Gonococcal Isolate Surveillance Project (GISP) Profiles, 2022
GISP Overview
The Gonococcal Isolate Surveillance Project (GISP) was established in 1986 to monitor trends in antimicrobial susceptibilities of N. gonorrhoeae strains in the United States in order to establish an evidence-based rationale for selection of gonococcal therapies. GISP is a sentinel surveillance system based on a collaboration between participating STD clinics and their state or local public health authorities, regional laboratories, and CDC.
N. gonorrhoeae isolates are collected monthly from up to the first 25 men with symptomatic gonococcal urethritis seeking care at participating STD clinics. Clinical and demographic data are abstracted from medical records. Isolates are shipped from participating clinics to the regional laboratories participating in the Antibiotic Resistance Laboratory Network (ARLN) for agar dilution antimicrobial susceptibility testing. In 2022, isolates were tested to determine minimum inhibitory concentrations (MICs) of penicillin, tetracycline, ceftriaxone, cefixime, ciprofloxacin, azithromycin, and gentamicin. Cefixime susceptibility testing was discontinued in 2007 and re-started in 2009. Gentamicin susceptibility testing was first started in 2015. Clinical, demographic, and laboratory data are compiled and analyzed at CDC for public use.
In 2017, GISP expanded in a subset of clinical sites to conduct N. gonorrhoeae surveillance in non-urethral isolates (i.e., pharyngeal, rectal, and endocervical isolates). The Enhanced Gonococcal Isolate Surveillance Program (eGISP) was established to help understand if the pharynx and/or rectum may be anatomic niches that select for or foster resistance and to evaluate if gonococcal susceptibility patterns vary between men and women.
N. gonorrhoeae isolates are collected monthly in eGISP from up to the first 25 men or women with gonococcal infections at the pharynx or rectum and from up to the first 25 women with cervical or vaginal gonococcal infections seeking care at participating STD clinics. The submission of isolates, susceptibility testing by agar dilution, and the transmission of clinical, demographic, and laboratory data are the same as in GISP.
GISP continues to be the core antimicrobial-resistant gonococcal surveillance system in the US. Findings from GISP have directly contributed to the CDC’s STD Treatment Guidelines released in 1993, 1998, 2002, 2006, 2010, 2015, and 2021 and the Gonorrhea Treatment recommendation updates released in 2000, 2004, 2007, 2012, and 2020. Findings from GISP have also directly contributed to CDC’s AR Threats Reports in 2013 and 2019. Expanding GISP supports the ability to detect changes in susceptibility patterns across different populations and to detect resistant infections sooner for improved surveillance and public health response.
|
Antimicrobial
|
MIC Value | MIC Interpretation |
| Ceftriaxone | MIC ≥0.125 µg/ml | Elevated MIC* |
| Cefixime | MIC ≥0.25 µg/ml | Elevated MIC* |
| Azithromycin | MIC ≥2.0 µg/ml | Elevated MIC* |
| Ciprofloxacin | MIC ≥1.0 µg/ml | Resistance |
| Penicillin | MIC ≥2.0 µg/ml or Beta lactamase positive | Resistance |
| Tetracycline | MIC ≥2.0 µg/ml | Resistance |
| Gentamicin | MIC values correlated with susceptibility and resistance have not been established* |
The majority of these criteria are consistent with Clinical and Laboratory Standards Institute (CLSI) criteria.
* As of December 2022, the CLSI criteria for resistance to ceftriaxone, cefixime, gentamicin, and azithromycin and for susceptibility to gentamicin have not been established for N. gonorrhoeae.
GISP and eGISP Site Profiles
The Site Profiles consist of figures depicting the demographic and clinical data and the antimicrobial susceptibility results associated with the N. gonorrhoeae isolates submitted for GISP and eGISP. Each figure is labeled with the participating site and the number of isolates on which the site’s data are based. The maximum number of male urethral gonococcal isolates submitted by each site annually in GISP is 300. The maximum number of female genital and extragenital (pharyngeal and rectal) isolates submitted by each site annually in eGISP is 600. The number of isolates submitted may be fewer for some sites located in areas with lower gonorrhea morbidity or clinics with limited specific populations.
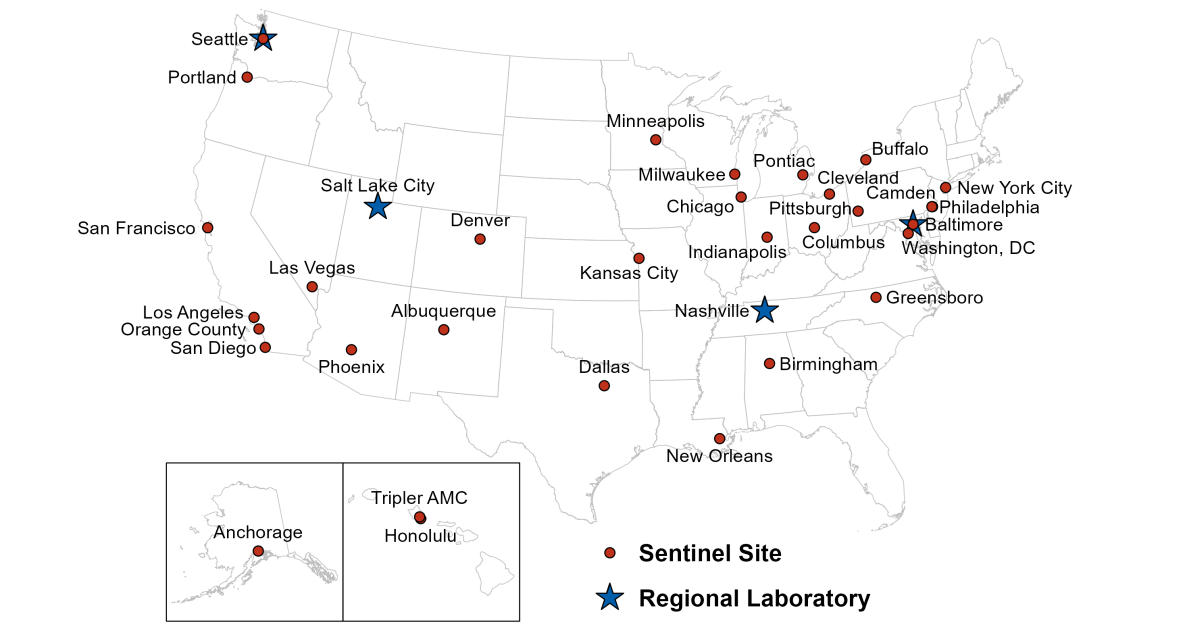
2022 GISP Clinical Sites and Regional Laboratories
STD clinics affiliated with 32 state or city health departments contributed 3,684 gonococcal isolates to GISP in 2022. Of these 32 sites, 10 current sites have participated continuously since 1987.
