COVID-19 Science Update released: September 17, 2021 Edition 105

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 database, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Estimates of COVID-19 cases and deaths among nursing home residents not reported in federal data. Shen et al. JAMA Network Open (September 9, 2021).
Key findings:
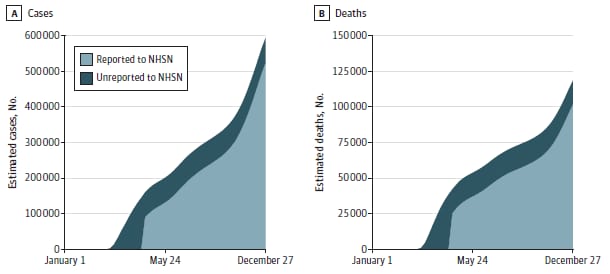
- Among US nursing homes, an estimated 43.7% of COVID-19 cases and 40.0% of COVID-19 deaths before May 24, 2020 were not reported to the federal National Healthcare Safety Network (NHSN), resulting in the omission of 68,613 cases and 16,623 deaths nationwide.
- The percentage of cumulative estimated cases and deaths that were unreported declined by the end of 2020, at the same time reported nursing home cases and deaths steeply increased (Figure).
- After accounting for unreported events (11.6%, cases; 14.0%, deaths), in 2020 estimated nursing home cases were 592,629 and deaths were 118,335 nationwide.
Methods: COVID-19 cases and deaths reported by nursing homes to 20 state health departments (15,415 nursing homes) were compared to those reported to NHSN. Weekly estimates of non-reporting states were extrapolated from the sample states and other data sources including the New York Times COVID-19 database. Limitations: Definitions of cases and deaths vary between states and NHSN, suggesting that underreporting could be overstated in states with broader definitions of cases and deaths.
Implications: A delay in state reporting to the federal NHSN in the early months of the pandemic might have resulted in an undercount in total US nursing home COVID-19 cases and deaths during 2020.
Figure:
Note: Adapted from Shen et al. Estimated and reported national cumulative COVID-19 cases (A) and deaths (B) in nursing homes, 2020. The dark blue area shows cumulative estimates of the cases and deaths unreported. The light blue area shows cumulative cases and deaths using the National Healthcare Safety Network data. Licensed under CC BY.
Clinical and pregnancy outcomes of COVID-19 among hospitalized pregnant women in the United States. Ackerman et al. Open Forum Infectious Diseases (September 3, 2021).
Key findings:
- Among hospitalized pregnant women, risk of poor outcomes was higher in women with COVID-19 compared with those without COVID-19:
- Supplemental oxygen use (Risk Ratio [RR] 2.7, 95% CI 2.5-3.0); critical illness (RR 4.3, 95% CI 4.0-4.5); ICU admission (RR 7.6, 95% CI 6.9-8.5); invasive mechanical ventilation or ECMO (RR 11.9, 95% CI 10.2-13.9); death (RR 13.3, 95% CI 7.3- 24.4).
- In subgroup analyses, risk of ICU admission increased with age and was highest among Asian Non-Hispanic women.
- Maternal deaths among pregnant women with COVID-19 (n = 13) occurred only among Hispanic and Non-Hispanic Black women.
Methods: Retrospective study of hospitalizations among pregnant women (n = 473,902) ages 15–44 years in the United States from April–November 2020, of whom 1.8% (n = 8,584) had a COVID-19 diagnosis. Outcomes were measured among all pregnant women and separately among those who delivered. Limitations: 20.5% of pregnant women were missing information on race/ethnicity; analysis did not account for trimester of pregnancy.
Implications: COVID-19 has adverse effects on maternal health and pregnancy outcomes, especially with increasing maternal age and among Hispanic, Asian Non-Hispanic, and Black Non-Hispanic women.
Note: Adapted from Goldberg et al. A) Rate of SARS-CoV-2 infections, by age group (years) and vaccination period in 2021.
B) Rate of severe COVID-19, by age group (in years) and vaccination period in 2021. White bars represent time periods during which groups were not yet eligible for vaccination in Israel. Permission request in process.
Two studies evaluate the risk of spontaneous abortion after COVID-19 vaccination, one based on vaccination safety data and the other based on large-scale US health system data.
PEER-REVIEWED
A. Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. Zauche et al. NEJM (September 8, 2021).
