FAQs for Clinicians about C. diff
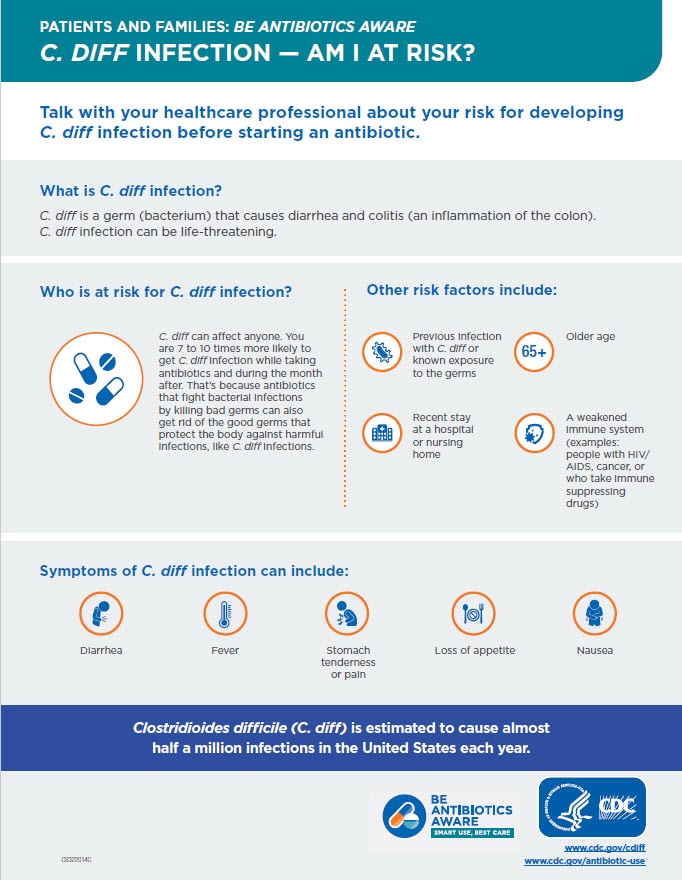
C. diff is a spore-forming, Gram-positive anaerobic bacillus that produces two exotoxins: toxin A and toxin B. It is a common cause of antibiotic-associated diarrhea (AAD) and accounts for 15 to 25% of all episodes of AAD.
- pseudomembranous colitis (PMC)
- toxic megacolon
- perforations of the colon
- sepsis
- death (rarely)
- watery diarrhea
- fever
- loss of appetite
- nausea
- abdominal pain/tenderness
The risk for disease increases in patients with:
-
- antibiotic exposure (e.g., fluoroquinolones, third/fourth generation cephalosporins, clindamycin, carbapenems)
- gastrointestinal surgery/manipulation
- long length of stay in healthcare settings
- a serious underlying illness
- immunocompromising conditions
- advanced age
- Colonization with C. diff is more common than infection. Colonized patients do not have disease caused by C. diff and often exhibit NO clinical symptoms (asymptomatic) of infection (e.g., diarrhea); colonized patients do test positive for the C. diff organism or its toxin.
Patients with infection exhibit clinical symptoms and test positive for the C. diff organism or its toxin.
- Prevention Recommendations
Molecular tests: FDA-approved PCR assays, which test for the genes encoding toxin, are same-day tests that are highly sensitive and specific for the presence of a toxin-producing C. diff organism. Molecular assays can be positive for C. diff in individuals who are asymptomatic and do not have infection. Patients with other causes of diarrhea might be positive, which could lead to over-diagnosis and treatment. When using multi-pathogen (multiplex) molecular methods, the results should be read with caution as the pre-test probability of C. diff infection might be less.
- Antigen detection for C. diff: These are rapid tests (<1 hour) that detect the presence of C. diff antigen glutamate dehydrogenase (GDH). Because results of antigen testing alone are nonspecific, antigen assays have been employed in combination with tests for toxin detection, PCR, or toxigenic culture in two-step testing algorithms.
See Figure 2 in the 2017 IDSA/SHEA Clinical Practice Guidelinesexternal icon
- Toxin testing for C. diff:
- Tissue culture cytotoxicity assay detects toxin B only. This assay requires technical expertise to perform, is costly, and requires 24 to 48 hours for a final result. It does provide specific and sensitive results for CDI. While it served as a historical gold standard for diagnosing clinically significant disease caused by C. diff, it is recognized as less sensitive than PCR or toxigenic culture for detecting the organism in patients with diarrhea.
- Enzyme immunoassay detects toxin A, toxin B, or both A and B. Due to concerns over toxin A-negative, B-positive strains causing disease, most laboratories employ a toxin B-only or A and B assay. Because these are same-day assays that are relatively inexpensive and easy to perform, they are popular with clinical laboratories. However, there are increasing concerns about their relative insensitivity (less than tissue culture cytotoxicity and much less than PCR or toxigenic culture).
