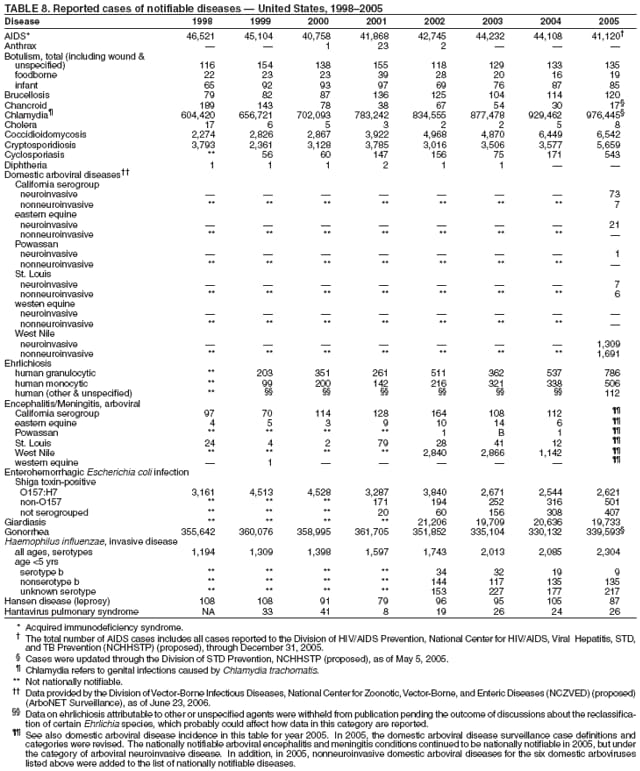
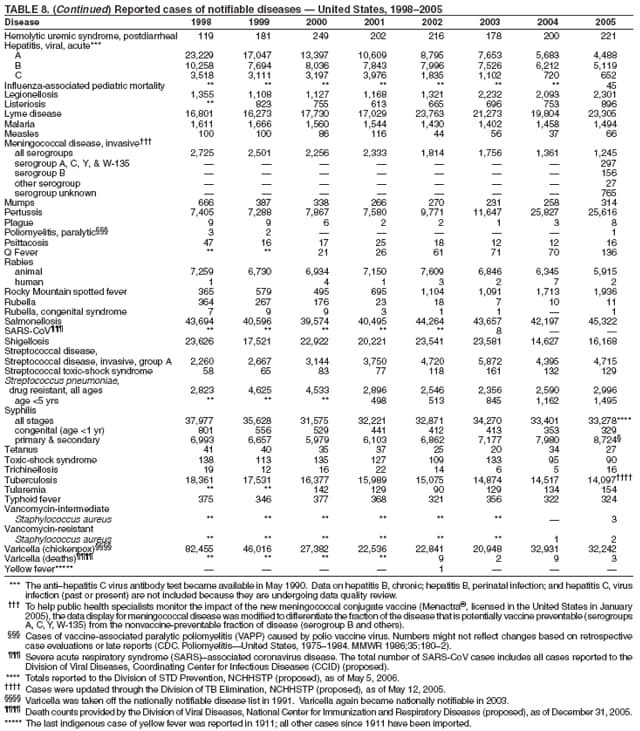
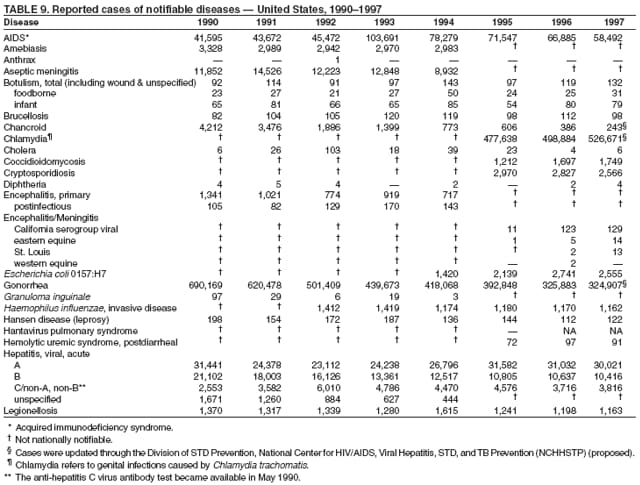
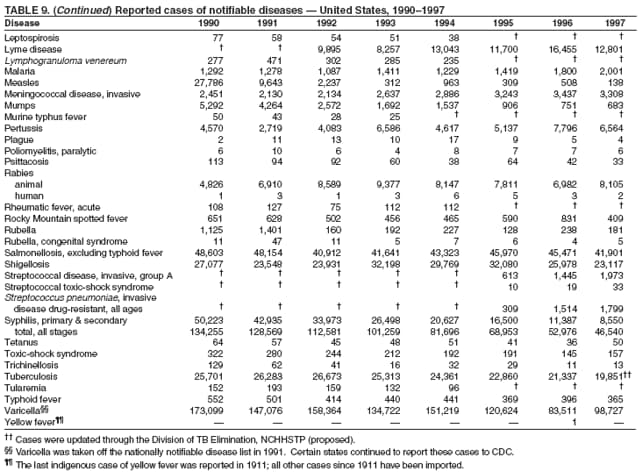
 |
|
|
|
|
|
|
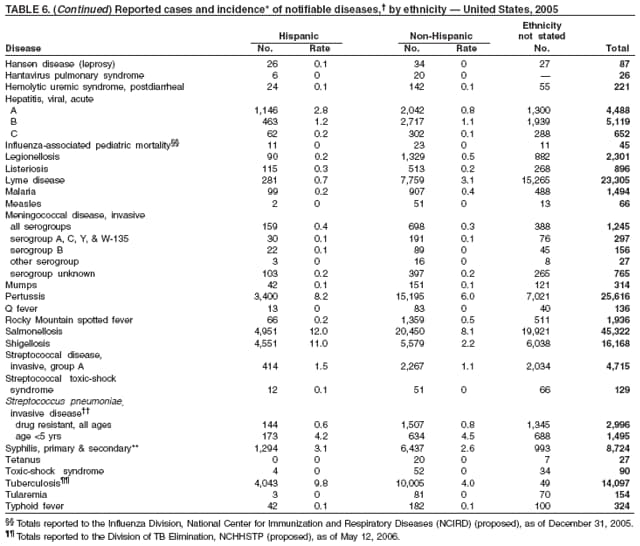
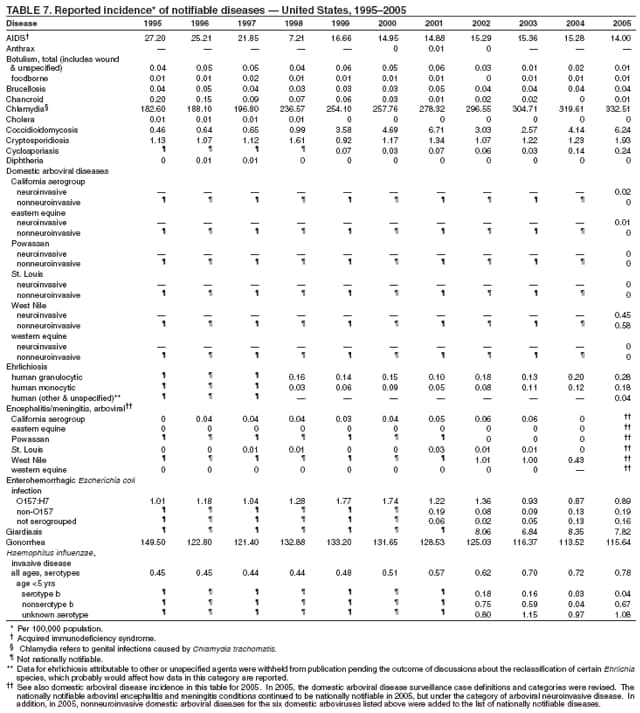
| ||||||||||
|
|
|
|
|
|
|
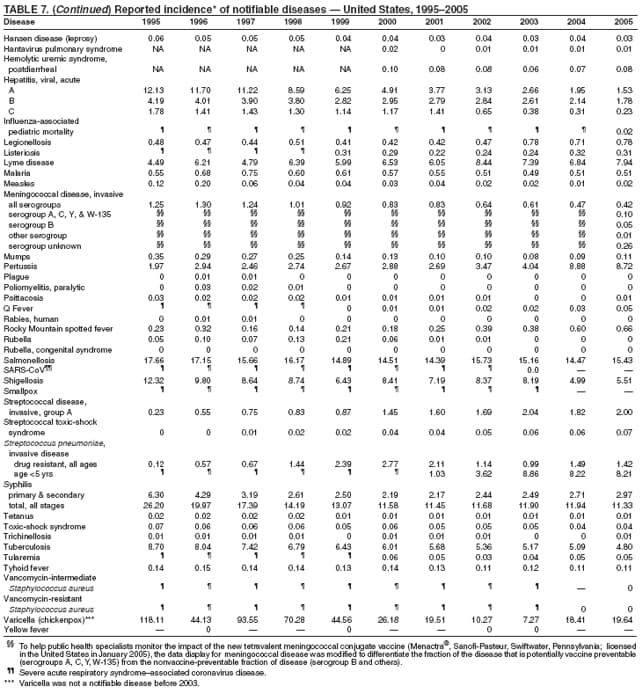
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Summary of Notifiable Diseases --- United States, 2005
Prepared by
PrefaceThe Summary of Notifiable Diseases --- United States, 2005 contains the official statistics, in tabular and graphic form, for the reported occurrence of nationally notifiable infectious diseases in the United States for 2005. Unless otherwise noted, the data are final totals for 2005 reported as of June 30, 2006. These statistics are collected and compiled from reports sent by state health departments to the National Notifiable Diseases Surveillance System (NNDSS), which is operated by CDC in collaboration with the Council of State and Territorial Epidemiologists (CSTE). The Summary is available at http://www.cdc.gov/mmwr/summary.html. This site also includes publications from previous years. The Highlights section presents noteworthy epidemiologic and prevention information for 2005 for selected diseases and additional information to aid in the interpretation of surveillance and disease-trend data. Part 1 contains tables showing incidence data for the nationally notifiable infectious diseases during 2005.* The tables provide the number of cases reported to CDC for 2005 as well as the distribution of cases by month, geographic location, and the patient's demographic characteristics (age, sex, race, and ethnicity). Part 2 contains graphs and maps that depict summary data for certain notifiable infectious diseases described in tabular form in Part 1. Part 3 contains tables that list the number of cases of notifiable diseases reported to CDC since 1973. This section also includes a table enumerating deaths associated with specified notifiable diseases reported to CDC's National Center for Health Statistics (NCHS) during 2002--2003. The Selected Reading section presents general and disease-specific references for notifiable infectious diseases. These references provide additional information on surveillance and epidemiologic concerns, diagnostic concerns, and disease-control activities. Comments and suggestions from readers are welcome. To increase the usefulness of future editions, comments about the current report and descriptions of how information is or could be used are invited. Comments should be sent to Public Health Surveillance Team --- NNDSS, Division of Integrated Surveillance Systems and Services, National Center for Public Health Informatics at soib@cdc.gov. * Because no cases of anthrax; diphtheria; domestic arboviral, western equine encephalitis virus, neuroinvasive and nonneuroinvasive, eastern equine nonneuroinvasive, and Powassen nonneuroinvasive; severe acute respiratory syndrome--associated coronavirus (SARS-CoV) disease; smallpox; or yellow fever were reported in the United States during 2005, these diseases do not appear in the tables in Part 1. For certain other nationally notifiable diseases, incidence data were reported to CDC but are not included in the tables or graphs of this Summary. Data on chronic hepatitis B and hepatitis C virus infection past or present are undergoing quality review. Data on human immunodeficiency virus (HIV) infections are not included because HIV infection (not acquired immunodeficiency syndrome [AIDS]) reporting has been implemented on different dates and using different methods than for AIDS case reporting; however, these data are summarized in the Highlights section. BackgroundThe infectious diseases designated as notifiable at the national level during 2005 are listed on page 3. A notifiable disease is one for which regular, frequent, and timely information regarding individual cases is considered necessary for the prevention and control of the disease. A brief history of the reporting of nationally notifiable infectious diseases in the United States is available at http://www.cdc.gov/epo/dphsi/nndsshis.htm. In 1961, CDC assumed responsibility for the collection and publication of data on nationally notifiable diseases. NNDSS is neither a single surveillance system nor a method of reporting. Certain NNDSS data are reported to CDC through separate surveillance information systems and through different reporting mechanisms; however, these data are aggregated and compiled for publication purposes. Notifiable disease reporting at the local level protects the public's health by ensuring the proper identification and follow-up of cases. Public health workers ensure that persons who are already ill receive appropriate treatment; trace contacts who need vaccines, treatment, quarantine, or education; investigate and halt outbreaks; eliminate environmental hazards; and close premises where spread has occurred. Surveillance of notifiable conditions helps public health authorities to monitor the impact of notifiable conditions, measure disease trends, assess the effectiveness of control and prevention measures, identify populations or geographic areas at high risk, allocate resources appropriately, formulate prevention strategies, and develop public health policies. Monitoring surveillance data enables public health authorities to detect sudden changes in disease occurrence and distribution, identify changes in agents and host factors, and detect changes in health-care practices. The list of nationally notifiable infectious diseases is revised periodically. A disease might be added to the list as a new pathogen emerges, or a disease might be deleted as its incidence declines. Public health officials at state health departments and CDC collaborate in determining which diseases should be nationally notifiable. CSTE, with input from CDC, makes recommendations annually for additions and deletions. Although disease reporting is mandated by legislation or regulation at the state and local levels, state reporting to CDC is voluntary. Reporting completeness of notifiable diseases is highly variable and related to the condition or disease being reported (1). The list of diseases considered notifiable varies by state and year. Current and historic national public health surveillance case definitions used for classifying and enumerating cases consistently across reporting jurisdictions are available at http://www.cdc.gov/epo/dphsi/nndsshis.htm. All states report conditions that were designated as internationally quarantinable and notifiable (i.e., cholera, plague, and yellow fever) in compliance with the International Health Regulations (IHR) issued by the World Health Organization (WHO). In May 2005, the World Health Assembly adopted revised IHR. The current IHR will be replaced by the 2005 IHR when it becomes official on June 15, 2007, unless an earlier implementation date is adopted. The 2005 IHR revision stipulates that smallpox, poliomyelitis caused by wild-type poliovirus, human influenza caused by a new subtype, and SARS-CoV are public health events of international concern (PHEIC) and are reportable to WHO. In addition, the 2005 IHR includes an open-ended algorithm to determine other conditions or events that require mandatory reporting to WHO because they might constitute a PHEIC. Conditions for which the algorithm is used to determine notifiability include, but are not limited to, cholera, pneumonic plague, yellow fever, West Nile fever, and meningococcal disease (2). On December 13, 2006, the United States formally accepted the 2005 IHR and is taking steps to implement these new international rules.