Key findings:
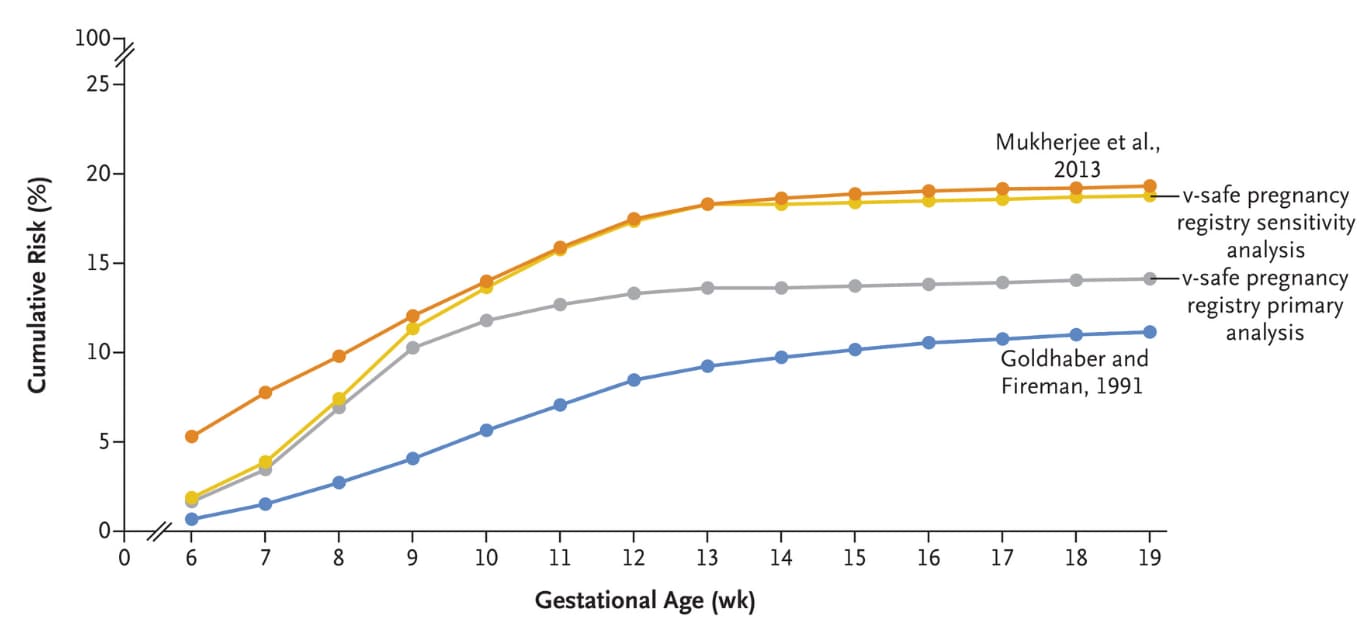
- Cumulative risk of spontaneous abortion from 6–<20 weeks of gestation among vaccinated persons was 14.1% (95% CI 12.1%-16.1%) in the primary analysis and 12.8% (95% CI 10.8%-14.8%) in maternal age standardized analysis, both within the expected range from historical cohorts (Figure).
- Comparable results in a sensitivity analysis were 18.8% (95% CI, 16.6%-20.9%) and 18.5% (95% CI, 16.1%-20.8%).
- 52.7% of participants received BNT162b2 (Comirnaty, Pfizer/BioNTech); 77.3% were ≥30 years of age; 78.3% were Non-Hispanic White; and 88.8% were healthcare personnel.
Methods: Data from CDC v-safe COVID-19 vaccine pregnancy registry were evaluated for cumulative risk of spontaneous abortion from 6–<20 weeks gestation among pregnant persons (n = 2,456). Singleton pregnancies among persons receiving at least 1 dose of mRNA COVID-19 vaccine before conception or up to 20 weeks gestation were included. Age standardized cumulative rates of spontaneous abortion were calculated by gestational week. A sensitivity analysis assumed that all 65 participants with most recent contact during the first trimester had a spontaneous abortion. Limitations: No control group of unvaccinated persons; lack of racial and ethnic diversity and voluntary enrollment in v-safe may limit generalizability.
Figure:
Note: Adapted from Zauche et al. Cumulative risk of spontaneous abortion in the v-safe COVID-19 Vaccine pregnancy registry sensitivity analysis, v-safe pregnancy registry primary analysis, and in two historical cohorts from 2013 and from 1991. Data from Mukherjee et al. included race-specific rates and are White women are provided here to maximize comparability with the v-safe pregnancy registry. From the New England Journal of Medicine, Zauche et al., Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. September 8, 2021, online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
B. Spontaneous abortion following COVID-19 vaccination during pregnancy. Kharbanda et al. JAMA (September 8, 2021).
Key findings:
- Among 105,446 pregnancies, COVID-19 vaccination within 28 days of event did not increase risk of spontaneous abortion compared with ongoing pregnancies (aOR 1.02; 95% CI 0.96-1.08; Figure).
- Results were consistent for mRNA-1273 (Moderna) and BNT162bs (Cominarty, Pfizer/BioNtech) vaccines, and by gestational age group (Figure).
- 8.6% of women with spontaneous abortions received a COVID-19 vaccine within 28 days of the event.
Methods: Validated pregnancies linked to vaccination data were identified in 8 US health systems, as part of the Vaccine Safety Datalink collaboration, from December 15, 2020–June 28, 2021. Spontaneous abortions among women vaccinated within 28 days of the event were categorized by gestational age and compared to same gestational age ongoing pregnancy events during 4-week surveillance periods. Limitations: Gestational age was not confirmed directly through chart review; Ad26.COV2.S (Johnson & Johnson/Janssen) could not be evaluated due small sample size.
Implications for Zauche et al. and Kharbanda et al.: COVID-19 vaccination in the US did not increase the risk of spontaneous abortion compared to expected rates in ongoing pregnancies. mRNA vaccines appear to be safe before and during pregnancy.
PREPRINTS (NOT PEER-REVIEWED)
Perceptions towards mask use in school children during the SARS-CoV-2 pandemic: The Ciao Corona Study. Ammann et al. medRxiv (September 8, 2021). Published in Swiss Medical Weekly as Perceptions towards mask use in school children during the SARS-CoV-2 pandemic: descriptive results from the longitudinal Ciao Corona cohort study (April 20, 2022).
Key findings:
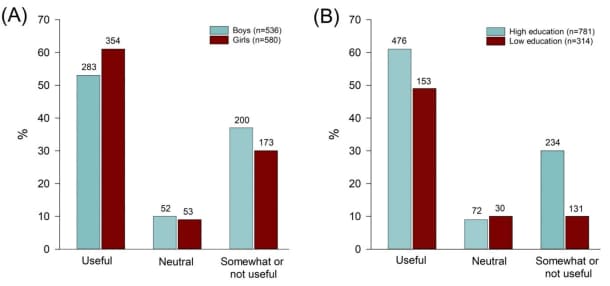
- 60% of school children perceived mask wearing as beneficial to reducing SARS-CoV-2 transmission at school and in public.