| Albuquerque, New Mexico (1987 – 2022) |
Greensboro, North Carolina (2002–2022) |
Philadelphia, Pennsylvania (1987 – 2022) |
| Anchorage, Alaska (1987-2003, 2018–2022) |
Honolulu, Hawaii (1987 – 2022) |
Phoenix, Arizona (1987 – 2022) |
| Birmingham, Alabama (1987 – 2022) |
Indianapolis, Indiana (2013–2022) |
Pittsburgh, Pennsylvania (2021-2022) |
| Baltimore, Maryland (1987–2013, 2019 – 2022) |
Kansas City, Missouri (1991–2001, 2007–2022) |
Pontiac, Michigan (2012–2022) |
| Buffalo, New York (2014–2022) |
Las Vegas, Nevada (2002–2022) |
Portland, Oregon (1987 – 2022) |
| Camden, New Jersey (2019 – 2022) |
Los Angeles, California (2003–2022) |
San Diego, California (1987 – 2022) |
| Chicago, Illinois (1996–2022) |
Milwaukee, Wisconsin (2018–2022) |
San Francisco, California (1987 – 2022) |
| Cleveland, Ohio (1991–2022) |
Minneapolis, Minnesota (1992–2022) |
Seattle, Washington (1987 – 2022) |
| Columbus, Ohio (2012–2022) |
New Orleans, Louisiana (1987 – 2022) |
Tripler Army Medical Center, Hawaii (2001–2022) |
| Dallas, Texas (2000–2022) |
New York, New York (2006–2022) |
Washington, District of Columbia (2018–2022) |
| Denver, Colorado (1987-2013, 2018–2022) |
Orange County, California (1991–2022) |
A subgroup of clinical sites began contributing gonococcal isolates to eGISP beginning in 2018. A total of 7 sites participated in eGISP in 2022 contributing 1,473 gonococcal isolates during the year.
| Columbus, Ohio (2018–2022) |
Orange County, California (2018 – 2022) |
Pontiac, Michigan (2018 – 2022) |
| Las Vegas, Nevada (2018–2022) |
Philadelphia, Pennsylvania (2018 – 2022) |
San Diego, California (2018 – 2022) |
| New Orleans, Louisiana (2018 – 2022) |
Phoenix, Arizona (2020 – 2022) |
Starting in 2017, four of the seven ARLN state public health laboratories began functioning as GISP Regional Laboratories. In 2022, antimicrobial susceptibility testing of all GISP and eGISP isolates was conducted by these four laboratories:
- Maryland Department of Health and Mental Hygiene (Baltimore, Maryland)
- Tennessee Department of Health (Nashville, Tennessee)
- Utah Department of Health (Salt Lake City, Utah)
- Washington State Department of Health (Seattle, Washington)
Summary
Susceptibility testing for cefixime began in 1992, was discontinued in GISP in 2007, and was re-started in 2009. The distribution of cefixime MICs each year during 2018–2022 is displayed in Figure 1 of the GISP National Profiles. Each year, approximately 90% of isolates exhibited cefixime MICs ≤0.03 µg/ml. The percentage of isolates with elevated cefixime MICs (≥0.25 µg/ml) fluctuated between approximately 0.1% and 0.4% during 2018 through 2022. Site-specific data are presented in the Site Profiles section of this report.
Susceptibility testing for ceftriaxone began in 1987. The distribution of ceftriaxone MICs each year during 2018–2022 is displayed in Figure 2 of the GISP National Profiles. Each year, approximately 90% of isolates exhibited ceftriaxone MICs ≤0.015 µg/ml. The percentage of GISP isolates that exhibited elevated ceftriaxone MICs, defined as ≥0.125 µg/ml, never went above 0.2% between 2018 and 2022. Site-specific data are presented in the Site Profiles section of this report.
Susceptibility testing for azithromycin began in 1992. The distribution of azithromycin MICs each year during 2018–2022 is displayed in Figure 3 of the GISP National Profiles. Most isolates had azithromycin MICs of 0.125–1.0 µg/ml. The proportion of GISP isolates with azithromycin MICs of ≥2.0 µg/ml peaked in 2020 at 5.8%. Site-specific data are presented in the Site Profiles section of this report.
Susceptibility testing for ciprofloxacin began in 1990. The distribution of ciprofloxacin MICs each year during 2018-2022 is displayed in Figure 4 of the GISP National Profiles. Each year, approximately 30% of isolates exhibited ciprofloxacin MICs ≥1.0 µg/ml. Site-specific data on resistance to ciprofloxacin are presented in the Site Profiles section of this report.