Infectious Diseases Designated as Notifiable at the National Level During
2005
|
| Acquired immunodeficiency syndrome (AIDS) | Influenza-associated pediatric mortality |
| Anthrax | Legionellosis |
| Botulism | Listeriosis |
| foodborne | Lyme disease |
| infant | Malaria |
| other (wound and unspecified) | Measles |
| Brucellosis | Meningococcal disease, invasive |
| Chancroid | Mumps |
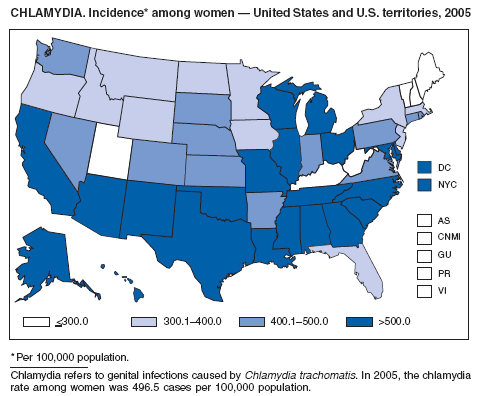
| Chlamydia trachomatis, genital infection | Pertussis |
| Cholera | Plague |
| Coccidioidomycosis | Poliomyelitis, paralytic |
| Cryptosporidiosis | Psittacosis |
| Cyclosporiasis | Q fever |
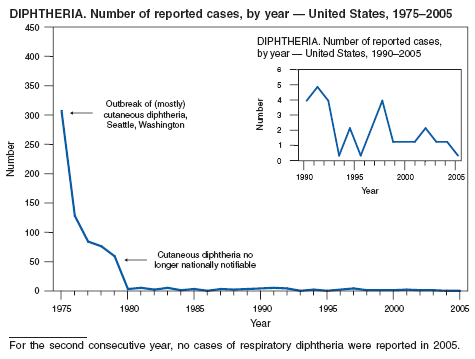
| Diphtheria | Rabies |
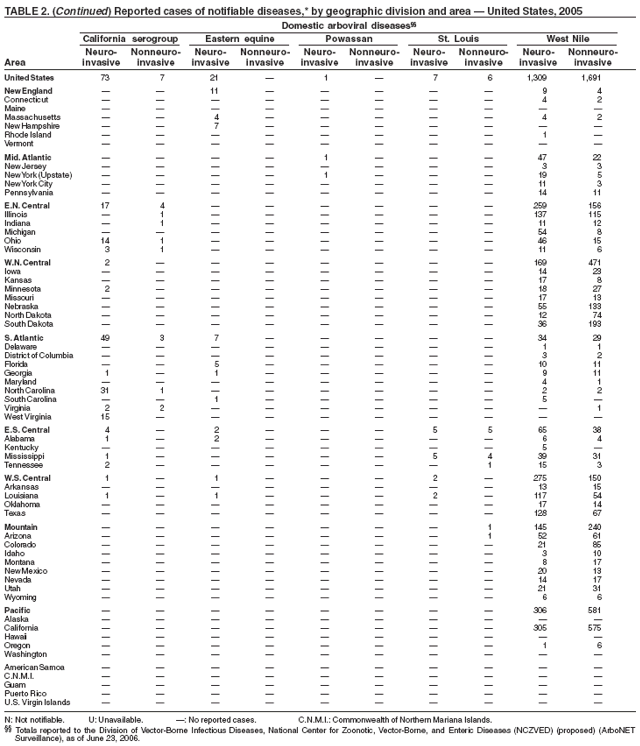
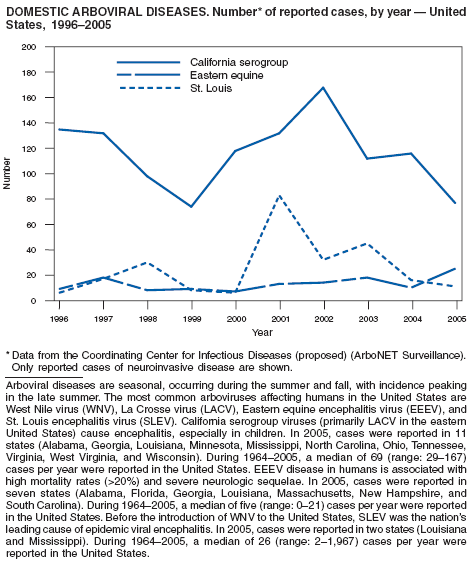
| Domestic arboviral diseases, neuroinvasive and non-neuroinvasive† | animal |
| California serogroup virus disease | human |
| eastern equine encephalitis virus disease | Rocky Mountain spotted fever |
| Powassan virus disease | Rubella |
| St. Louis encephalitis virus disease | Rubella, congenital syndrome |
| West Nile virus disease | Salmonellosis |
| western equine encephalitis virus disease | Severe acute respiratory syndrome--associated coronavirus (SARS-CoV) disease |
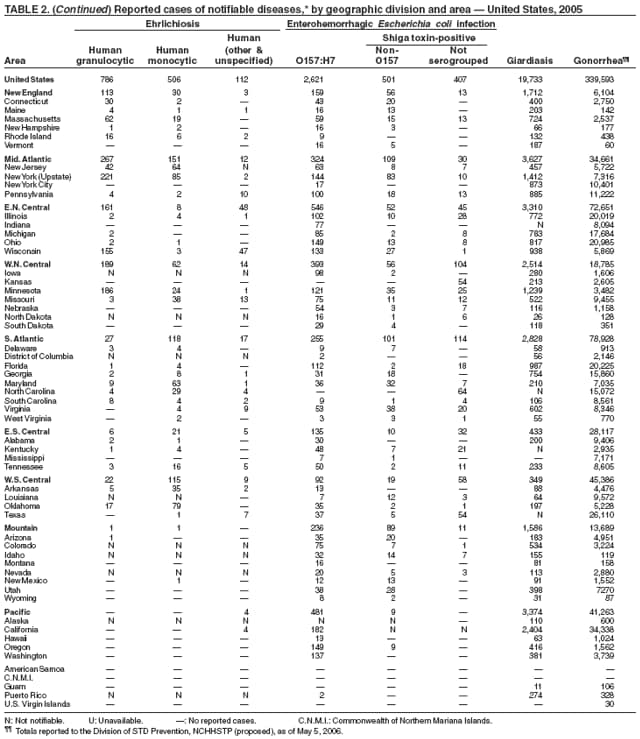
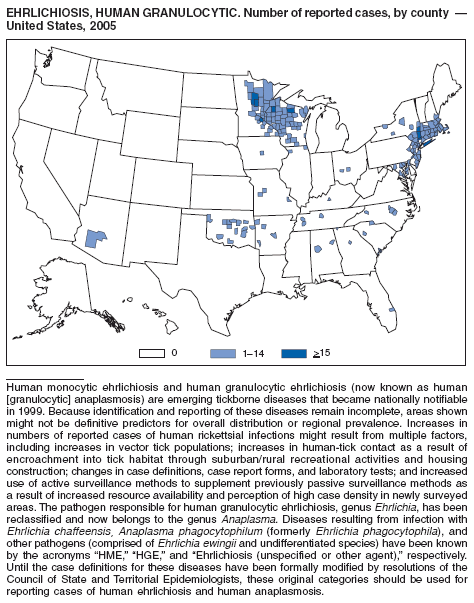
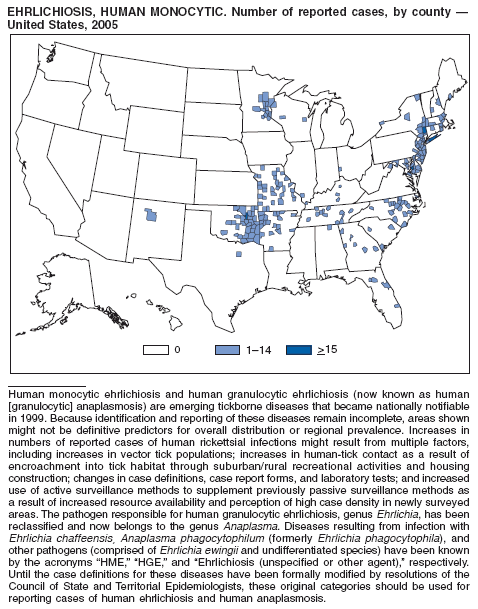
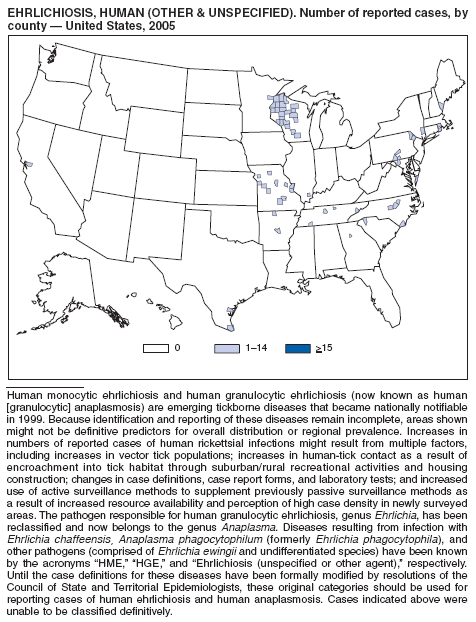
| Ehrlichiosis | Shigellosis |
| human granulocytic | Smallpox |
| human monocytic | Streptococcal disease, invasive, group A |
| human, other or unspecified agent | Streptococcal toxic-shock syndrome |
| Enterohemorrhagic Escherichia coli (EHEC) infection | Streptococcus pneumoniae, invasive disease |
| EHEC O157:H7 | drug resistant, all ages |
| EHEC Shiga toxin-positive, serogroup non-O157 | age <5 years |
| EHEC Shiga toxin-positive, not serogrouped | Syphilis |
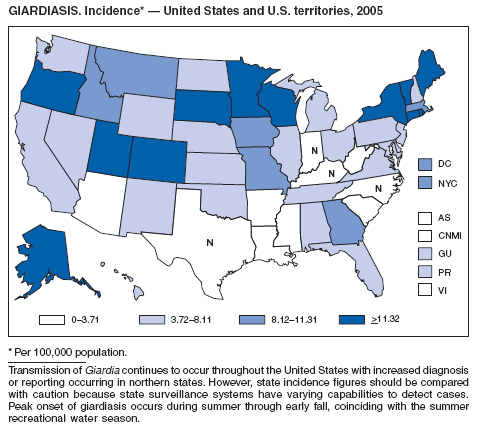
| Giardiasis | Syphilis, congenital |
| Gonorrhea | Tetanus |
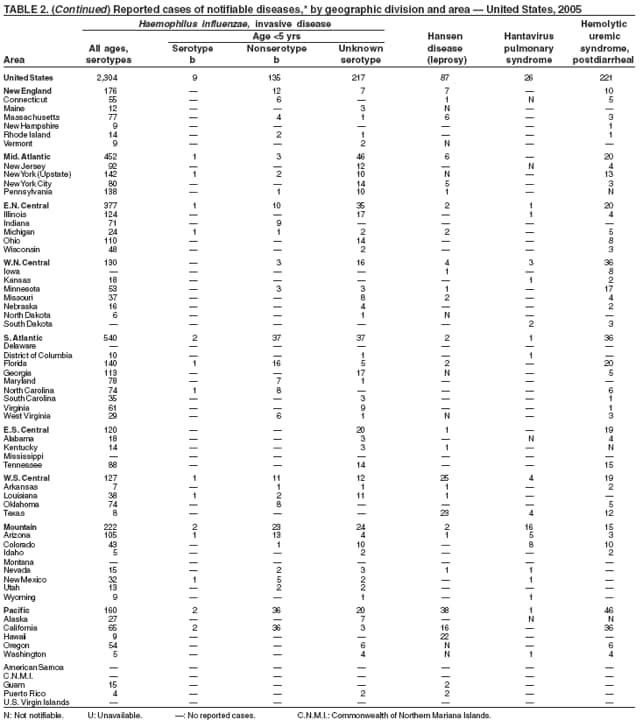
| Haemophilus influenzae, invasive disease | Toxic-shock syndrome (other than streptococcal) |
| Hansen disease (leprosy) | Trichinellosis |
| Hantavirus pulmonary syndrome | Tuberculosis |
| Hemolytic uremic syndrome, postdiarrheal | Tularemia |
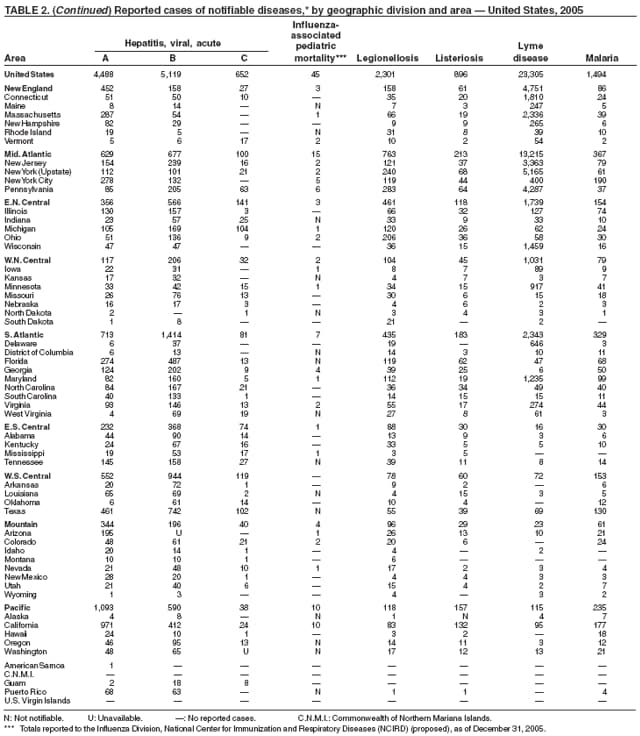
| Hepatitis A, viral, acute | Typhoid fever |
| Hepatitis B, viral, acute | Vancomycin-intermediate Staphylococcus aureus |
| Hepatitis B, chronic | infection (VISA) |
| Hepatitis B virus infection, perinatal | Vancomycin-resistant Staphylococcus aureus infection (VRSA) |
| Hepatitis C, viral, acute | Varicella infection (morbidity) |
| Hepatitis C virus infection (past or present) | Varicella deaths |
| Human immunodeficiency virus (HIV) infection | Yellow fever |
| adult (age >13 yrs) | |
| pediatric (age <13 yrs) |
† The national surveillance case definition for the arboviral diseases was revised in 2005, and nonneuroinvasive arboviral diseases were added to the list of nationally notifiable infectious diseases.
Provisional data concerning the reported occurrence of nationally notifiable infectious diseases are published weekly in MMWR. After each reporting year, staff in state health departments finalize reports of cases for that year with local or county health departments and reconcile the data with reports previously sent to CDC throughout the year. These data are compiled in final form in the Summary.
Notifiable disease reports are the authoritative and archival counts of cases. They are approved by the appropriate chief epidemiologist from each submitting state or territory before being published in the Summary. Data published in MMWR Surveillance Summaries or other surveillance reports produced by CDC programs might not agree exactly with data reported in the annual Summary because of differences in the timing of reports, the source of the data, or surveillance methodology.