- Girls were more likely than boys (61% vs. 53%; p = .018), and students of parents with higher education were more likely than those with less education (61% vs 49%; p< .001) to perceive masks as useful at school (Figure).
- Primary and secondary students had similar perceptions about masks.
- 7–9% of students reported side-effects (skin irritations, headache, or difficulties breathing during physical education) from wearing masks.
Methods: School children (age 10-17 years) in Zurich, Switzerland responded to online questionnaires (Q1 and Q2) as part of a prospective school-based cohort study of SARS-CoV-2; Q1: January–March 2021 (n = 595, secondary, 8th–9th grades); Q2: March–May 2021 (n = 596, primary, 5th–6th grades; and n = 522 secondary). Limitations: 44% item response rate for the mask questionnaires; more than 60% of parents had higher education, not representative of the general population.
Implications: For a safer in-person learning experience, mask wearing at school has been recommended as one prevention strategy against COVID-19 in K–12 schools. Understanding youth perception of masks, including benefits and harms, is important to ensure compliance at school and in public.
Figure:
Note: Adapted from Ammann et al. Perceived use of mask wearing at school A) between girls and boys B) according to parents’ educational level: low education, high education; primary and secondary school students in Zurich, Switzerland, March–May 2021. Numbers on top of bars are counts. Licensed under CC-BY-NC 4.0.
PEER-REVIEWED
Inequities in COVID-19 vaccination rates in the 9 largest US cities. Sacarny et al. JAMA Health Forum (September 3, 2021).
Key findings:
- As of April 2021, the mean COVID-19 vaccination rate (≥1 dose) across neighborhoods in the 9 largest US cities was 42.3% (Interquartile range 27.6%-59.7%).
- Higher vaccination rates were observed in neighborhoods with a higher proportion of White and Asian people, and with socioeconomic advantages by income and 4-year college completion rates.
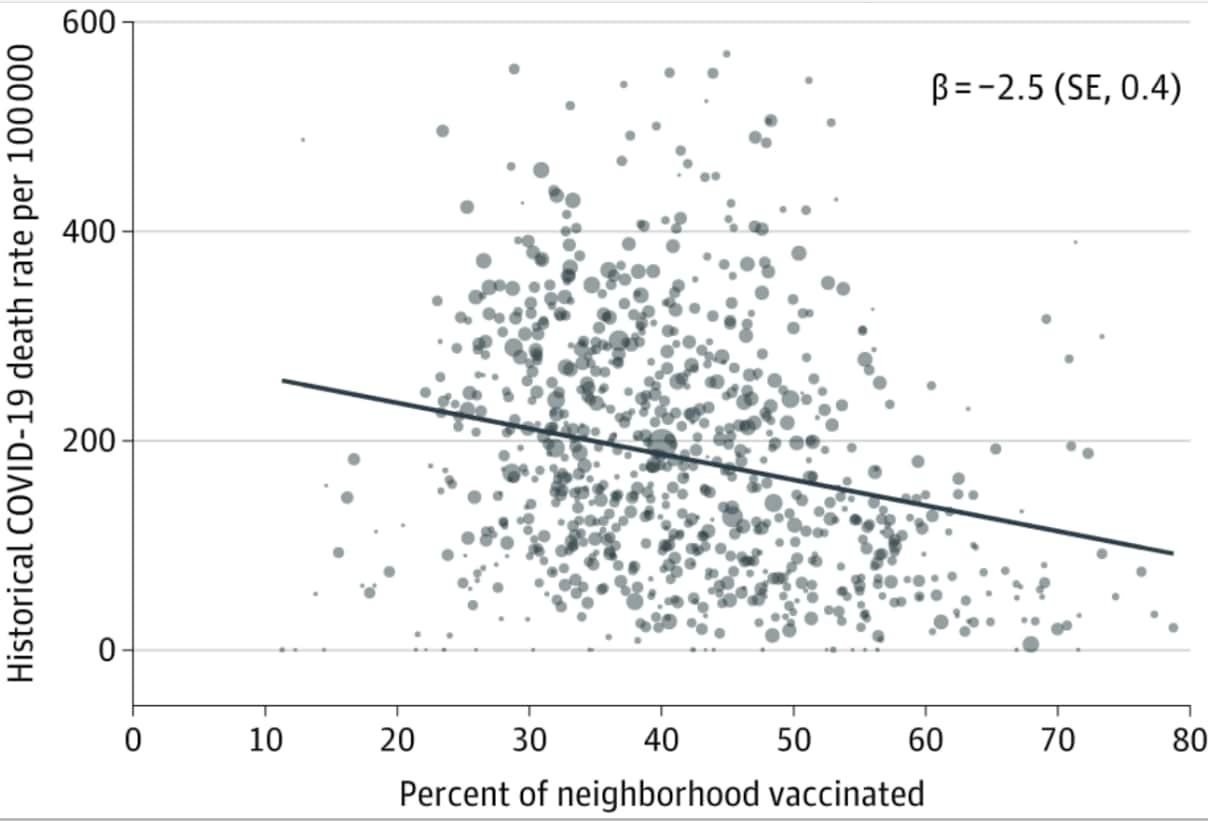
- A 10% increase in the neighborhood vaccination rate was associated with 25 fewer COVID-19 deaths/100,000 population (P < .001) (Figure).
