhea, and trichomonas for women and chlamydia and gonorrhea for men.
- Emergency contraception should be considered when the assault could result in pregnancy (see Emergency Contraception).
- Postexposure hepatitis B vaccination (without HBIG) if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. If the assailant is known to be HBsAg positive, unvaccinated survivors should receive both hepatitis B vaccine and HBIG. The vaccine and HBIG, if indicated, should be administered to sexual assault survivors at the time of the initial examination, and follow-up doses of vaccine should be administered 1–2 and 4–6 months after the first dose. Survivors who were previously vaccinated but did not receive postvaccination testing should receive a single vaccine booster dose (see Hepatitis B Virus Infection).
- HPV vaccination for female and male survivors aged 9–26 years who have not been vaccinated or are incompletely vaccinated (11) (https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/hpv.html). The vaccine should be administered to sexual assault survivors at the time of the initial examination, and follow-up doses should be administered at 1–2 months and 6 months after the first dose. A 2-dose schedule (0 and 6–12 months) is recommended for persons initiating vaccination before age 15 years.
- Recommendations for HIV PEP are made on a case-by-case basis according to risk (see Risk for Acquiring HIV Infection; Recommendations for Postexposure HIV Risk Assessment of Adolescents and Adults <72 Hours After Sexual Assault).
Ceftriaxone 500 mg* IM in a single dose
PLUS
Doxycycline 100 mg 2 times/day orally for 7 days
PLUS
Metronidazole 500 mg orally 2 times/day orally for 7 days
* For persons weighing ≥150 kg, 1 g of ceftriaxone should be administered.
Ceftriaxone 500 mg* IM in a single dose
PLUS
Doxycycline 100 mg 2 times/day orally for 7 days
* For persons weighing ≥150 kg, 1 g of ceftriaxone should be administered.
Clinicians should counsel persons regarding the possible benefits and toxicities associated with these treatment regimens; gastrointestinal side effects can occur with this combination. The efficacy of these regimens in preventing infections after sexual assault has not been evaluated. For those requiring alternative treatments, refer to the specific sections in this report relevant to the specific organisms.
Other Management Considerations
At the initial examination and, if indicated, at follow-up examinations, patients should be counseled regarding symptoms of STIs and the need for immediate examination if symptoms occur. Further, they should be instructed to abstain from sexual intercourse until STI prophylactic treatment is completed.
Follow-Up
After the initial postassault examination, follow-up examinations provide an opportunity to detect new infections acquired during or after the assault, complete hepatitis B and HPV vaccinations, if indicated, complete counseling and treatment for other STIs, and monitor side effects and adherence to PEP, if prescribed. If initial testing was performed, follow-up evaluation should be conducted in <1 week to ensure that results of positive tests can be discussed promptly with the survivor, treatment is provided if not administered at the initial visit, and any follow-up for infections can be arranged. If initial tests are negative and treatment was not provided, examination for STIs can be repeated 1–2 weeks after the assault; repeat testing detects infectious organisms that might not have reached sufficient concentrations to produce positive test results at the time of initial examination. For survivors who are treated during the initial visit, regardless of whether testing was performed, posttreatment testing should be conducted only if the person reports having symptoms. If initial test results were negative and infection in the assailant cannot be ruled out, serologic tests for syphilis can be repeated at 4–6 weeks and 3 months; HIV testing can be repeated at 6 weeks and at 3 months by using methods to identify acute HIV infection.
Risk for Acquiring HIV Infection
HIV seroconversion has occurred among persons whose only known risk factor was sexual assault or sexual abuse; however, the frequency of this occurrence likely is low (1428,1429). In consensual sex, the per-act risk for HIV transmission from vaginal intercourse is 0.08%, and for receptive anal intercourse, 1.38% (192). The per-act risk for HIV transmission from oral sex is substantially lower. Specific circumstances of an assault (e.g., bleeding, which often accompanies trauma) might increase risk for HIV transmission in cases involving vaginal, anal, or oral penetration. Site of exposure to ejaculate, viral load in ejaculate, and the presence of an STI or genital lesions in the assailant or survivor also might increase risk for HIV acquisition.
PEP with a 28-day course of zidovudine was associated with an 81% reduction in risk for acquiring HIV in a study of health care workers who had percutaneous exposures to HIV-infected blood (1430). On the basis of these results and results from animal studies, PEP has been recommended for health care workers who have occupational exposures to HIV (1431). These findings have been extrapolated to nonoccupational injecting drug and sexual HIV exposures, including sexual assault. The possibility of HIV exposure from the assault should be assessed at the initial examination; survivors determined to be at risk for acquiring HIV should be informed about the possible benefit of PEP in preventing HIV infection. Initiation of PEP as soon as possible after the exposure increases the likelihood of prophylactic benefit.
Multiple factors affect the medical recommendation for PEP and affect the assault survivor’s acceptance of that recommendation. These factors include the likelihood of the assailant having HIV, any exposure characteristics that might increase the risk for HIV transmission, the time elapsed after the event, and the potential benefits and risks associated with PEP (1431). Determination of the assailant’s HIV status at the time of the postassault examination is usually not possible. Therefore, health care providers should assess any available information concerning the characteristics and HIV risk behaviors of the assailant (e.g., being an MSM or using injecting drugs), local epidemiology of HIV/AIDS, and exposure characteristics of the assault. When an assailant’s HIV status is unknown, determinations about risk for HIV transmission to the survivor should be based on whether vaginal or anal penetration occurred; whether ejaculation occurred on mucous membranes; whether multiple assailants were involved; whether mucosal lesions were present in the assailant or survivor; and any other characteristics of the assault, survivor, or assailant that might increase risk for HIV transmission.