Data in the Summary were derived primarily from reports transmitted to CDC from health departments in the 50 states, five territories, New York City, and the District of Columbia. Data were reported for MMWR weeks 1--52, which correspond to the period for the week ending January 8, 2005, through the week ending December 31, 2005. More information regarding infectious notifiable diseases, including case definitions, is available at http://www.cdc.gov/epo/dphsi/phs.htm. Policies for reporting notifiable disease cases can vary by disease or reporting jurisdiction. The case-status categories used to determine which cases reported to NNDSS are published, by disease or condition, and are listed in the print criteria column of the 2006 NNDSS event code list (available at http://www.cdc.gov/epo/dphsi/phs/files/NNDSSeventcodelistJanuary2006.pdf).
Final data for certain diseases are derived from the surveillance records of the CDC programs listed below. Requests for further information regarding these data should be directed to the appropriate program.
Coordinating Center for Health Information and Service National Center for Health Statistics (NCHS)
Office of Vital and Health Statistics Systems (deaths from selected notifiable diseases).
Coordinating Center for Infectious Diseases (proposed)
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (proposed)
Division of HIV/AIDS Prevention (AIDS and HIV infection).
Division of STD Prevention (chancroid; Chlamydia trachomatis, genital infection; gonorrhea; and syphilis).
Division of Tuberculosis Elimination (tuberculosis).
National Center for Immunization and Respiratory Diseases (proposed)
Influenza Division (proposed) (influenza-associated pediatric mortality).
Division of Viral Diseases (proposed) (poliomyelitis, varicella deaths, and SARS-CoV).
National Center for Zoonotic, Vector-Borne, and Enteric Diseases (proposed)
Division of Vector-Borne Infectious Diseases (arboviral diseases).
Division of Viral and Rickettsial Diseases (animal rabies).
Population estimates for the states are from the NCHS bridged-race estimates of the July 1, 2004, U.S. resident population from the vintage 2004 postcensal series by year, county, age, sex, race, and Hispanic origin, prepared under a collaborative arrangement with the U.S. Census Bureau. This data set was released on September 9, 2005, and is available at http://www.cdc.gov/nchs/about/major/dvs/popbridge/popbridge.htm. Populations for territories are 2004 estimates from the U.S. Census Bureau International Data Base Data Access--Display Mode, available at http://www.census.gov/ipc/www/idbprint.html. The choice of population denominators for incidence reported in the MMWR is based on 1) the availability of census population data at the time of preparation for publication and 2) the desire for consistent use of the same population data to compute incidence reported by different CDC programs. Incidence in the Summary is calculated as the number of reported cases for each disease or condition divided by either the U.S. resident population for the specified demographic population or the total U.S. residential population, multiplied by 100,000. When a nationally notifiable disease is associated with a specific age restriction, the same age restriction is applied to the population in the denominator of the incidence calculation. In addition, population data from states in which the disease or condition was not notifiable or was not available were excluded from incidence calculations. Unless otherwise stated, disease totals for the United States do not include data for American Samoa, Guam, Puerto Rico, the Commonwealth of the Northern Mariana Islands, or the U.S. Virgin Islands.
Incidence data in the Summary are presented by the date of report to CDC as determined by the MMWR week and year assigned by the state or territorial health department. Data are reported by the state in which the patient resided at the time of diagnosis. For certain nationally notifiable infectious diseases, surveillance data are reported independently to different CDC programs. Thus, surveillance data reported by other CDC programs might vary from data reported in the Summary because of differences in 1) the date used to aggregate data (e.g., date of report or date of disease occurrence), 2) the timing of reports, 3) the source of the data, 4) surveillance case definitions, and 5) policies regarding case jurisdiction (i.e., which state should report the case to CDC).
The data reported in the Summary are useful for analyzing disease trends and determining relative disease burdens. However, reporting practices affect how these data should be interpreted. Disease reporting is likely incomplete, and completeness might vary depending on the disease. The degree of completeness of data reporting might be influenced by the diagnostic facilities available; control measures in effect; public awareness of a specific disease; and the interests, resources, and priorities of state and local officials responsible for disease control and public health surveillance. Finally, factors such as changes in methods for public health surveillance, introduction of new diagnostic tests, or discovery of new disease entities can cause changes in disease reporting that are independent of the true incidence of disease.
Public health surveillance data are published for selected racial/ethnic populations because these variables can be risk markers for certain notifiable diseases. Race and ethnicity data also can be used to highlight populations for focused prevention efforts. However, caution must be used when drawing conclusions from reported race and ethnicity data. Different racial/ethnic populations might have different patterns of access to health care, potentially resulting in data that are not representative of actual disease incidence among specific racial/ethnic populations. Surveillance data reported to NNDSS are in either individual case-specific form or summary form (i.e., aggregated data for a group of cases). Summary data often lack demographic information (e.g., race); therefore, the demographic-specific rates presented in the Summary might be underestimated.
In addition, not all race and ethnicity data are collected uniformly for all diseases. For example, certain disease programs collect data on race and ethnicity using one or two variables, based on the 1977 standards for collecting such data issued by the Office of Management and the Budget (OMB). However, beginning in 2003, certain CDC programs, such as the tuberculosis program, implemented OMB's 1997 revised standards for collecting such data; these programs collect data on multiple races per person using multiple race variables. In addition, although the recommended standard for classifying a person's race or ethnicity is based on self-reporting, this procedure might not always be followed.
Before 1990, data were reported to CDC as cumulative counts rather than individual case reports. In 1990, states began electronically capturing and reporting individual case reports (without personal identifiers) to CDC using the National Electronic Telecommunication System for Surveillance (NETSS). In 2001, CDC launched the National Electronic Disease Surveillance System (NEDSS), now a component of the Public Health Information Network, to promote the use of data and information system standards that advance the development of efficient, integrated, and interoperable surveillance information systems at the local, state, and federal levels. One of the objectives of NEDSS is to improve the accuracy, completeness, and timeliness of disease reporting at the local, state, and national level. CDC has developed the NEDSS Base System (NBS), a public health surveillance information system that can be used by states that do not wish to develop their own NEDSS-based systems. A major feature of NBS is the ability to capture data already in electronic form (e.g., electronic laboratory results, which are needed for case confirmation) rather than enter these data manually as in NETSS. In 2005, NBS was used by 10 states to transmit nationally notifiable infectious diseases to CDC; as of January 1, 2007, NBS was used by 16 states to transmit these data to CDC. Additional information concerning NEDSS is available at http://www.cdc.gov/NEDSS.
Below are summary highlights for certain national notifiable diseases. Highlights are intended to assist in the interpretation of major occurrences that affect disease incidence or surveillance trends (e.g., outbreaks, vaccine licensure, or policy changes).
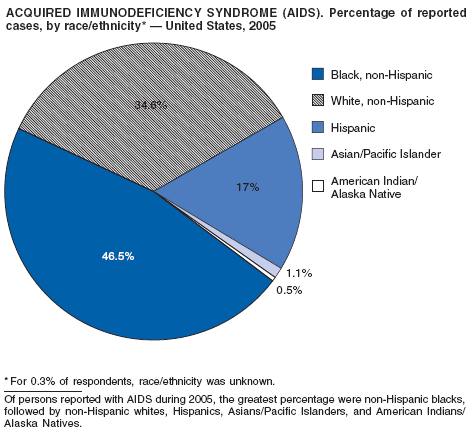
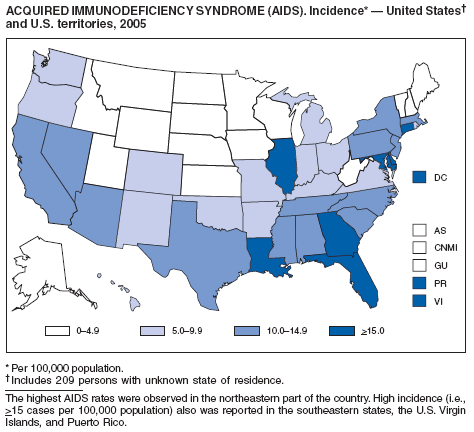
Since 1981, confidential name-based AIDS surveillance has been the cornerstone of national, state, and local efforts to monitor the scope and impact of the HIV epidemic. The data have multiple uses, including developing policy to help prevent and control AIDS. However, because of the introduction of therapies that effectively slow the progression of the infection, AIDS data no longer adequately represent the populations affected by the epidemic. By helping researchers to understand the epidemic at an earlier stage, HIV data, combined with AIDS data, better represent the overall impact. As of the end of 2005, a total of 43 areas (38 states, Puerto Rico, and four U.S. territories) had implemented confidential name-based HIV reporting. These 43 areas have integrated name-based HIV surveillance into their AIDS surveillance systems, whereas other jurisdictions have used other methods for reporting cases of HIV infection. Under no configuration are names or other personal identifying information collected at the national level.
During 1998--1999, declines in AIDS rates began to level. This trend followed a period of sharp declines in reported cases after 1996, when highly effective antiretroviral therapies were introduced. At the end of 2005, an estimated 437,982 persons were living with AIDS. After a substantial decrease in the number of deaths among persons with AIDS during the late 1990s, the rate of decrease declined through 2004. The number of deaths among persons with AIDS decreased 66% during 1995--2000. During 2001--2003, the number of reported deaths decreased an average of 5% annually; however, in 2004, the number of deaths increased 3% compared with the number reported in 2001. In 2005, reported deaths resumed a downward trend and decreased 17% compared with 2004.
No human cases of anthrax were reported in the United States during 2005. Naturally occurring anthrax epizootics are commonly reported in the United States; in 2005, epizootics were reported in four states, affecting livestock in Montana, North Dakota, and South Dakota, and livestock and game animals in Texas.
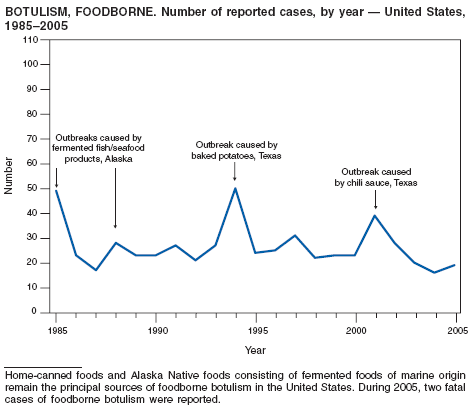
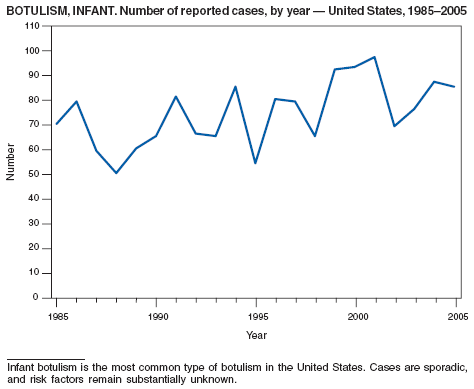
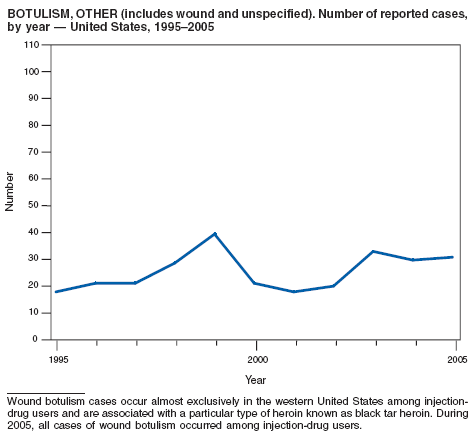
Botulism is a severe paralytic illness caused by the toxins of Clostridium botulinum. Exposure to toxin can occur by ingestion (foodborne botulism) or by in situ production from C. botulinum colonization of a wound (wound botulism) or the gastrointestinal tract (infant botulism and adult intestinal colonization botulism) (1). In addition to the National Notifiable Diseases Surveillance System, CDC maintains intensive surveillance for cases of botulism in the United States. In 2005, cases were attributed to foodborne botulism, wound botulism, infant botulism, and intestinal colonization (2).
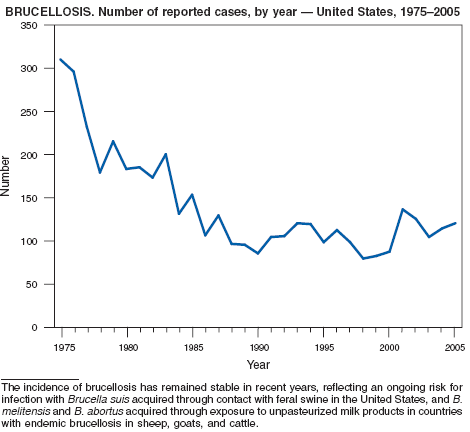
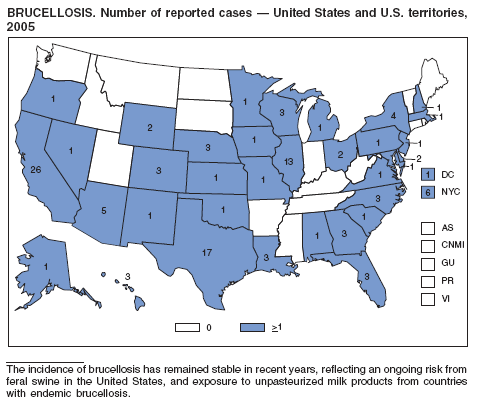
In 2005, three cattle herds in two states, and two swine herds in two states were reported by the U.S. Department of Agriculture (USDA) to be affected by brucellosis. Overall, 48 states remain designated free of cattle brucellosis by USDA (1). Brucella abortus remains enzootic in elk and bison in the greater Yellowstone National Park area, and Brucella suis is enzootic in feral swine in the southeast. Hunters exposed to these animals might be at increased risk for infection. Human cases also can occur among returned travelers or immigrants from countries with endemic brucellosis and are associated with consumption of unpasteurized milk or soft cheeses. Pathogenic Brucella species are considered category B biologic threat agents because of a high potential for aerosol transmission (2). For the same reason, biosafety level 3 practices, containment, and equipment are recommended for laboratory manipulation of isolates (3).