Methods: Ecologic study of COVID-19 vaccination and cumulative death rates in 1,127 neighborhoods in 9 large US cities and surrounding counties (New York, Los Angeles, Chicago, Houston, Phoenix, Philadelphia, San Antonio, San Diego, Dallas) using data obtained from health department websites and the American Community Survey. Neighborhoods (except in Los Angeles) were defined by zip codes. Limitations: Possible inaccuracies in vaccination and death reporting data; vaccine supply and vaccination eligibility during the study period was not considered; findings not generalizable to all US cities.
Implications: During the first five months of vaccine availability, inequities in vaccination were observed in neighborhoods in large cities across the U.S. Increasing uptake of vaccination in neighborhoods that have been marginalized is important to decrease morbidity and mortality of COVID-19.
Figure:
Note: Adapted from Sacarny et al. Association between neighborhood historical (cumulative) COVID-19 death rate and vaccination rate (December 2020–April 2021). The line of best fit controls for city and is weighted by total population. β is the slope coefficient with robust SE reported in parentheses. Licensed under CC BY.
Vaccines
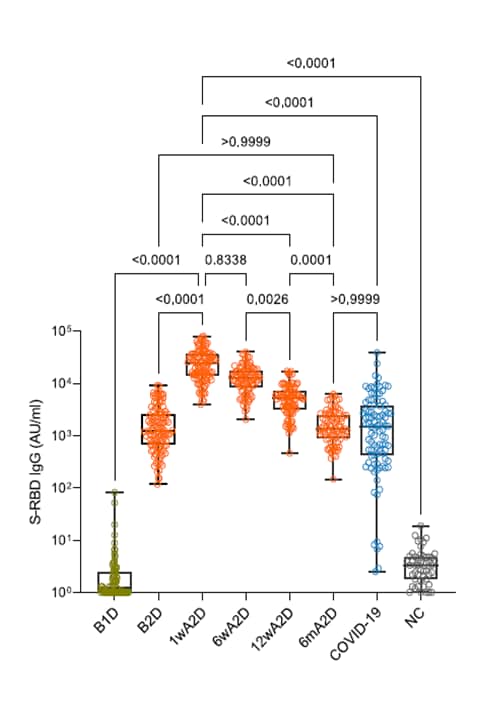
- Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Naaber et al. The Lancet Regional Health Europe (September 5, 2021). Six months after 2 doses of BNT162b2 (Comirnaty, Pfizer/BioNTech) vaccine, spike IgG antibody levels in 122 SYNLAB Estonia employee volunteers (100% white, 83% female, median age 34 years) were similar to levels in persons vaccinated with 1 dose or in COVID-19 convalescent individuals.
Note: Adapted from Naaber et al. Antibody responses S-RBD IgG in individuals vaccinated with BNT162b2 (Comirnaty, Pfizer/BioNTech), before vaccination (B1D, n = 88), after 1 dose (B2D, n = 111), 1 week after 2 doses (1wA2D, n = 106); 6 weeks after 2 doses (6wA2D, n = 89); 12 weeks after 2 doses (12wA2D, n = 90); 6 months after 2 doses (6mA2D; n = 84); compared with post-infection levels in patients recovered from COVID-19 (COVID-19, n = 97); and pre-COVID-19 negative controls (NC, n = 50). Licensed under CC-BY-NC-ND.
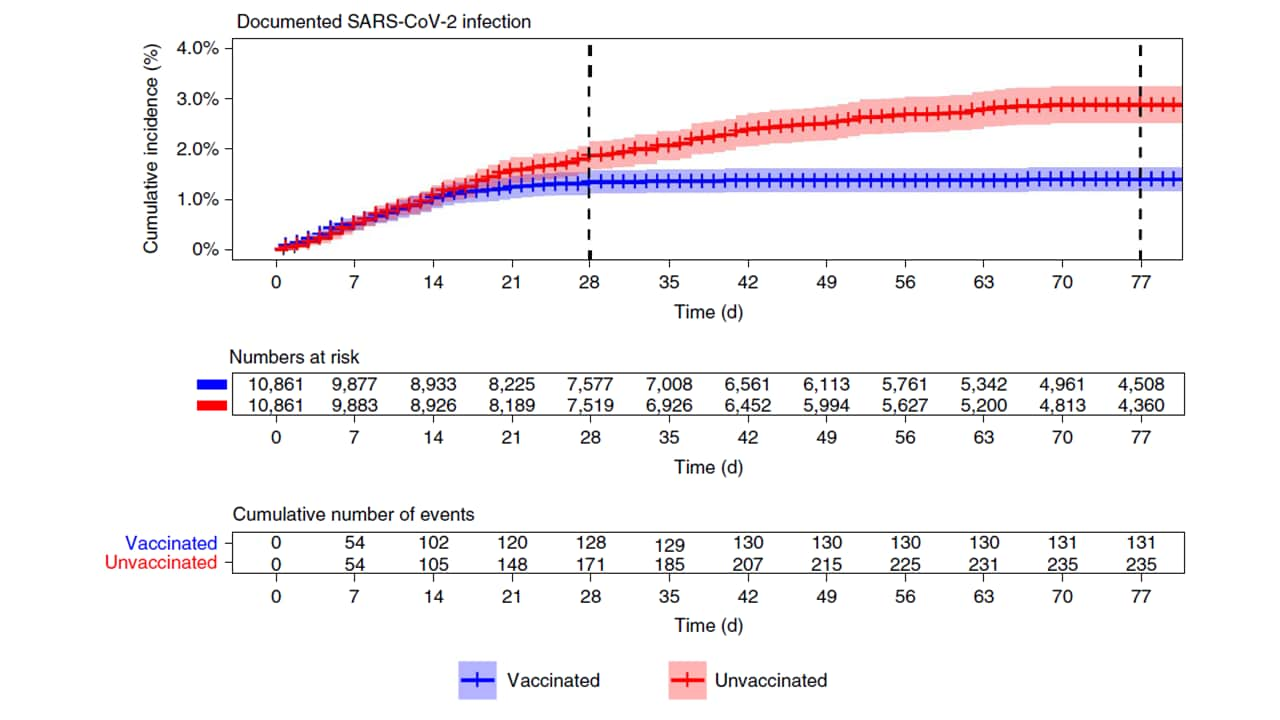
- Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Dagan et al. Nature Medicine (September 7, 2021). Among 21,722 (half vaccinated, half matched controls) pregnant women in Israel, BNT162b2 (Comirnaty, Pfizer/BioNTech) vaccine effectiveness was 96% (95% CI 89%-100%) for all infections up to 56 days after 2nd dose, 97% (95% CI 91%-100%) for symptomatic infections and 89% (95% CI 43%-100%) for hospitalization. Results reflect effectiveness mainly against original SARS-CoV-2 and Alpha (B.1.1.7), the dominant strains during the study period.