- C. diff toxin is very unstable. The toxin degrades at room temperature and might be undetectable within two hours after collection of a stool specimen. False-negative results occur when specimens are not promptly tested or kept refrigerated until testing can be done.
- Stool culture for C. diff: While this is the most sensitive test available, it is the one most often associated with false-positive results due to the presence of nontoxigenic C. diff strains. However, this can be overcome by testing isolates for toxin production (i.e. so-called “toxigenic culture”). Nonetheless, stool cultures for C. diff are labor-intensive, require an appropriate culture environment to grow anaerobic microorganisms, and have a relatively slow turnaround time (i.e. results available in 48 to 96 hours), making them less clinically useful overall. Results of toxigenic cultures do serve as a gold standard against which other test modalities are compared in clinical trials of performance.
C. diff is shed in feces. Any surface, device, or material (such as commodes, bathtubs, and electronic rectal thermometers) that becomes contaminated with feces could serve as a reservoir for the C. diff spores. C. diff spores can also be transferred to patients via the hands of healthcare personnel who have touched a contaminated surface or item.
Although in about 20% of patients, CDI will resolve within two to three days of discontinuing the antibiotic to which the patient was previously exposed, CDI should usually be treated with an appropriate course (about 10 days) of treatment, including oral vancomycin or fidaxomicin. After treatment, repeat C. diff testing is not recommended if the patient’s symptoms have resolved, as patients often remain colonized.
If a patient has had ≥ 3 stools in 24 hours:
-
-
- Order a C. diff test if other etiologies of diarrhea (e.g., stool softener or laxative use) are considered unlikely.
- Isolate patients with possible C. diff immediately, even if you only suspect CDI.
- Wear gloves and a gown when treating patients with C. diff, even during short visits. Gloves are important because hand sanitizer doesn’t kill C. diff and handwashing might not be sufficient alone to eliminate all C. diff spores.
- In patient being evaluated for C. diff, reassess appropriateness of antibiotics.
-
If the patient is positive for CDI:
-
-
- Continue isolation and contact precautions.
- Use antibiotics appropriately.
- Clean room surfaces thoroughly on a daily basis while treating a patient with C. diff and upon patient discharge or transfer using an EPA-approved spore-killing disinfectant.
- When a patient transfers, notify the new facility if the patient has or had a C. diff infection. (Inter-Facility Infection Control Transfer Form pdf icon[PDF – 3 pages])
-
CDI can be prevented by using antibiotics appropriately and implementing infection control recommendations to prevent transmission.
-
-
- Improving Diagnosis and Management
Use antibiotics appropriately.
- Use contact precautions for patients with known or suspected CDI:
- Place these patients in private rooms. If private rooms are not available, they can be placed in rooms (cohorted) with other CDI patients.
- Wear gloves and a gown when entering CDI patient rooms and during their care.
- As no single method of hand hygiene will eliminate all C. diff spores, using gloves to prevent hand contamination remains the cornerstone for preventing C. diff transmission via the hands of healthcare personnel.
- Always perform hand hygiene after removing gloves.
-
- If your institution experiences an outbreak, consider using soap and water instead of alcohol-based hand sanitizers for hand hygiene after removing gloves while caring for patients with CDI.
- Dedicate or perform cleaning and disinfection of any shared medical equipment between patients.
- Continue CDI precautions at least until diarrhea ceases.
- Because CDI patients continue to shed the organism for a number of days following cessation of diarrhea, some institutions routinely continue isolation and contact precautions for either several days beyond symptom resolution or until discharge, depending upon the type of setting and average length of stay.
- Implement an environmental cleaning and disinfection strategy.
- Ensure adequate cleaning and disinfection of environmental surfaces and reusable devices, especially items likely to be contaminated with feces and surfaces that are touched frequently.
- Ensure daily and terminal cleaning of patient rooms.
- Use an Environmental Protection Agency (EPA)-registered disinfectant with a sporicidal claim for environmental surface disinfection after cleaning in accordance with label instructions. (Note: Only hospital surface disinfectants listed on EPA’s List K are registered as effective against C. diff spores).
- Follow the manufacturer’s instructions for disinfection of endoscopes and other devices.
- Ensure adequate cleaning and disinfection of environmental surfaces and reusable devices, especially items likely to be contaminated with feces and surfaces that are touched frequently.
-
Surfaces should be kept clean, and body substance spills should be managed promptly, as outlined in CDC’s Guidelines for Environmental Infection Control in Health-Care Facilities. Routine cleaning should be performed prior to disinfection. EPA-registered disinfectants with a sporicidal claim have been used with success for environmental surface disinfection in those patient-care areas where surveillance and epidemiology indicate ongoing transmission of C. diff.
Note: EPA-registered disinfectants (List K) are recommended for use in patient-care areas. When choosing a disinfectant, check product labels for inactivation claims, indications for use, and instructions.