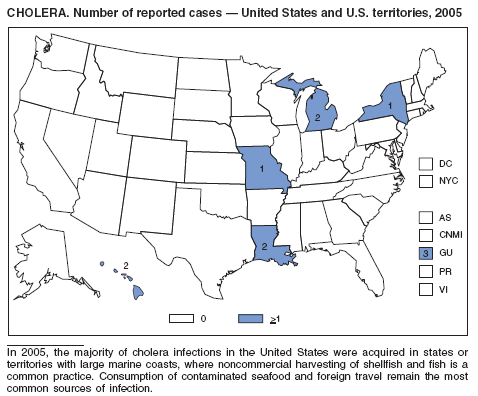
In 2005, the largest number of laboratory-confirmed cases of toxigenic Vibrio cholerae O1 infection were reported since 1998. The average annual number of cases of cholera reported during 1995--2000 and 2001--2005 was 10.2 and 4.6 per year, respectively (1). None of the patients hospitalized for cholera died. Approximately 36% of cases were acquired outside the United States, 36% were attributable to consumption of domestic seafood, and for 27% (residents of Guam),
no source was identified (3). Crabs harvested from the U.S. Gulf Coast after Hurricane Katrina were the source of illness for certain cases associated with domestic seafood (2). Certain cases associated with domestic seafood were attributed to consumption of raw seafood at a restaurant. Foreign travel and consumption of undercooked seafood continue to be the main sources of illness. Crabs harvested from the U.S. Gulf Coast are a common source of cholera, especially during warmer months, when environmental conditions favor the growth and survival of V. cholerae in brackish and coastal waters.
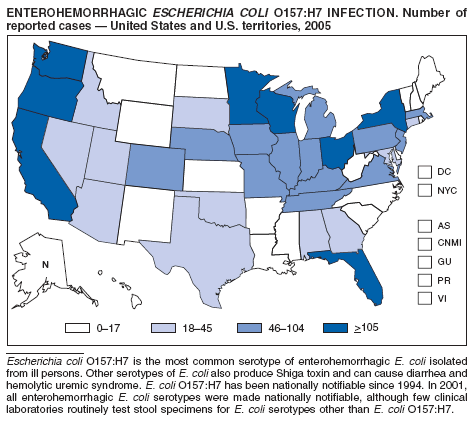
Escherichia coli O157:H7 has been nationally notifiable since 1994 (1). National surveillance for all Shiga toxin-producing E. coli (STEC), under the name enterohemorrhagic E. coli (EHEC), began in 2001. Surveillance categories for EHEC infection include 1) EHEC O157:H7; 2) serogroup non-O157; and 3) EHEC, not serogrouped. During 2005, cases of EHEC infection were reported from 50 states, the District of Columbia, and Puerto Rico. Of these, 74% were classified as EHEC O157:H7; 14% as EHEC, serogroup non-O157; and 12% as EHEC, not serogrouped. The majority of cases were reported during July--October.
Healthy cattle, which harbor the organism as part of their bowel flora, are the main animal reservoir for STEC. The majority of reported outbreaks are caused by contaminated food or water. The substantial decline in cases since 2002 coincided with industry and regulatory control activities and with a decrease in the contamination of ground beef (2). Direct transmission from animals and their environments to humans in settings such as petting zoos remains a public health concern (3), and prevention recommendations have been developed and disseminated (4).
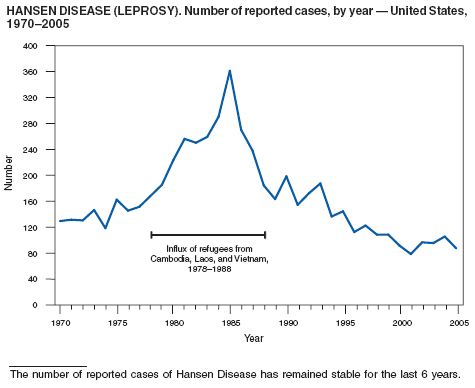
The number of reported cases of Hansen disease (HD) in the United States peaked at 361 in 1985 and has declined since 1988. HD outpatient clinics operated under the guidance and direction of the U.S. Department of Health and Human Services, Health Resources and Services Administration exist in Phoenix, Arizona; Los Angeles, Martinez, and San Diego, California; Miami, Florida; Chicago, Illinois; Baton Rouge, Louisiana; Boston, Massachusetts; New York City, New York; San Juan, Puerto Rico; Austin, Dallas, Harlingen, Houston, and San Antonio, Texas; and Seattle, Washington. Services provided to HD patients include diagnosis, treatment, follow-up of patients and contacts, disability prevention and monitoring, education, and a referral system for HD health-care services. More information is available at http://bphc.hrsa.gov/nhdp/default.htm.
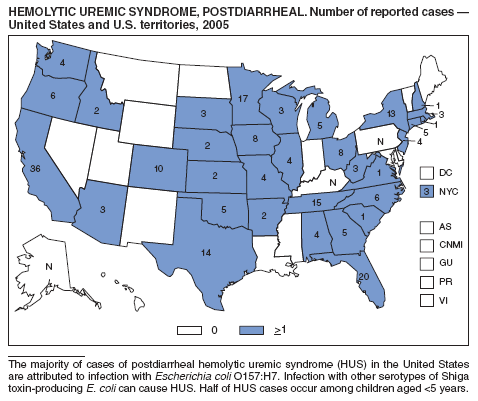
Hemolytic uremic syndrome (HUS) is characterized by the triad of hemolytic anemia, thrombocytopenia, and renal insufficiency. The most common etiology of HUS in the United States is infection with Shiga toxin-producing Escherichia coli, principally E. coli O157:H7 (1). Approximately 8% of persons infected with E. coli O157:H7 progress to HUS (2). During 2005, the majority of reported cases occurred among children aged <5 years.
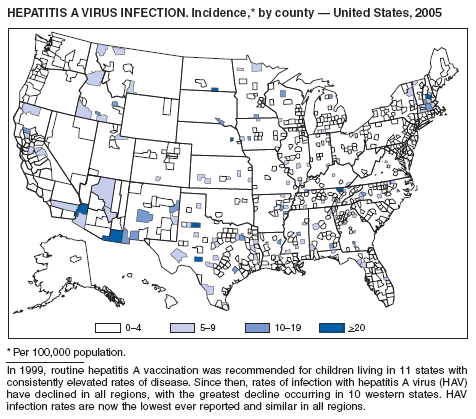
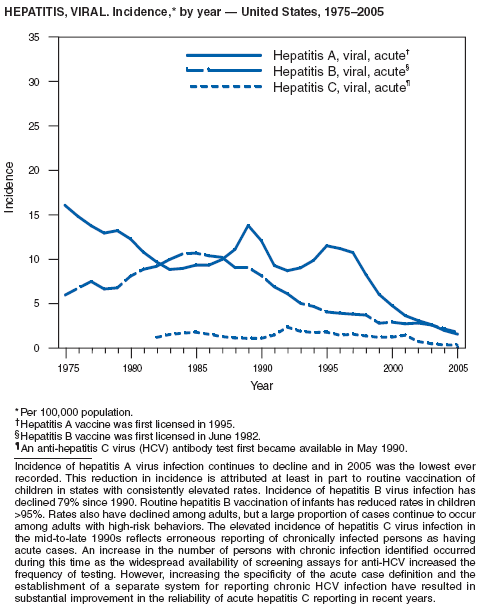
In 2005, to further reduce morbidity and mortality from hepatitis A virus infections in the United States, CDC expanded recommendations for hepatitis A vaccination published previously (1). Hepatitis A vaccination is now recommended routinely for children aged 1 year (1) and for persons who are at increased risk for infection (e.g., international travelers, men who have sex with men [MSM], users of illicit drugs, persons working with nonhuman primates or with hepatitis A virus [HAV] in a laboratory, persons with clotting-factor disorders, and persons who have chronic liver disease), and for any person wishing to become immune (2).
Since routine childhood vaccination was recommended in 1999, the overall hepatitis A rate has declined dramatically, especially in the western states. In 2005, the rate of infection (1.5 per 100,000 population) was the lowest yet recorded. Declines have been greater in the age groups and regions for which targeted vaccination was recommended previously (1), reflecting the success of the targeted vaccination strategy.
Although rates among children have declined among all races and ethnicities, the decline among Hispanic children has been less than that among non-Hispanics. The highest rates among children are now among children in states in which morbidity was low historically and that were not included in the initial recommendations for routine childhood hepatitis A vaccination.
The decline in rates among children has resulted in a substantial shift in the epidemiologic profile of this disease in the United States. Rates in the western states, which historically have been higher than in other regions, are now similar to the rest of the country, and rates among adults are higher than those among children. These declines also have been accompanied by a shift in the pattern of reported risk factors, with an increasing proportion of cases occurring among adults at high risk for hepatitis A, including MSM and users of injection and noninjection drugs. In addition, as transmission of HAV has declined within the United States, the proportion of cases attributed to travel to countries in which hepatitis A is endemic has increased for all age groups, and travel is now the most frequently reported risk factor among persons with HAV aged <15 years.
Since 1990, the number of acute hepatitis B cases has declined 80%; the rate reported in 2005 was 1.8 per 100,000 population. This steady decline has coincided with the implementation of a national strategy to achieve the elimination of hepatitis B (1). The primary elements of this strategy are the screening of all pregnant women for hepatitis B virus (HBV) infection with the provision of postexposure prophylaxis to infants born to infected women; routine vaccination of all infants and children aged <19 years; and vaccination of others at increased risk for hepatitis B (e.g., health-care workers, men who have sex with men [MSM], injection-drug users [IDUs], and household and sex contacts of persons with chronic HBV infection).
In 2005, the rate of infection among children aged <13 years, the cohort born since routine infant vaccination was implemented, was 0.02 per 100,000 population, representing a 98% decline in that age group since 1990. By race and ethnicity, the highest rates among children continue to be among Asian/Pacific Islanders (APIs), followed by blacks, American Indians/Alaska Natives, and whites; however, since 1990, the disparity between the highest incidence group (APIs) and the lowest (whites) has been reduced 98%. A substantial number of confirmed cases in children born after 1991 occurred among children born outside the United States, including international adoptees (2). Rates among adolescents aged 12--19 years also have declined approximately 97% since 1990, but the 2005 rate (0.2 per 100,000 population) remains substantially higher than for younger children.
During 1990--2005, acute hepatitis B rates among adults declined 76%. Among adults, a high proportion of cases occur among persons in identified risk groups (i.e., IDUs, MSM, and persons with multiple sex partners), indicating a need to strengthen efforts to reach these populations with vaccine.
By December 2003, all 50 states and the District of Columbia had implemented HIV surveillance systems, including both name-based and nonname-based systems. Since 2001, a total of 37 areas (33 states, American Samoa, Guam, the Commonwealth of the Northern Mariana Islands, and the U.S. Virgin Islands) have had laws or regulations requiring name-based confidential reporting for adults and adolescents with confirmed HIV infection, in addition to reporting of persons with AIDS. In 2002, CDC initiated a system to monitor HIV incidence; in 2003, CDC expanded this system and also initiated a national HIV behavioral surveillance system. CDC will assess the implementation and effectiveness of prevention activities through multiple monitoring systems, including use of new performance indicators for state and local health departments and community-based organizations (1).
At the end of 2005, a total of 212,579 adults and adolescents in the 37 areas were living with HIV infection (not AIDS). The estimated prevalence of HIV infection (not AIDS) in this group was 136.5 per 100,000 population (2). In these areas, 2005 was the first year in which mature HIV surveillance data (i.e., available since at least 2001) could be used to allow for stabilization of data collection and for adjustment of the data to monitor trends. Data from additional areas will be included in analyses when >4 years of case reports have accrued.
At the end of 2005, in the 37 areas (33 states, American Samoa, Guam, the Commonwealth of the Northern Mariana Islands, and the U.S. Virgin Islands) that have had laws or regulations since 2001 requiring confidential name-based reporting for children aged <13 years with confirmed HIV infection, an estimated 2,460 children were living with HIV infection. Estimated prevalence of HIV infection (not AIDS) in this group was 7.4 per 100,000 population (1).
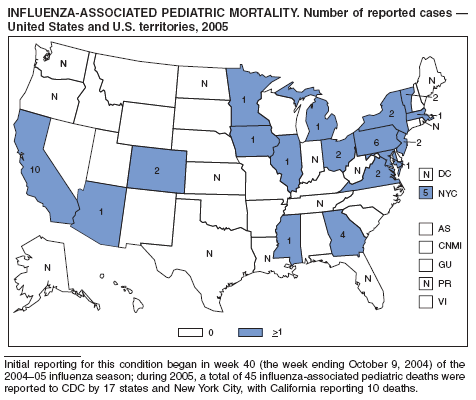
Early outbreaks of influenza during the 2003--04 season were associated with deaths of children in 31 states, prompting CDC to request that all state, territorial, and local health departments report laboratory-confirmed influenza-associated deaths in children aged <18 years (1,2). During the 2003--04 influenza season, 153 pediatric influenza-associated deaths were reported to CDC by 40 state health departments (3). Histopathologic and immunohistochemical features of fatal influenza virus infection were described in 47 of these children (4). As a result, the Council of State and Territorial Epidemiologists (CSTE) and CDC worked together to draft recommendations for national reporting of pediatric deaths with laboratory confirmation of influenza; these recommendations were approved at the 2004 CSTE annual meeting (5). In October 2004, CDC added influenza-associated pediatric mortality to the list of conditions voluntarily reportable to the National Notifiable Diseases Surveillance System (6). Reporting for this condition began in week 40 (week ending October 9, 2004) of the 2004--05 influenza season. The cumulative year-to-date incidence is published each week in the MMWR Table I for low-incidence nationally notifiable diseases.
During 2005, a total of 45 influenza-associated pediatric deaths were reported to CDC by 17 states and New York City, with California reporting 10 deaths. The median age of the deceased children was 5 years (range: 23 days--17 years); 21 (47%) were aged <5 years. Although the majority of deaths occurred in a hospital setting, six children (13%) died outside a hospital setting. Of the 45 children, 31 (69%) had an underlying or chronic condition, and 14 (31%) were previously healthy. Chronic conditions included seizure disorder, prematurity, neurologic disease, neuromuscular disorders, chronic pulmonary disease, immunosuppression, congenital anomalies, and developmental delay. Bacterial coinfections were confirmed in four children. The current recommendations of the Advisory Committee on Immunization Practices (7) highlight the importance of administering 2 doses of influenza vaccine for previously unvaccinated children aged 6 months--<9 years. Continued surveillance of severe influenza-related mortality is important to monitor the impact of influenza and the possible effects of interventions, including influenza vaccination in children.
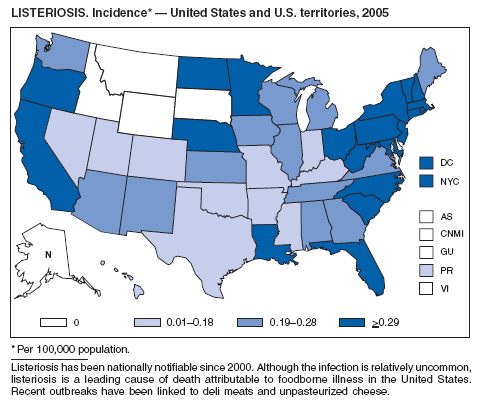
Listeriosis is a rare but severe infection caused by Listeria monocytogenes that has been nationally notifiable since 2000. Listeriosis is primarily foodborne and occurs most frequently among persons who are older, pregnant, or immuno-compromised. During 2005, the majority of cases occurred among persons aged >60 years.