Note: Adapted from Dagan et al. Cumulative incidence of SARS-CoV-2 infection by time among vaccinated and unvaccinated pregnant women, December 20, 2020–June 3, 2021. Permission request in process.
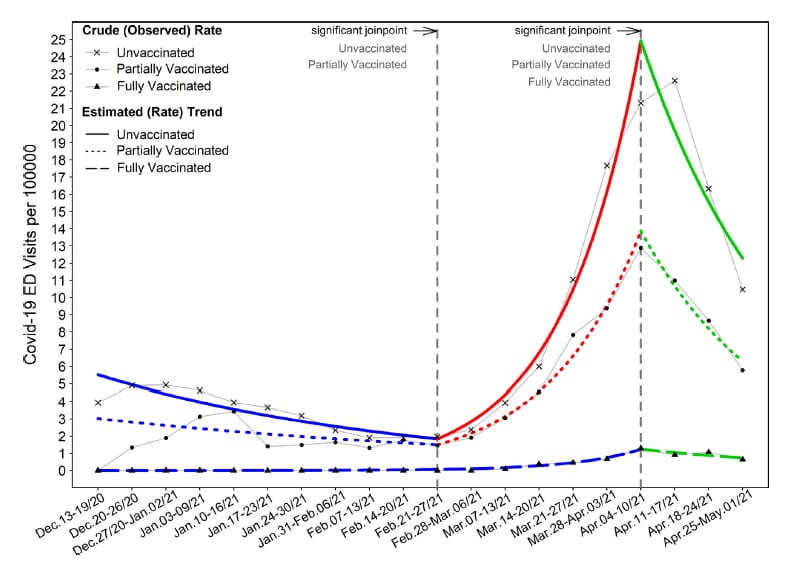
- Vaccination reduces need for emergency care in breakthrough COVID-19 infections: A multicenter cohort study. Bahl et al. The Lancet Regional Health Americas (September 9, 2021). From December–April 2021, the COVID-19-related emergency department (ED) encounter rate in Michigan was 96% lower in fully vaccinated (n = 129) than in unvaccinated (n = 10,880) patients. ED rates peaked from April 4–17, 2021 at 22.61/100000 for unvaccinated, 12.88/100000 for partially vaccinated, and 1.29/100000 for fully vaccinated patients.
Note: Adapted from Bahl et al. Trends (crude and estimated rates) in weekly emergency department (ED) encounters of COVID-19 patients by vaccination status. The point at which a line changes to a different color indicates the week that a significant change in trend occurred. Permission request in process.
Variants
- Breakthrough infections with multiple lineages of SARS-CoV-2 variants reveals continued risk of severe disease in immunosuppressed patients. Deng et al. Viruses (September 1, 2021). Between March 29 and April 29, 2021, 50% (7/14) of the confirmed SARS-CoV-2 infections among fully vaccinated individuals (n = 692, mean age 60.3 years) at a Chicago hospital were immunocompromised. Of these, 6 patients were solid organ transplant recipients, and were hospitalized (3 ICU); 1 died. Immunosuppressive treatment was associated with hospitalization (p = 0.047).