If PEP is offered, the following information should be discussed with the survivor: the necessity of early initiation of PEP to optimize potential benefits (i.e., as soon as possible after and <72 hours after the assault), the importance of close follow-up, the benefit of adherence to recommended dosing, and potential adverse effects of antiretroviral medications. Providers should emphasize that severe adverse effects are rare from PEP (1431–1435). Clinical management of the survivor should be implemented according to the HIV PEP guidelines and in collaboration with specialists (1436). Health care providers should provide an initial course of 3–7 days of medication (i.e., a starter pack) with a prescription for the remainder of the course, or, if starter packs are unavailable, they should provide a prescription for an entire 28-day course. Provision of the entire 28-day PEP medication supply at the initial visit has been reported to increase likelihood of adherence, especially when patients have difficulty returning for multiple follow-up visits (1437). Routinely providing starter packs or the entire 28-day course requires that health care providers stock PEP drugs in their practice setting or have an established agreement with a pharmacy to stock, package, and urgently dispense PEP drugs with required administration instructions. Uninsured patients or those with high copayments can be enrolled in a patient-assistance program to ensure access to PEP medications. An early follow-up visit should be scheduled at which health care providers can discuss the results of HIV and STI testing, provide additional counseling and support, provide indicated vaccines not administered at the initial evaluation, assess medication side effects and adherence, or provide an altered PEP medication regimen if indicated by side effects or laboratory test results.
Recommendations for Postexposure HIV Risk Assessment of Adolescents and Adults <72 Hours After Sexual Assault
Health care providers should do the following:
- Assess risk for HIV infection in the assailant, and test that person for HIV whenever possible.
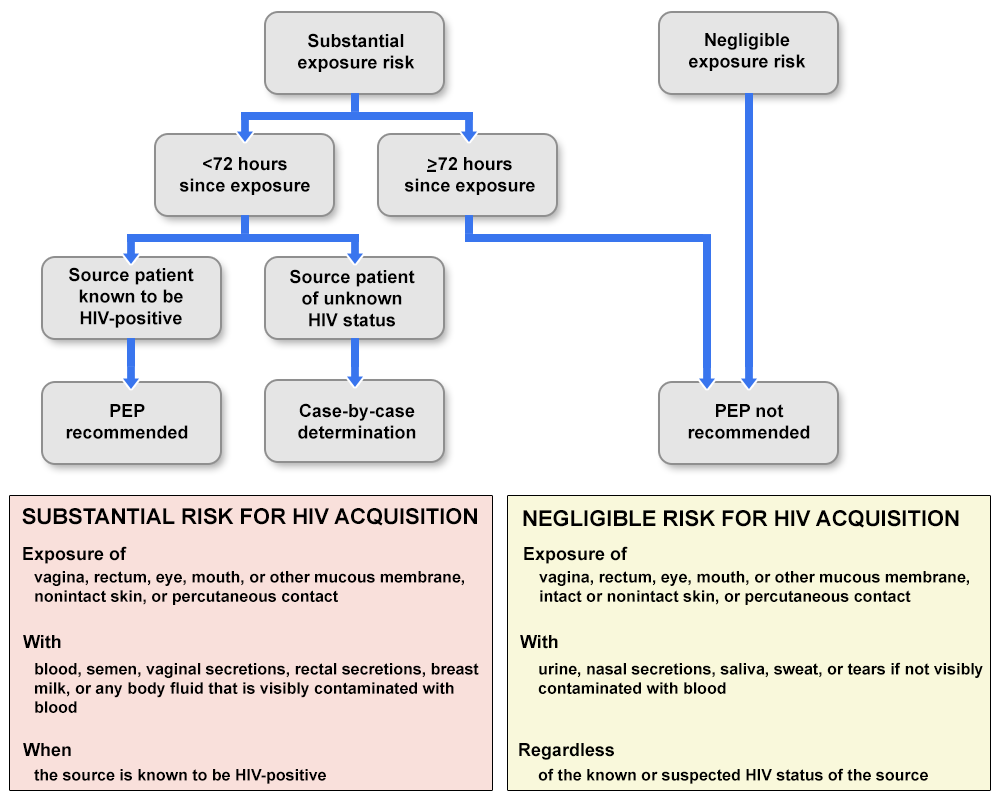
- Use the algorithm to evaluate the survivor for the need for HIV PEP (Figure) (1436).
- Consult with a specialist in HIV treatment if PEP is being considered.
- If the survivor appears to be at risk for acquiring HIV from the assault, discuss PEP, including benefits and risks.
- If the survivor chooses to start PEP, provide an initial course of 3–7 days of medication (i.e., a starter pack) with a prescription for the remainder of the course or provide a prescription for an entire 28-day course. Schedule an early follow-up visit to discuss test results and provide additional counseling (1438).
- If PEP is started, obtain serum creatinine, AST, and alanine aminotransferase at baseline.
- Perform an HIV antibody test at original assessment; repeat at 6 weeks and 3 months.
- Counsel the survivor regarding ongoing risk for HIV acquisition and about HIV PrEP, and provide referrals to a PrEP provider.
Assistance with PEP-related decisions can be obtained by calling the National Clinician’s Post Exposure Prophylaxis Hotline (PEP Line) (telephone: 888-448-4911).
FIGURE 1. Algorithm for evaluation and treatment of possible nonoccupational HIV exposures
Source: Updated Guidelines for Antiretroviral Postexposure Prophylaxis after Sexual, Injection-Drug Use, or Other Nonoccupational Exposure to HIV – United States, 2016. MMWR Morb Mortal Wkly Rep, 2016. 65(17): p. 458.