Molecular subtyping of L. monocytogenes isolates and sharing of that information through PulseNet (2) has enhanced the ability of public health officials to detect and investigate outbreaks of listeriosis. Recent outbreaks have been linked to ready-to-eat deli meat (1) and unpasteurized cheese (3). During 2005, incidence of listeriosis as reported to FoodNet active surveillance was 0.27 per 100,000 population, representing a decrease of 32% compared with 1996--1998 (4).
All clinical isolates should be submitted to state public health laboratories for pulsed-field gel electrophoresis (PFGE) pattern determination, and all persons with listeriosis should be interviewed by a public health official or health-care provider using a standard Listeria case form, available at http://www.cdc.gov/foodborneoutbreaks/documents/ListeriaCaseReportFormOMB0920-0004.pdf. Rapid analysis of surveillance data will allow identification of possible food sources of outbreaks. In 2005, an outbreak linked to turkey deli meat was detected by this method (CDC, unpublished data, 2005).
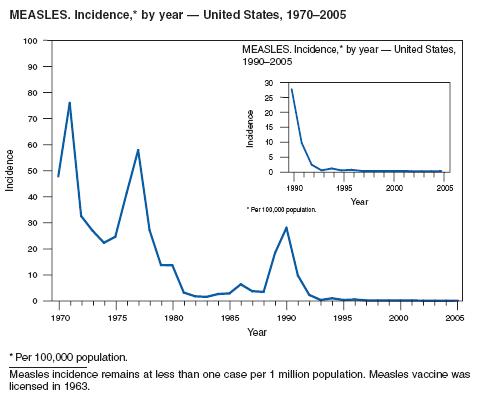
Nearly all of confirmed measles cases reported in 2005 were import-associated. Half of all cases occurred among children aged 5--19 years. Overall measles morbidity increased 79% after a record low number of cases in 2004. The increase was the result primarily of an outbreak in Indiana among a group of members of a single church who had not been vaccinated for measles. This outbreak was the largest outbreak in the United States since 1996 and the largest in Indiana since 1990. The source of the outbreak was an unvaccinated U.S. resident who had acquired measles infection while traveling in Romania (1). The majority of all cases among U.S. residents can be prevented by following current recommendations for vaccination, including specific guidelines for travelers (2,3). Although the elimination of endemic measles in the United States has been achieved, and population immunity remains high (4), an outbreak can occur when measles is introduced into a susceptible group. Indiana public health officials estimated that the cost of containing the disease was approximately $168,000 (5).
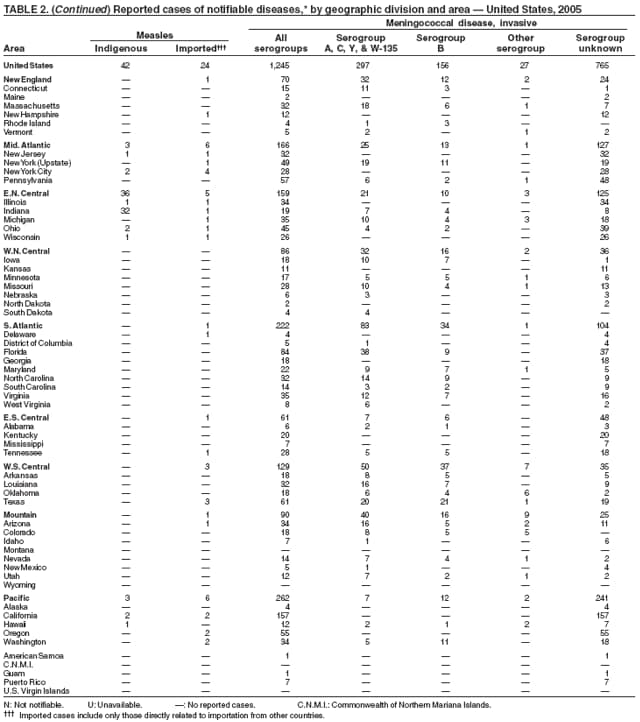
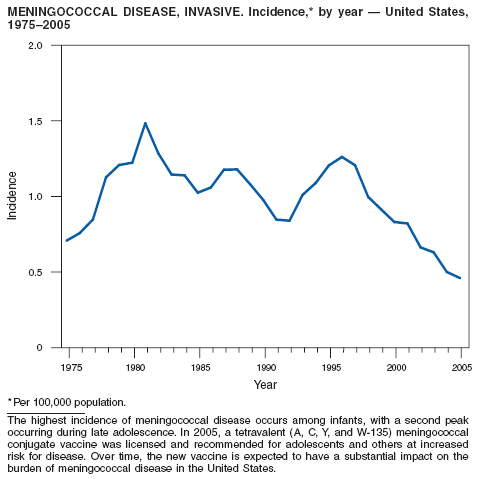
Neisseria meningitidis is a leading cause of bacterial meningitis and sepsis in the United States. Despite declining incidence, the case-fatality ratio (10%--14%) remains high, and 11%--19% of survivors have serious health sequelae, including hearing loss, amputations, and cognitive impairment. Rates of meningococcal disease are highest among infants, with a second peak at age 18 years (1). The proportion of cases caused by each serogroup of N. meningitidis varies by age group. The majority of cases in infants are caused by serogroup B, for which no vaccine is licensed in the United States.
A new tetravalent (A, C, Y, W-135) meningococcal conjugate vaccine ([MCV4] Menactra®; manufactured by Sanofi Pasteur, Swiftwater, Pennsylvania) was licensed in January 2005 for persons aged 11--55 years. CDC's Advisory Committee on Immunization Practices recommends routine vaccination with MCV4 of young adolescents aged 11--12 years, adolescents at high school entry if not vaccinated previously, college freshmen living in dormitories, and other populations at increased risk for meningococcal disease (1). The new conjugate vaccine is an important addition to meningococcal disease prevention strategies. Further reductions in meningococcal disease could be achieved with the development of an effective serogroup B vaccine.
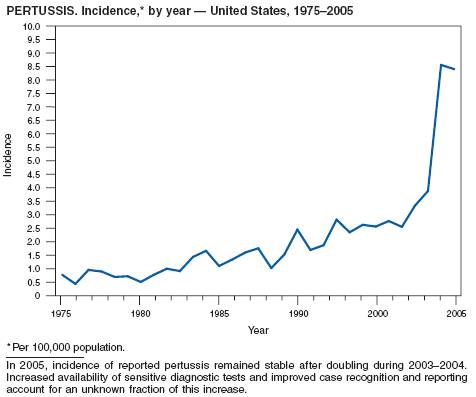
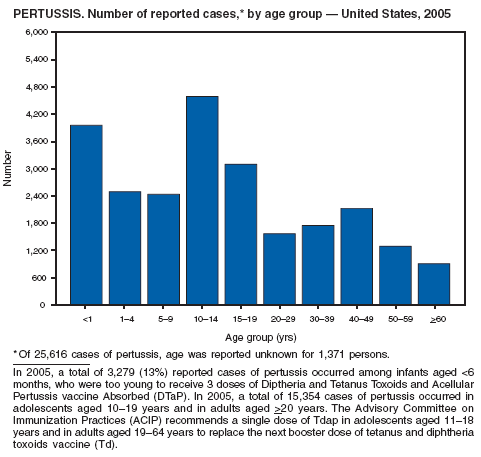
In 2005, incidence of reported pertussis remained stable at 8.7 cases per 100,000 population after doubling during 2003--2004. Infants aged <6 months, who are too young to be fully vaccinated, had the highest reported rate of pertussis (160.81 per 100,000 population), but adolescents aged 10--19 years and adults aged >20 years contributed the greatest number of reported cases (60%). Adolescents and adults might be a source of transmission of pertussis to young infants who are at higher risk for severe disease and death (1). In addition to routine use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in adolescents aged 11--18 years as recommended by the Advisory Committee on Immunization Practices (ACIP) in 2005, ACIP recommends use of Tdap for a single dose to replace the next dose of Td for adults aged 19--64 years (2,3). Use of Tdap also is recommended for certain populations of adults, including health-care workers and persons in close contact with infants aged <12 months (3,4).
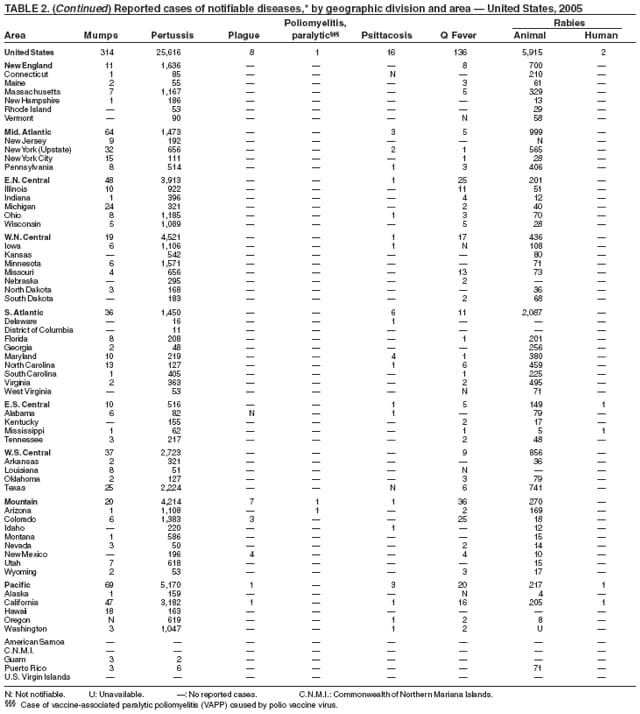
In 2005, an imported case of vaccine-associated paralytic poliomyelitis (VAPP) was reported to the National Notifiable Diseases Surveillance System. In addition, type 1 vaccine-derived poliovirus (VDPV) infections were reported to CDC. The VAPP case occurred in an unvaccinated U.S. college student aged 22 years who was residing temporarily in Costa Rica, where she likely was exposed through contact with an infant who had recently been vaccinated with oral polio vaccine (OPV) (1). Although the risk is extremely low, health-care providers should be aware of contact VAPP; be alert to the diagnosis of polio, especially in unvaccinated persons with onset of acute flaccid paralysis; and obtain stool cultures for poliovirus testing. Electrodiagnostic studies can assist in differentiating polio from demyelinating conditions such as Guillain-Barré syndrome. The VDPV infections occurred among an Amish population in Minnesota. The index case-patient was an Amish infant with severe combined immune deficiency who underwent stool culture examination for diarrhea and failure to thrive. Community investigations demonstrated circulation of VDPV infection in the local Amish community but not in other related communities in the United States and Canada. No cases of paralytic disease or other clinically compatible illnesses caused by poliovirus were identified (2). VDPVs emerge from OPV viruses as a result of continuous replication in immune-deficient persons or their circulation in populations with low vaccination coverage. Because OPV has not been used in the United States since 2000 and in Canada since 1997, the original source of the VPDV infection was likely a person who received OPV in another country. Both situations highlight the risks for U.S. citizens of not being vaccinated and the importance of continued polio surveillance.
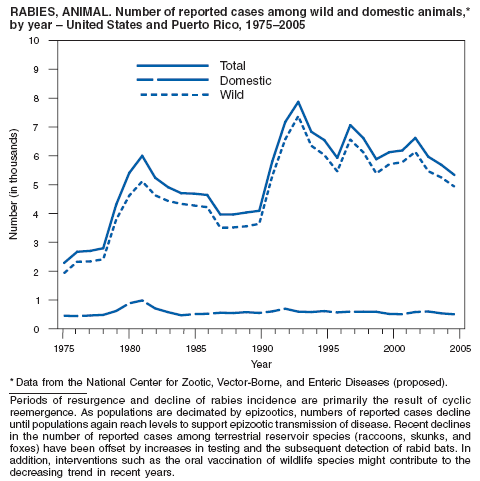
During 2005, the majority (92%) of animal rabies cases were reported in wild animal species. Overall, 6.2% fewer cases of animal rabies were reported in 2005 compared with 2004 (1). In the United States, five animal species are recognized as reservoir species for various rabies virus variants over defined geographic regions: raccoons (eastern United States), skunks (north and south central United States and California), bats (various species in all U.S. states except Hawaii), foxes (in Alaska, Arizona, and Texas), and mongoose (in Puerto Rico). The reported number of cases decreased among all wildlife species, except bats and mongooses. Reported cases of rabies in domestic animals remain low in part because of high vaccination rates. As in the preceding decade, cats were the most commonly reported domestic animal with rabies during 2005. Vaccination programs to control rabies in wild carnivores were ongoing through the distribution of baits containing an oral rabies vaccine in the eastern United States and Texas. Oral rabies vaccination programs in Texas have demonstrated continued success (2). These programs appear to have eliminated a rabies virus variant associated with coyotes and dogs along the U.S.-Mexico border and have reduced the area affected by a variant associated with gray foxes. No cases associated with the coyote/dog variant and few cases of the gray fox variant were reported during 2005. Oral rabies vaccination programs also are being conducted in the eastern United States in an attempt to stop the westward spread of the raccoon rabies virus variant. Active surveillance efforts conducted by the United States Department of Agriculture (USDA) to monitor oral rabies vaccination programs were further enhanced by USDA's use of the Direct Rapid Immunohistochemical Test (DRIT) in the second half of 2005 after training at CDC. This test is used for screening samples collected by USDA, reducing the burden on state laboratories, and permitting faster processing of surveillance samples (3). One case of rabies was identified in a human in Mississippi during 2005. This case was identified retrospectively after the Mississippi Department of Health submitted samples to CDC's unexplained deaths project (4).
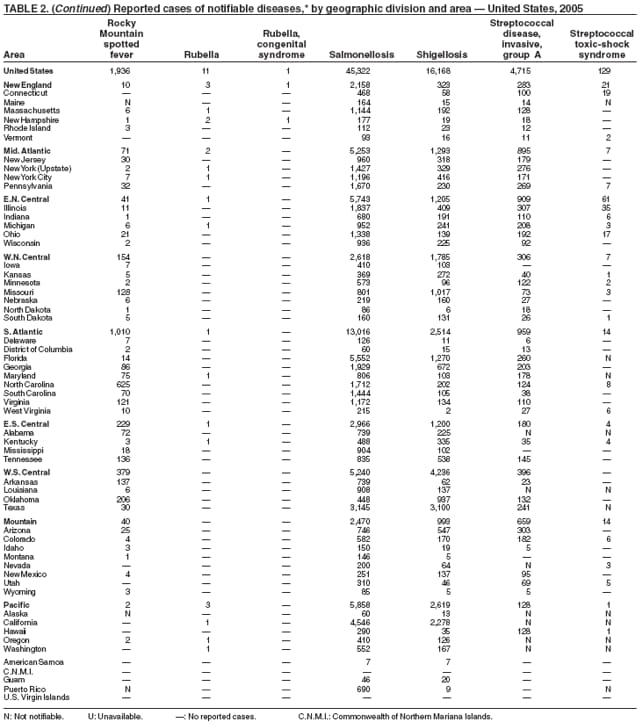
During 2005, as in previous years, the majority of salmonellosis cases occurred among persons aged <5 years. Since 1993, the most frequently reported isolates have been Salmonella enterica serotype Typhimurium and S. enterica serotype Enteritidis (1). The epidemiology of Salmonella has been changing. S. enterica serotype Typhimurium has decreased in incidence, while the incidence of serotypes Newport, Mississippi, and Javiana have increased. Specific control programs (e.g., farm-based egg--quality assurance programs) have led to reduction of serotype Enteritidis infections, which have been associated with the consumption of internally contaminated eggs. Rates of antibiotic resistance among certain serotypes have been increasing: a substantial proportion of serotypes Typhimurium and Newport isolates are resistant to multiple drugs (2).