Natural History, Reinfection, and Health Impact
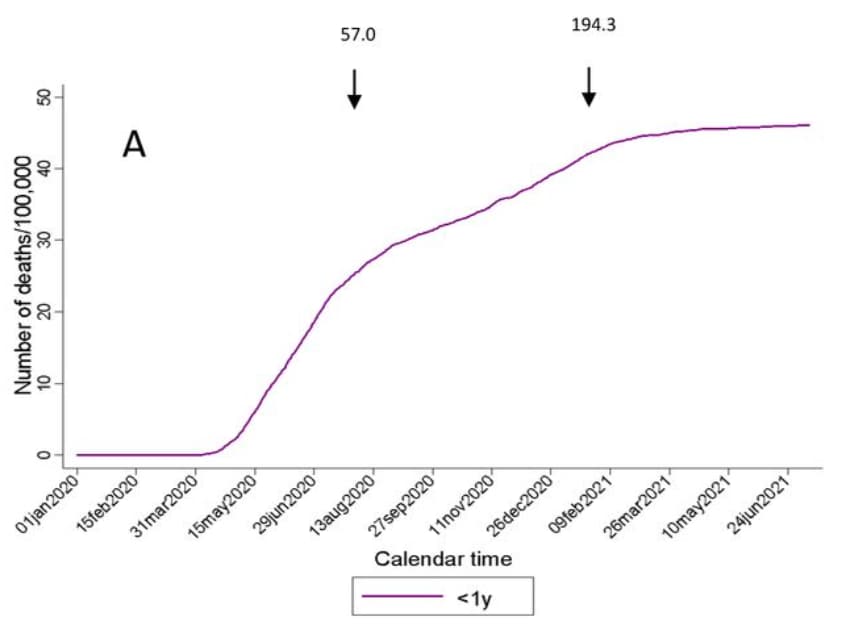
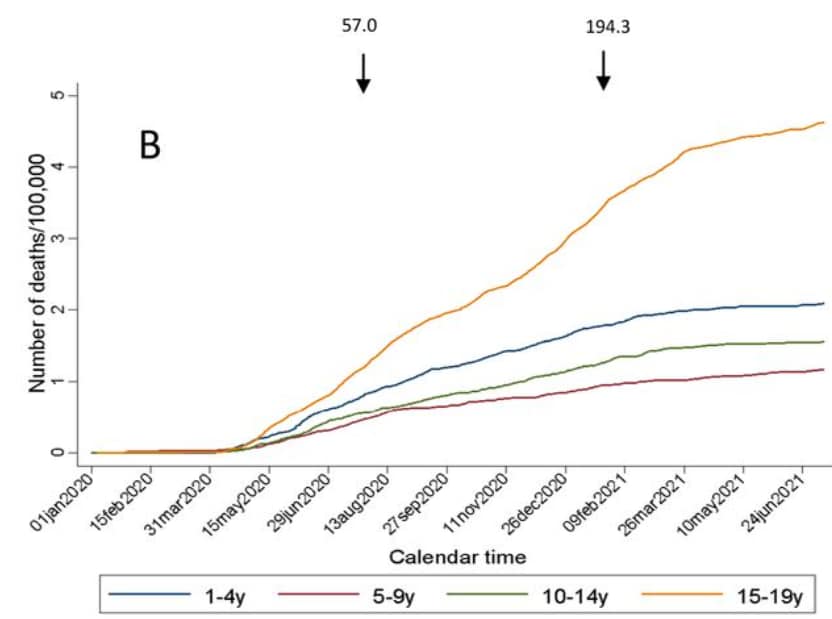
- COVID-19 mortality in children and young people in Mexico. Stern et al. medRxiv (Preprint; September 5, 2021). In 2020, COVID-19 accounted for 4.4% (95% CI 4.1%-4.6%) of deaths in children ages 0–9 years and 3.7% (95% CI 3.4%-4.1%) in adolescents ages 10–19 years in Mexico. From January 2020 to July 2021, the largest increase in cumulative COVID-19 mortality was among children <1 year and adolescents 15–19 years.
Note: Adapted from Stern et al. Mortality trends of children and adolescents in Mexico January 1, 2020–July 10, 2021 A) <1 year old children B) children and adolescents aged 1–19 years. Arrows indicate date of peak all-age reported mortality per day from COVID-19. Numbers above arrows correspond to the cumulative mortality/100,000 adults on the dates of peak (July 2020 and January 2021). Used by permission of authors.
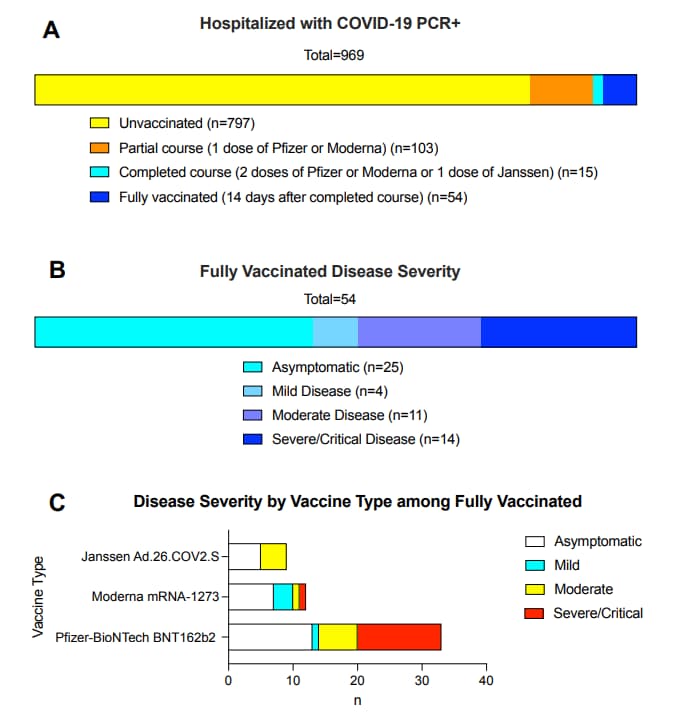
- Hospitalisation among vaccine breakthrough COVID-19 infections. Juthani et al. The Lancet Infectious Diseases (September 7, 2021). Among 969 patients hospitalized with confirmed SARS-CoV-2, 14/54 (26%) of fully vaccinated patients were severely or critically ill (median age 80·5 years); 3 died. Among those with severe/critical disease, pre-existing comorbidities were overweight (n = 9), cardiovascular disease (n = 12), lung disease (n = 7), malignancy (n = 4), type 2 diabetes (n = 7), immunosuppressants (n = 4). 13/14 patients had been vaccinated with BNT162b2 (Comirnaty, Pfizer/BioNTech) at least 14 days before symptom onset or a positive SARS-CoV-2 test.
Note: Adapted from Juthani et al. A) Classification of all patients admitted to a Connecticut hospital March 23, 2021–July 1, 2021 with positive SARS-CoV-2 PCR test by vaccination status. B) Disease severity among fully vaccinated, hospitalized patients with positive SARS-CoV-2 PCR test. C) Disease severity among fully vaccinated individuals by vaccine type. Severe/critical disease includes respiratory failure, septic shock, and/or multiple organ dysfunction. Reprinted from The Lancet Infectious Diseases, September 7, 2021, Juthani et al., Hospitalisation among vaccine breakthrough COVID-19 infections, online ahead of print. Copyright 2021, with permission from Elsevier.
Prevention Strategies and Non-Pharmaceutical Interventions
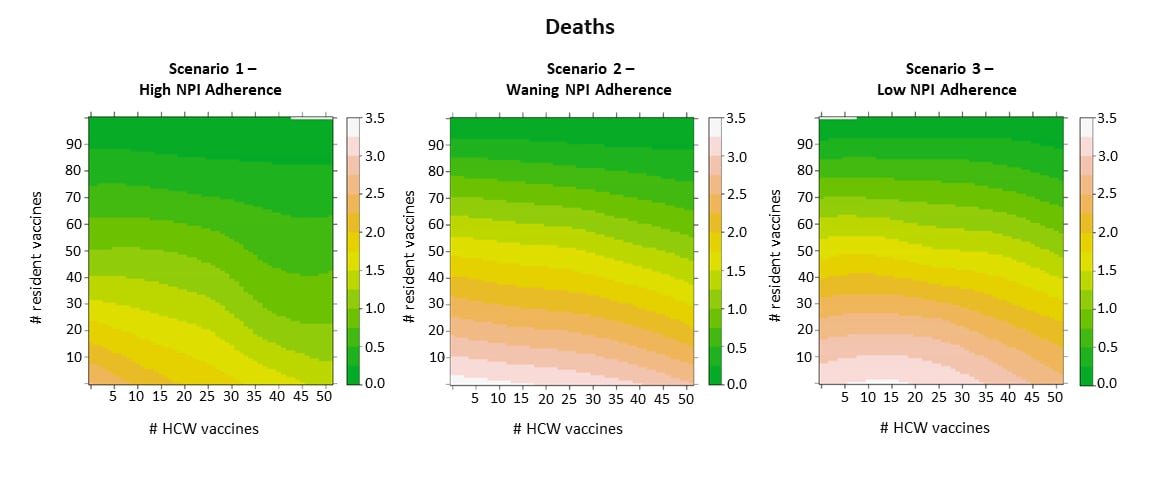
- Continued need for non-pharmaceutical interventions after COVID-19 vaccination in long-term-care facilities. Love et al. Scientific Reports (September 10, 2021). In a stochastic model of outbreak scenarios in long-term care facilities (LTCF), vaccine deployment plus strong adherence to non-pharmaceutical interventions (NPIs) produced 37% fewer infections, 61% fewer severe infections and hospitalizations, and 62% fewer deaths than baseline. Vaccinating healthcare workers prevented more deaths when NPIs adherence was high; that benefit diminished with lower adherence.
Note: Adapted from Love et al. Heatmaps of the impact of vaccine coverage on COVID-19 deaths. Warmer colors indicate more deaths/infections. Licensed under CC BY.
Testing
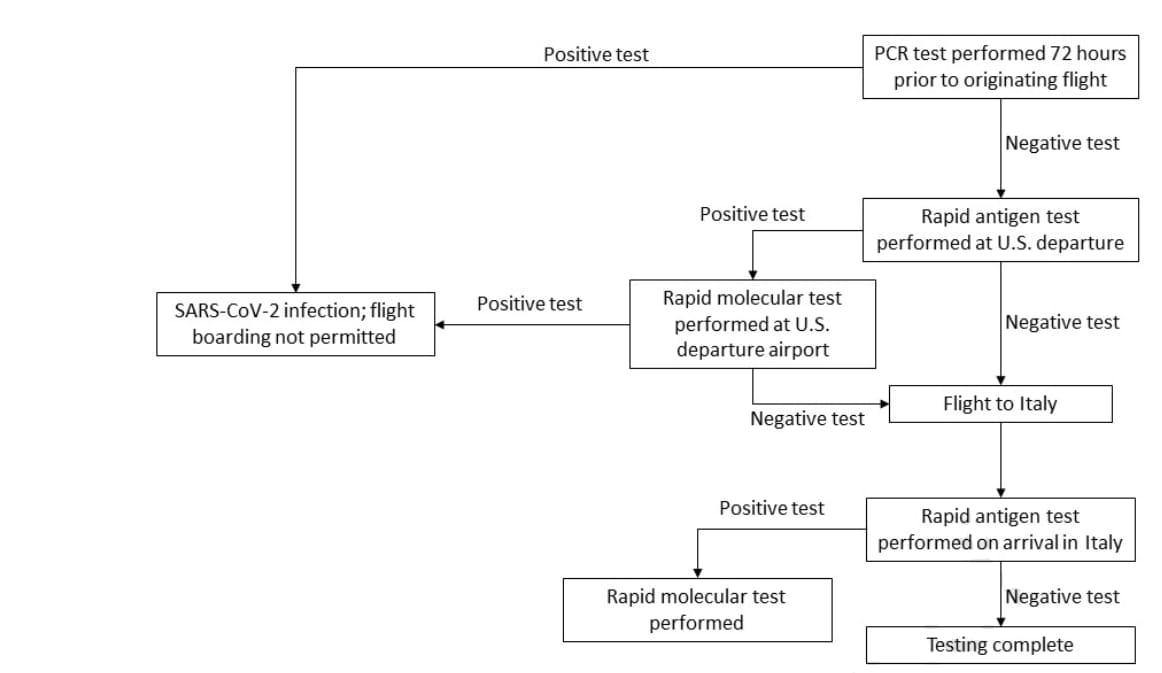
- SARS-CoV-2 testing prior to international airline travel, December 2020-May 2021. Tande et al. Mayo Clinic Proceedings (September 2, 2021). Among 9,853 passengers with a negative SARS-CoV-2 PCR test conducted ≤ 72 hours of departure, 5 passengers (0.05%) were identified as SARS-CoV-2 positive from rapid antigen tests and confirmed with rapid molecular tests performed both before and after commercial flights from the US to Italy between December 2020 and May 2021. This translates to a low case detection rate of 1/1970 travelers during a time of high SARS-CoV-2 infection rates in the US.