The epidemiology of Salmonella infections is based on serotype characterization, and in 2005, the Council of State and Territorial Epidemiologists adopted a position statement for serotype-specific reporting of laboratory confirmed salmonellosis cases (3). However, reporting through the National Notifiable Diseases Surveillance System (NNDSS) does not include serotype, and for users of NNDSS, serotype for Salmonella isolates are reported through the Public Health Laboratory Information System (PHLIS). NEDSS or compatible systems eventually will replace PHLIS; users of NEDSS or compatible systems should report serotype in NEDSS.
The approximately 16,000 cases of shigellosis reported to CDC in 2005 represent an increase over the all-time low of approximately 14,000 cases reported in 2004. Reported annual totals during 1978--2003, with the exception of 2004, have consistently exceeded 17,000 cases. Shigella sonnei infections continue to account for >75% of shigellosis in the United States (1,2). In 2005, a strain of S. sonnei resistant to ampicillin and trimethoprim-sulfamethoxazole emerged as a cause of prolonged, community-wide outbreaks of shigellosis associated with day care centers in three states (3). Antimicrobial treatment options for children infected with this strain are limited and include oral azithromycin, "off-label" use of fluoroquinolones, or intramuscular agents such as ceftriaxone (3,4). In addition to spread from one person to another, shigellae can be transmitted through contaminated foods, sexual contact, and water used for drinking or recreational purposes (1).
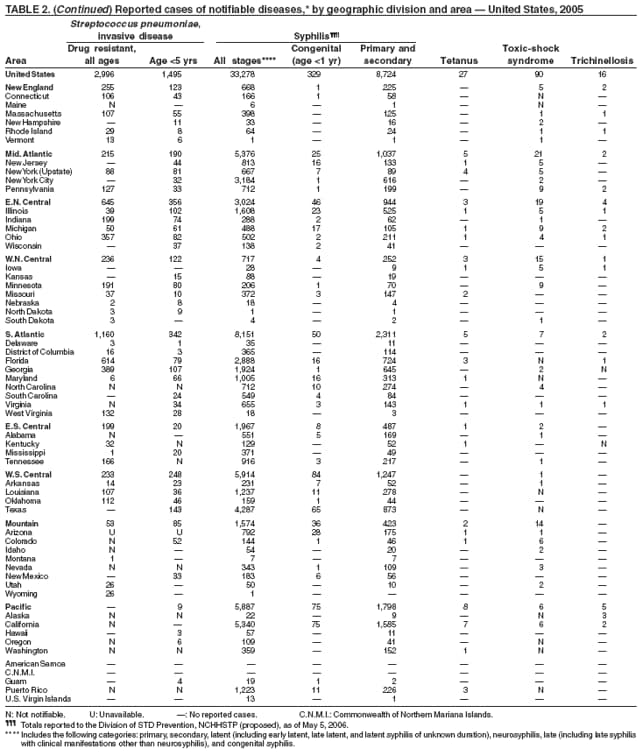
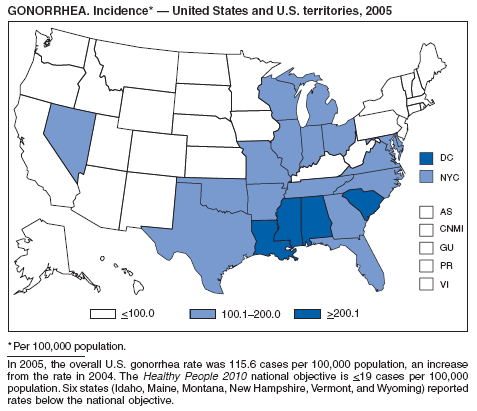
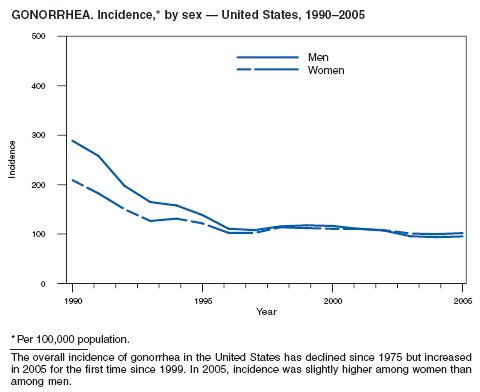
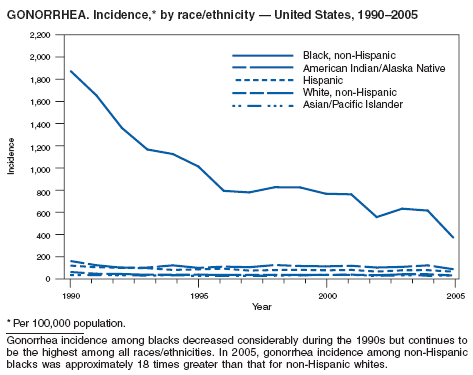
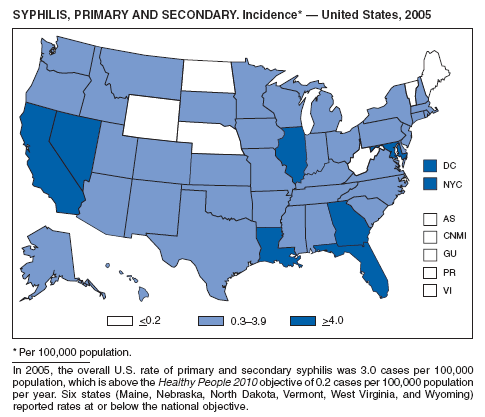
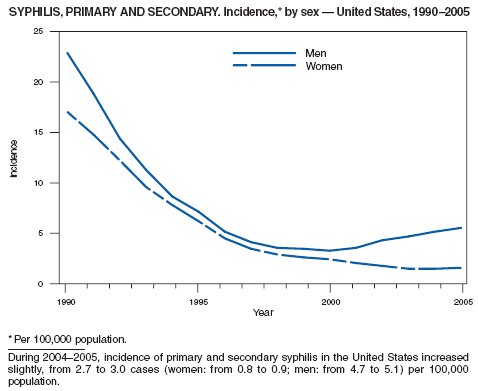
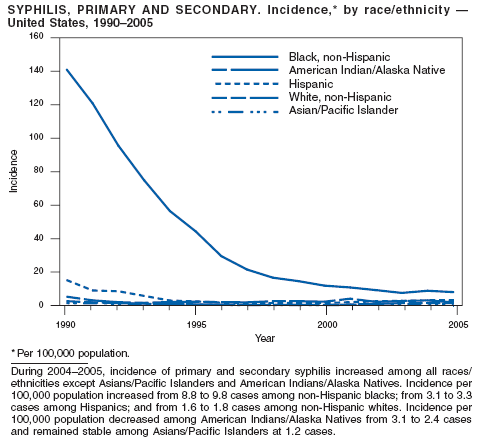
In 2005, primary and secondary syphilis cases reported to CDC increased for the fifth consecutive year (1). The overall increase in 2005 was 9.3%. Although the rate of syphilis infection increased mostly among men, for the first time in >10 years, the rate also increased among women. Rates increased among black, white, and Hispanic men and women. In collaboration with partners throughout the United States, CDC updated the Syphilis Elimination Plan for 2005--2010 and is now working to implement it (2).
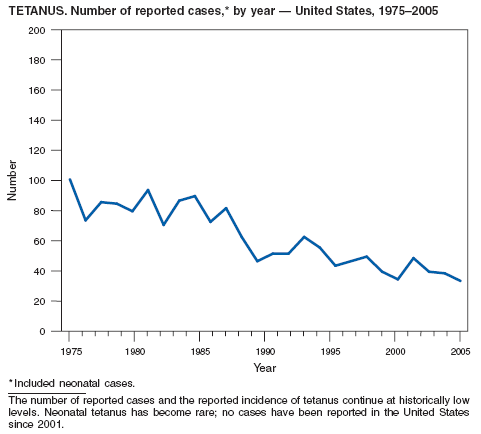
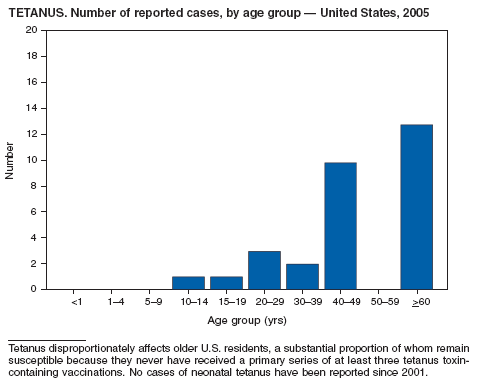
Rates of reported tetanus in 2005 (0.095 cases per 1 million population) continue at historically low levels. Two fatalities were attributed to tetanus in 2005: a woman aged 94 years who had never received tetanus toxoid vaccination, and a woman aged 73 years with an unknown vaccination history. The majority (85%) of tetanus cases occurred among persons aged >25 years; 44% occurred among persons aged 25--59 years, and 41% occurred among persons aged >60 years. No neonatal cases were reported.
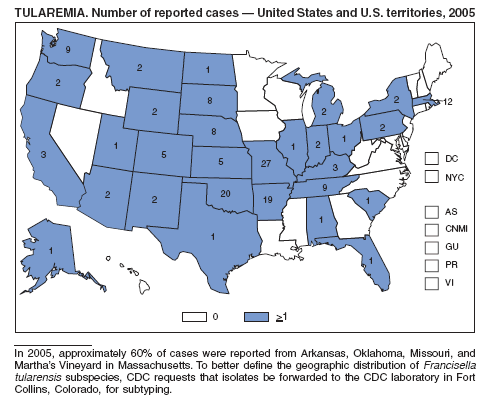
In the United States, tularemia is caused by two subspecies of Francisella tularensis: subspecies tularensis (type A) and subspecies holarctica (type B). A recent analysis combining national surveillance data with laboratory testing demonstrated marked differences in the demographic and geographic distribution of type A and type B infections (1). Patients with type A infections were younger and less likely to have a reported immunocompromising condition than patients with type B infections. Type A infections were predominant on the eastern seaboard, in and around Arkansas and Oklahoma, and from the Rocky Mountains in Colorado west to the Sierra Nevada mountains in California. Infections reported from the northern Pacific Coast and along tributaries of the Mississippi River typically were type B. Further subtyping of type A isolates by pulsed-field gel electrophoresis identified two distinct genetic groups, one causing infections east of the 100th meridian (East) and the other to the west (West). Mortality among patients with type A-East infections was 14%, compared with 9% for patients with type B infections, and 0 for patients with type A-West infections. To define the epidemiology of tularemia in the United States further, CDC encourages reporting of cases and submission of F. tularensis isolates to the CDC laboratory in Fort Collins, Colorado.
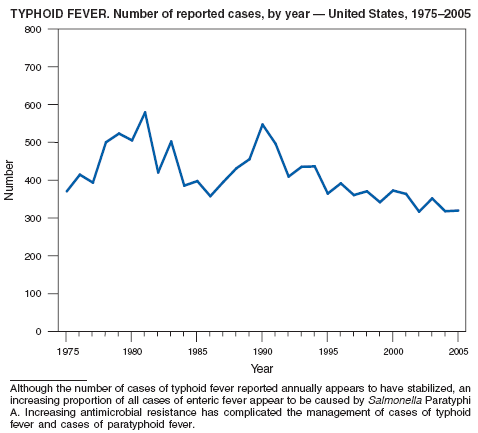
In 2005, the number of cases of typhoid fever in the United States reported to CDC remained essentially stable. Despite recommendations that travelers to countries in which typhoid fever is endemic should be vaccinated with either of two effective vaccines available in the United States, approximately three fourths of all cases occur among persons who report international travel during the preceding month. Persons visiting friends and relatives in south Asia appear to be at particular risk, even during short visits (1,2). Salmonella Typhi strains with decreased susceptibility to ciprofloxacin are increasingly frequent in that region and might require treatment with alternative antimicrobial agents (3). In 2005, the first case of truly ciprofloxacin-resistant S. Typhi infection in the United States was identified. Cases of paratyphoid fever caused by Salmonella Paratyphi A make up an increasing proportion of all cases of enteric fever diagnosed in the United States (CDC, unpublished data, 2006). During 2004--2005, patients with paratyphoid fever were even more likely than those patients with typhoid fever to have acquired their infections in south Asia and to be infected with fluoroquinolone-resistant strains.
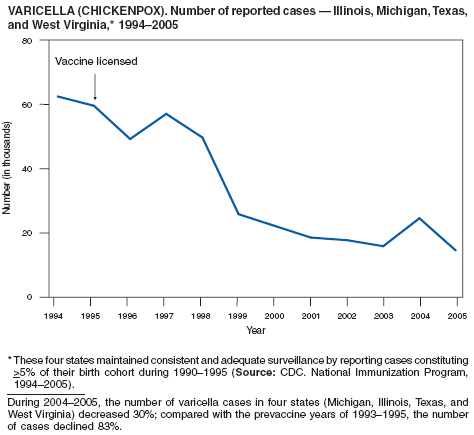
In 2003, varicella infection was again added to the nationally notifiable disease list with the recommendation that states implement statewide individual case reporting by 2005 (1). The objectives of varicella surveillance at state and national levels are to monitor the impact of the varicella vaccination program on the epidemiology of varicella by person (e.g., age, vaccination status, and severity), place, and time, and to evaluate vaccine policy. As of 2005, a total of 30 states and the District of Columbia were conducting either statewide or sentinel case-based surveillance for varicella.
Part 1
Summaries of Notifiable Diseases in the United States, 2005
Part 2
Graphs and Maps for Selected Notifiable Diseases in the United States, 2005


Part 3
Historical Summaries of Notifiable Diseases in the United States, 1974-2005
General
Adekoya N. Nationally notifable disease surveilance (NNDSS) and the Healthy People 2010 objectives. The eJournal of the South Carolina Medical Association 2005;101:e68--72. Available at http://www.scmanet.org/Downloads/e-Journal/SCMA_eJournal_March05.pdf.
Baker MG, Fidler DP. Global public health surveillance under new international health regulations. Emerg Infect Dis 2006;12:1058--65.
Bayer R, Fairchild AL. Public health: surveillance and privacy. Science 2000;290:1898--9.
CDC. Racial disparities in nationally notifiable diseases---United States, 2002. MMWR 2005;54:9--11.
CDC. Case definitions for infectious conditions under public health surveillance. MMWR 1997;46(No. RR-10). Additional information available at http://www.cdc.gov/epo/dphsi/casedef/index.htm.