Note: Adapted from Tande et al. SARS-CoV-2 testing algorithm used for commercial flights from the United States to Italy, December 2020–May 2021. Licensed under CC BY 4.0.
Transmission Risk and Dynamics
- Characteristics of SARS-CoV-2 infections in Israeli children during the circulation of different SARS-CoV-2 variants. Somekh et al. JAMA Network Open (September 7, 2021). Weekly SARS-CoV-2 incidence among children ages 0–9 years in Israel was higher during December 2020–February 2021 when B.1.1.7 was predominant (50,811 infected) than during August–October 2020 (21,615 infected). Transmission to children’s secondary contacts was also higher (rate ratio (RR) 2.24, 95% CI 2.20-2.29) in December 2020–February 2021.
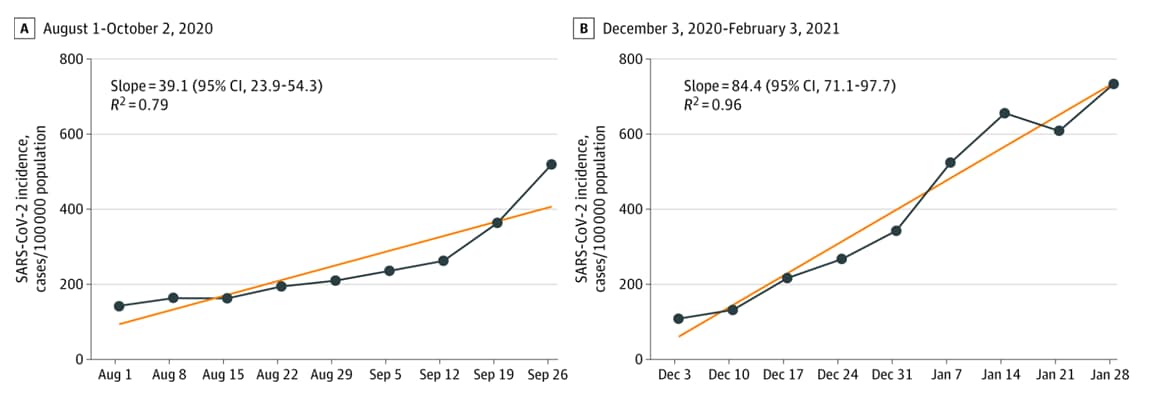
Note: Adapted from Somekh et al. Weekly adjusted incidence (new SARS-CoV-2 cases/100,000 in children aged 0–9 years). Linear regression curves are the lines connecting the dots. Dates represent day 1 of the study week. Licensed under CC BY.
Health Equity
- COVID-19 vaccine efficacy in a diverse urban healthcare worker population. Iliaki et al. medRxiv (Preprint; September 6, 2021). Published in Mayo Clinic Proceedings (October 19, 2021). Among 4,317 Massachusetts healthcare workers (HCWs), 3,249 were vaccinated between December 2020 and March 2021 and vaccine effectiveness was 95.5% (95% CI 88.2%-98.3%) for those fully vaccinated. Vaccinated HCWs were older, more likely to be non-Hispanic White persons, and to be medical providers. Vaccine uptake among African-American HCWs was lower (54%) than among White HCWs (83%).
From the Morbidity and Mortality Weekly Report (September 17, 2021).
- Disaggregating Data to Measure Racial Disparities in COVID-19 Outcomes and Guide Community Response — Hawaii, March 1, 2020–February 28, 2021
- Post-Acute Sequelae of SARS-CoV-2 Infection Among Adults Aged ≥18 Years — Long Beach, California, April 1–December 10, 2020
- Longitudinal Trends in Body Mass Index Before and During the COVID-19 Pandemic Among Persons Aged 2–19 Years — United States, 2018–2020
- Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status — 13 U.S. Jurisdictions, April 4–July 17, 2021
- Interim Estimates of COVID-19 Vaccine Effectiveness Against COVID-19–Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations Among Adults During SARS-CoV-2 B.1.617.2 (Delta) Variant Predominance — Nine States, June–August 2021
- Effectiveness of COVID-19 mRNA Vaccines Against COVID-19–Associated Hospitalization — Five Veterans Affairs Medical Centers, United States, February 1–August 6, 2021
- New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status — New York, May 3–July 25, 2021
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.