CDC. Framework for program evaluation in public health. MMWR 1999;48(No. RR-11).
CDC. Manual of procedures for the reporting of nationally notifiable diseases to CDC. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC; 1995.
CDC. Manual for the surveillance of vaccine-preventable diseases. 3rd ed. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC; 2002. Available at http://www.cdc.gov/nip/publications/surv-manual.
CDC. National Electronic Disease Surveillance System (NEDSS): a standards-based approach to connect public health and clinical medicine. J Public Health Management and Practice 2001;7:43--50.
CDC. Public Health Information Network (PHIN): overview. Atlanta, GA: US Department of Health and Human Services, CDC; 2006. Available at http://www.cdc.gov/phin/overview.html.
CDC. Sexually transmitted disease surveillance, 2005. Atlanta, GA: US Department of Health and Human Services, CDC; 2006.
CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR 2006;55(No. RR-11).
Chang M-H, Glynn MK, Groseclose SL. Endemic, notifiable bioterrorism-related diseases, United States, 1992--1999. Emerg Infect Dis 2003;9:556--64.
Chin JE, ed. Control of communicable diseases manual. 17th ed. Washington, DC: American Public Health Association; 2000.
Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol 2002;155: 866--74.
Effler P, Ching-Lee M, Bogard A, Ieong M-C, Nekomoto T, Jernigan D. Statewide system of electronic notifiable disease reporting from clinical laboratories: comparing automated reporting with conventional methods. JAMA 1999;282:1845--50.
Freimuth V, Linnan HW, Potter P. Communicating the threat of emerging infections to the public. Emerg Infect Dis 2000;6:337--47.
German R. Sensitivity and predictive value positive measurements for public health surveillance systems. Epidemiology 2000;11:720--7.
Government Accountability Office. Emerging infectious diseases: review of state and federal disease surveillance efforts. Washington, DC: Government Accountability Office; 2004. GAO-04-877. Available at http://www.gao.gov/new.items/d04877.pdf.
Hopkins RS. Design and operation of state and local infectious disease surveillance systems. J Public Health Management Practice 2005;11:184--90.
Jajosky RA, Groseclose SL. Evaluation of reporting timeliness of public health surveillance systems for infectious diseases. BMC Public Health 2004;4:29.
Koo D, Caldwell B. The role of providers and health plans in infectious disease surveillance. Eff Clin Pract 1999;2:247--52. Available at http://www.acponline.org/journals/ecp/sepoct99/koo.htm.
Koo D, Wetterhall S. History and current status of the National Notifiable Diseases Surveillance System. J Public Health Management Practice 1996;2:4--10.
Krause G, Brodhun B, Altmann D, Claus H, Benzler J. Reliability of case definitions for public health surveillance assessed by Round-Robin test methodology. BMC Public Health 2006;6:129.
Lin SS, Kelsey JL. Use of race and ethnicity in epidemiologic research: concepts, methodological issues, and suggestions for research. Epidemiol Rev 2000;22:187--202.
Martin SM, Bean NH. Data management issues for emerging diseases and new tools for managing surveillance and laboratory data. Emerg Infect Dis 1995;1:124--8.
McNabb S, Chungong S, Ryan M, et al. Conceptual framework of public health surveillance and action and its application in health sector reform. BMC Public Health 2002;2:2.
McNabb S, Surdo A, Redmond A, et al. Applying a new conceptual framework to evaluate tuberculosis surveillance and action performance and measure the costs, Hillsborough County, Florida, 2002. Ann Epidemiol 2004;14:640--5.
Niskar AS, Koo D. Differences in notifiable infectious disease morbidity among adult women---United States, 1992--1994. J Womens Health 1998;7:451--8.
Panackal AA, M'ikanatha NM, Tsui FC, et al. Automatic electronic laboratory-based reporting of notifiable infectious diseases at a large health system. Emerg Infect Dis 2002;8:685--91.
Pinner RW, Koo D, Berkelman RL. Surveillance of infectious diseases. In: Lederberg J, Alexander M, Bloom RB, eds. Encyclopedia of microbiology. 2nd ed. San Diego, CA: Academic Press; 2000.
Pinner RW, Jernigan DB, Sutliff SM. Electronic laboratory-based reporting for public health. Mil Med 2000;165 (Suppl 2):20--4.
Roush S, Birkhead G, Koo D, Cobb A, Fleming D. Mandatory reporting of diseases and conditions by health care professionals and laboratories. JAMA 1999;282:164--70.
Silk, BJ, Berkelman RL. A review of strategies for enhancing the completeness of notifiable disease reporting. J Public Health Management Practice 2005;11:191--200.
Teutsch SM, Churchill RE, eds. Principles and practice of public health surveillance. 2nd ed. New York, NY: Oxford University Press; 2000.
Thacker SB, Choi K, Brachman PS. The surveillance of infectious diseases. JAMA 1983;249:1181--5.
CDC. HIV/AIDS surveillance report, 2005. Atlanta, GA: US Department of Health and Human Services, CDC, Vol. 17; 2006. Available at http://www.cdc.gov/hiv/stats/hasrlink.htm.
Nakashima AK, Fleming PL. HIV/AIDS surveillance in the United States, 1981--2001. J Acquir Immune Defic Syndr 2003;32:68--85.
Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med 2006;144:270--80.
Howell JM, Mayer TA, Hanfling D, et al. Screening for inhalational anthrax due to bioterrorism: evaluating proposed screening protocols. Clin Infect Dis 2004;39:1842.
Hugh-Jones M. 1996--97 global anthrax report. J Appl Microbiol 1999;87:189--91.
Inglesby TV, O'Toole T, Henderson DA, et al. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 2002;287:2236--52.
Kyriacou DN, Stein AC, Yarnold PR, et al. Clinical predictors of bioterrorism-related inhalational anthrax. Lancet 2004;364(9432):449--52.
Angulo FJ, St. Louis ME. Botulism. In: Evans AS, Brachman PS, eds. Bacterial infections of humans. New York, NY: Plenum; 1998:131--53.
CDC. Infant botulism---New York City, 2001--2002. MMWR 2003;52:21--4.
Shapiro RL, Hatheway C, Becher J, Swerdlow DL. Botulism surveillance and emergency response: a public health strategy for a global challenge. JAMA 1997;278:433--5.
Shapiro RL, Hatheway C, Swerdlow DL. Botulism in the United States: a clinical and epidemiologic review. Ann Intern Med 1998;129:221--8.
Sobel J, Tucker N, McLaughlin J, Maslanka S. Foodborne botulism in the United States, 1999--2000. Emerg Infect Dis 2004;10:1606--12.
Sobel J. Botulism. Clin Infect Dis 2005;41:1167--73.
CDC. Brucellosis (Brucella melitensis, abortus, suis, and canis). Atlanta, GA: US Department of Health and Human Services, CDC; 2005. Available at http://www.cdc.gov/ncidod/dbmd/diseaseinfo/brucellosis_g.htm.
CDC. Brucellosis case definition. Atlanta, GA: US Department of Health and Human Services, CDC; 2001. Available at http://www.bt.cdc.gov/Agent/Brucellosis/CaseDef.asp.
CDC. Human exposure to Brucella abortus strain RB51---Kansas, 1997. MMWR 1998;47:172--5.
Stevens, MG, Olsen SC, Palmer MV, Cheville NF. US Department of Agriculture, Agricultural Research Service National Animal Disease Center, Iowa State University. Brucella abortus strain RB51: a new brucellosis vaccine for cattle. Compendium 1997;19:766--74.
Yagupsky P, Baron EJ. Laboratory exposures to Brucellae and implications for bioterrorism. Emerg Infect Dis 2005;11:1180--5.
Chomel BB, DeBess EE, Mangiamele DM, et al. Changing trends in the epidemiology of human brucellosis in California from 1973 to 1992: a shift toward foodborne transmission. J Infect Dis 1994;170:1216--23.
DiCarlo RP, Armentor BS, Martin DH. Chancroid epidemiology in New Orleans men. J Infect Dis 1995;172:446--52.
Mertz KJ, Weiss JB, Webb RM, et al. An investigation of genital ulcers in Jackson, Mississippi, with use of a multiplex polymerase chain reaction assay: high prevalence of chancroid and human immunodeficiency virus infection. J Infect Dis 1998;178:1060--6.
Mertz KJ, Trees D, Levine WC, et al. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis 1998;178:1795--8.
CDC. Sexually transmitted disease surveillance 2005 supplement: Chlamydia Prevalence Monitoring Project, annual report 2005. Atlanta, GA: US Department of Health and Human Services, CDC. In press.
Gaydos CA, Howell MR, Pare B, et al. Chlamydia trachomatis infections in female military recruits. N Engl J Med 1998;339:739--44.
Mertz KJ, McQuillian GM, Levine WC, et al. A pilot study of chlamydial infection in a national household survey. Sex Transm Dis 1998;25:225--8.
Miller WC, Ford CA, Handcock MS, et al. Prevalance of chlamydial and gonococcal infections among young adults in the United States. JAMA 2004;291:2229--36.
Steinberg EB, Greene KD, Bopp CA, Cameron DN, Wells JG, Mintz ED. Cholera in the United States, 1995--2000: trends at the end of the millennium. J Infect Dis 2001;184:799--802.
World Health Organization. Cholera, 2005. Wkly Epidemiol Rec 2006;81:297--308.
Mintz ED, Tauxe RV, Levine MM. The global resurgence of cholera. In: Noah ND, O'Mahony M, eds. Communicable disease epidemiology and control. Chichester, UK: John Wiley & Sons; 1998:63--104.
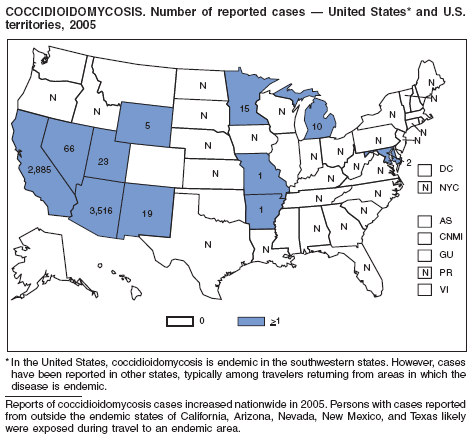
Park BJ, Sigel K, Vaz V et al. An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998--2001. J Infect Dis 2005;191:1981--7.
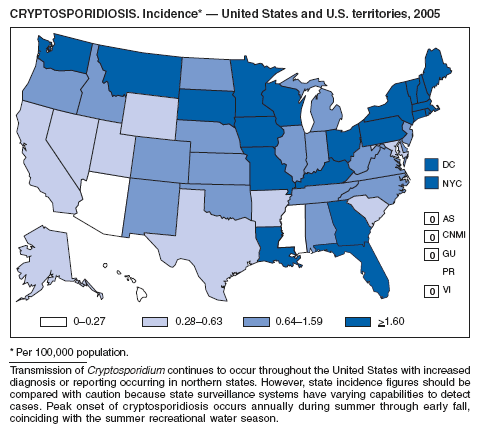
Hlavsa MC, Watson JC, Beach MJ. Cryptosporidiosis surveillance---United States 1999--2002. In: CDC Surveillance Summaries, January 28, 2005. MMWR 2005;54(No. SS-1): 1--8.
Yoder JS, Blackburn BG, Craun GF, et al. Surveillance for waterborne-disease outbreaks associated with recreational water---United States, 2001--2002. In: CDC Surveillance Summaries, October 22, 2004. MMWR 2004;53(No. SS-8): 1--21.
Roy SL, DeLong SM, Stenzel SA, et al. Risk factors for sporadic cryptosporidiosis among immunocompetent persons in the United States from 1999 to 2001. J Clin Microbiol 2004;42:2944--51.
CDC. Diagnostic procedures for stool specimens; detection of parasite antigens. Atlanta, GA: US Department of Health and Human Services, CDC; 2004. Available at http://www.dpd.cdc.gov/dpdx/html/diagnosticprocedures.htm.
Herwaldt BL. The ongoing saga of U.S. outbreaks of cyclosporiasis associated with imported fresh produce: what Cyclospora cayetanensis has taught us and what we have yet to learn. In: Institute of Medicine. Addressing foodborne threats to health: policies, practices, and global coordination. Washington, DC: The National Academies Press; 2006:85--115, 133--40. Available at http://newton.nap.edu/catalog/11745.html#toc.
Herwaldt BL. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis 2000;31:1040--57.
Dewinter LM, Bernard KA, Romney MG. Human clinical isolates of Corynebacterium diphtheriae and Corynebacterium ulcerans collected in Canada from 1999 to 2003 but not fitting reporting criteria for cases of diphtheria. Clin Microbiol 2005;43:3447--9.
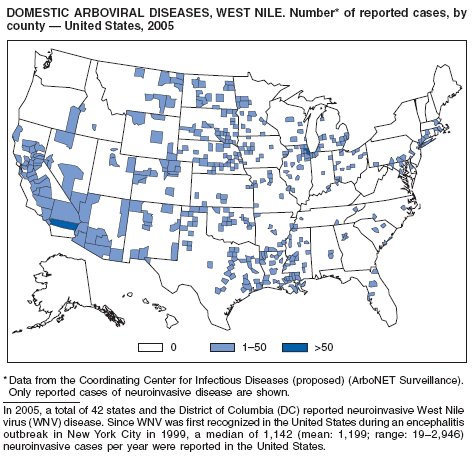
CDC. West Nile virus activity---United States, January 1--December 1, 2005. MMWR 2005;54:1253--6.
Hayes EB, Komar N, Nasci RS, et al. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis 2005;11:1167--73.
Bender JB, Hedberg CW, Besser JM, et al. Surveillance for Escherichia coli O157:H7 infections in Minnesota by molecular subtyping. N Engl J Med 1997;337:388--94.
Brooks JT, Sowers EG, Wells JB, et al. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983--2002. J Infect Dis 2005;192:1422--9.
Crump JA, Sulka AC, Langer AJ, et al. An outbreak of Escherichia coli O157:H7 among visitors to a dairy farm. N Engl J Med 2002;347:555--60.
Griffin PM, Mead PS, Sivapalasingam S. Escherichia coli O157:H7 and other enterohemorrhagic E. coli. In: Blaser MJ, Smith PD, Ravdin JI, Greenberg HB, Guerrant RL, eds. Infections of the gastrointestinal tract. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:627--42.
Mead PS, Griffin PM. Escherichia coli O157:H7. Lancet 1998;352:1207--12.
Demma LJ, Holman RC, McQuiston JH, Krebs JW, Swerdlow DL. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001--2002. Am J Trop Med Hyg 2005;73:400--9.
Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen [Review]. J Clin Microbiol 2003;16: 37--64.
IJdo JW, Meek JI, Cartter ML, et al. The emergence of another tick-borne infection in the 12-town area around Lyme, Connecticut: human granulocytic ehrlichiosis. J Infect Dis 2000;181:1388--93.
Stuart JM, Orr HJ, Warburton FG, et al. Risk factors for sporadic giardiasis: a case-control study in Southwestern England. Emerg Infect Dis 2003;9:229--33.
CDC. Diagnostic procedures for stool specimens; detection of parasite antigens. Atlanta, GA: US Department of Health and Human Services, CDC; 2004. Available at http://www.dpd.cdc.gov/dpdx/html/diagnosticprocedures.htm.
CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR 2006;55(No. RR-11).
CDC. Sexually transmitted diseases surveillance 2005 supplement: Gonococcal Isolate Surveillance Project (GISP) annual report 2005. Atlanta, GA: US Department of Health and Human Services, CDC. In press.
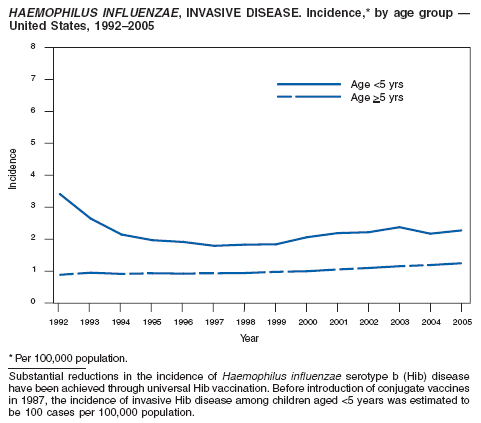
Fry AM, Lurie P, Gidley M, Schmink S, Lingappa J, Rosenstein NE. Haemophilus influenzae type b (Hib) disease among Amish children in Pennsylvania: reasons for persistent disease. Pediatrics 2001;108:1--6.
Britton WJ, Lockwood NJ. Leprosy. Lancet 2004;363:1209--19.
Hartzell JD, Zapor M, Peng S, Straight T. Leprosy: a case series and review. South Med J 2004;97:1252--6.
Hastings R, Ed. Leprosy. 2nd ed. New York, NY: Churchill Livingstone; 1994.
Joyce MP, Scollard DM. Leprosy (Hansen's disease). In: Rakel RE, Bope ET, eds. Conn's current therapy 2004: latest approved methods of treatment for the practicing physician. 56th ed. Philadelphia, PA: Saunders; 2004:100--5.
Ooi WW, Moschella SL. Update on leprosy in immigrants in the United States: status in the year 2000. Clin Infect Dis 2001;32:930--7.
Bruce S, Schroeder TL, Ellner K, Rubin H, Williams T, Wolf JE Jr. Armadillo exposure and Hansen's disease: an epidemiologic survey in southern Texas. J Am Acad Dermatol 2000;43(2 Pt1):223--8.
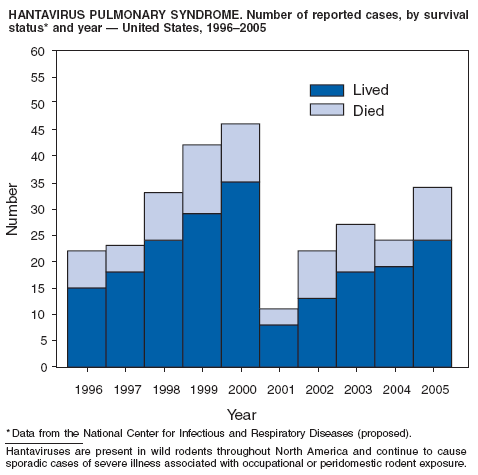
CDC. Hantavirus pulmonary syndrome---five states, 2006. MMWR 2006;55:627--9.
Banatvala N, Griffin PM, Greene KED, et al. The United States Prospective Hemolytic Uremic Syndrome Study; microbiologic, serologic, clinical, and epidemiologic findings. J Infect Dis 2001;183:1063--70.
Mahon BE, Griffin PM, Mead PS, Tauxe RV. Hemolytic uremic syndrome surveillance to monitor trends in infection with Escherichia coli O157:H7 and other Shiga toxin-producing E. coli [Letter]. Emerg Infect Dis 1997;3: 409--12.
Armstrong GL, Bell BP. Hepatitis A virus infections in the United States: model-based estimates and implications for childhood immunization. Pediatrics 2002;109:839--45.
Bell BP, Kruszon-Moran D, Shapiro CN, Lambert SB, McQuillan GM, Margolis HS. Hepatitis A virus infection in the United States: serologic results from the Third National Health and Nutrition Examination Survey. Vaccine 2005;23:5798--806.
Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. JAMA 2005;294:194--201.
Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev 2006;28:101--11.
Armstrong GL, Mast EE, Wojczynski M, Margolis HS. Childhood hepatitis B virus infections in the United States before hepatitis B immunization. Pediatrics 2001;108:1123--8.
Shepard CW, Simard EP, Finelli L, Fiore A, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006;28:112--25.
Goldstein ST, Alter MJ, Williams IT, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982--1998: implications for vaccination programs. J Infect Dis 2002;185:713--9.
McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: The National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health 1999;89:14--8.
Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006;144):705--14.
Armstrong GA, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31:777--82.
Shepard CW, Finelli L, Alter MJ. The global epidemiology of hepatitis C. Lancet Infect Dis 2005;5:558--67.
Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003--2004. N Engl J Med 2005;352:2559--67.
Council of State and Territorial Epidemiologists. Influenza-associated pediatric mortality, 2004. Atlanta, GA: Council of State and Territorial Epidemiologists; 2004. Available at http://www.cste.org/PositionStatementsResolutions2.htm.
Council of State and Territorial Epidemiologists. Position statement 04-ID-04: influenza-associated pediatric mortality 2004. Atlanta, GA: Council of State and Territorial Epidemiologists; 2004. Available at http://www.cste.org/ps/2004pdf/04-ID-04-final.pdf.
Guarner J, Paddock CD, Shieh WJ, et al. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003--2004 season. Clin Infect Dis 2006:43;132--40.
Cowgill KD, Lucas CE, Benson RF, et al. Recurrence of legionnaires disease at a hotel in the United States Virgin Islands over a 20-year period. Clin Infect Dis 2005;40:1205--7.
Fields BS, Benson RF, Besser RE. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev 2002;15:506--26.
European Working Group on Legionella Infections. European guidelines for control and prevention of travel associated Legionnaires' disease. London, UK: United Kingdom Health Protection Agency; 2005.
Joseph CA. Legionnaires' disease in Europe 2000--2002. Epidemiol Infect 2004;132:417--24.
Marston BJ, Lipman HB, Breiman RF. Surveillance for Legionnaires' disease: risk factors for morbidity and mortality. Arch Intern Med 1994;154:2417--22.
Gottlieb SL, Newbern EC, Griffin PM, et al. Multistate outbreak of listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin Infect Dis 2006;42:29--36.
Mead PS, Dunne EF, Graves L, et al. Nationwide outbreak of listeriosis due to contaminated meat. Epidemiol Infect 2006;134:744--51.
Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerg Infect Dis 1998;5: 607--25.
Slutsker L, Schuchat A. Listeriosis in humans. In: Ryser ET Marth EH, eds. Listeria, listeriosis, and food safety. 2nd ed. New York, NY: Marcel Dekker, Inc.; Little, Brown and Company; 1999:75--95.
Tappero J, Schuchat A, Deaver K, Mascola L, Wenger J, for the Listeriosis Study Group. Reduction in the incidence of human listeriosis in the United States: effectiveness of prevention efforts. JAMA 1995;273:1118--22.
Stafford KC III. Tick management handbook: an integrated guide for homeowners, pest control operators, and public health officials for the prevention of tick-associated disease. New Haven, CT: Connecticut Agricultural Experiment Station; 2004. Available at http://www.cdc.gov/ncidod/dvbid/lyme/resources/handbook.pdf.
Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med 2003;348:2424--30.
Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 2005;18:484--509.
Medical Letter. Treatment of Lyme disease. Med Lett Drugs Ther 2005;47:41--3.
CDC. Caution regarding testing for Lyme disease. MMWR 2005;54:125.
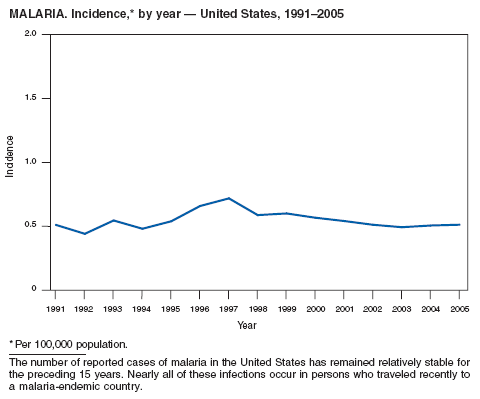
Baird JK. Effectiveness of antimalarial drugs. N Engl J Med 2005;352:1565--77.
Chen LH, Keystone JS. New strategies for the prevention of malaria in travelers. Infect Dis Clin N Amer 2005;19: 185--210.
Guinovart C, Navia MM, Tanner M, et al. Malaria: burden of disease. Curr Mol Med 2006;6:137--40.
Leder K, Black J, O'Brien D, et al. Malaria in travelers: a review of the GeoSentinel Surveillance Network. Clin Infect Dis 2004;39:1104--12.
Papania M, Hinman A, Katz S, Orenstein W, McCauley M, eds. Progress toward measles elimination---absence of measles as an endemic disease in the United States. J Infect Dis 2004;189(Suppl 1):S1--257.
Rota PA, Liffick SL, Rota JS, et al. Molecular epidemiology of measles viruses in the United States, 1997--2001. Emerg Infect Dis 2002;8:902--8.
De Serres G, Gay NJ, Farrington CP. Epidemiology of transmissible diseases after elimination. Am J Epidemiol 2000;151:1039--48.
Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med 2001;344:1378--88.
Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of meningococcal disease in the United States, 1992--1996. J Infect Dis 1999;180:1894--901.
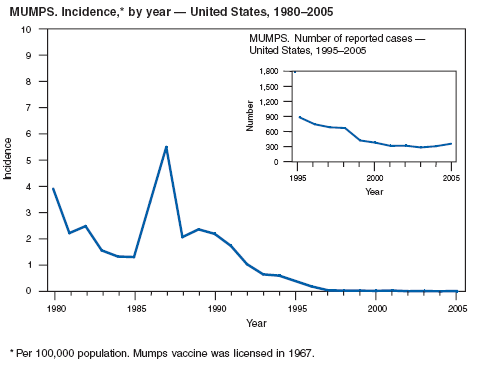
Harling R, White JM, Ramsay ME, et al. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine 2005;23:4070--4.
CDC. Mumps outbreak at a summer camp---New York, 2005. MMWR 2006;55:175--7.
Bisgard KM, Rhodes P, Connelly BL, et al. Pertussis vaccine effectiveness among children 6 to 59 months of age in the United States, 1998--2001. Pediatrics 2005;116:e285--94.
Bisgard KM, Pascual FB, Ehresmann KR, et al. Infant pertussis: who was the source? Pediatr Infect Dis J 2004;23:985--9.
CDC. Pertussis---United States, 2001--2003. MMWR 2005;54:1283--6.
Lee GM, Lebaron C, Murphy TV, Lett S, Schauer S, Lieu TA. Pertussis in adolescents and adults: should we vaccinate? Pediatrics 2005;115:1675--84.
CDC. Imported plague---New York City, 2002. MMWR 2003;53:725--8.
Enscore RE, Biggerstaff BJ, Brown TL, et al. Modeling relationships between climate and the frequency of human plague cases in the southwestern United States, 1960--1997. Am J Trop Med Hyg 2002;66:186--96.
Inglesby TV, Dennis DT, Henderson DA, et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense [Review]. JAMA 2000;283:2281--90.
Dennis DT, Gage KL, Gratz N, Poland JD, Tikhomirov E. Plague manual: epidemiology, distribution, surveillance and control. Geneva, Switzerland: World Health Organization; 1999.
CDC. Imported vaccine-associated paralytic poliomyelitis---United States, 2005. MMWR 2006;55:97--9.
Alexander LN, Seward JF, Santibanez TA, et al. Vaccine policy changes and epidemiology of polio in the United States. JAMA 2004;292:1696--702.
Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol 2005; 59:587--635.
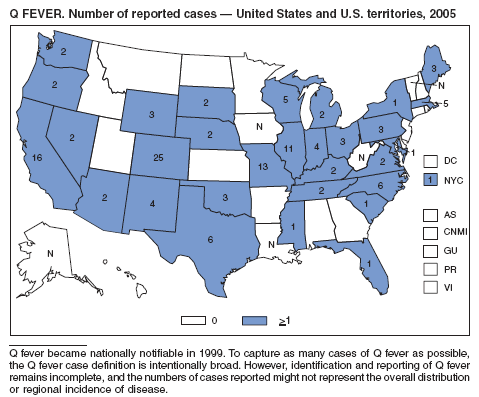
McQuiston JH, Holman RC, McCall CL, Childs JE, Swerdlow DL, Thompson HA. National surveillance and the epidemiology of Q fever in the United States, 1978--2004. Am J Trop Med Hyg 2006;75:36--40.
Mcquiston JH, Nargund VN, Miller JD, Priestly R, Shaw EI, Thompson HA. Prevalence of antibodies to Coxiella burnetii among veterinary school dairy herds in the United States, 2003. Vector Borne Zoonotic Dis 2005;5:90--1.
Raoult D, Tissot-Dupont H, Foucault C, et al. Q fever 1985--1998. Clinical and epidemiologic features of 1,383 infections [Review]. Medicine 2000;79:109--25.
Bernard KW, Parham GL, Winkler WG, Helmick CG. Q fever control measures: recommendations for research facilities using sheep. Infect Control 1982;3:461--5.
Krebs JW, Mandel EJ, Swerdlow DL, Rupprecht CE. Rabies surveillance in the United States during 2004. J Am Vet Med Assoc 2005;227:1912--25.
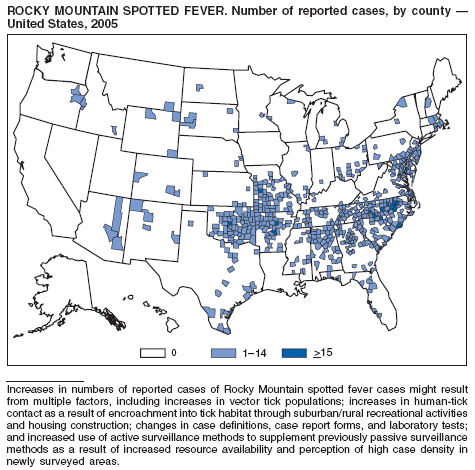
Chapman AS, Murphy SM, Demma LJ, et al. Rocky Mountain spotted fever in the United States, 1997--2002. Vector Borne Zoonotic Dis 2006;6:170--8.
Demma LJ, Traeger MS, Nicholson WL, et al. Rocky Mountain spotted fever from an unexpected tick reservoir in Arizona. N Engl J Med 2005;353:587--94.
Thorner AR, Walker DH, Petri WA. Rocky Mountain spotted fever [Review]. Clin Infect Dis 1998;27:1353--60.
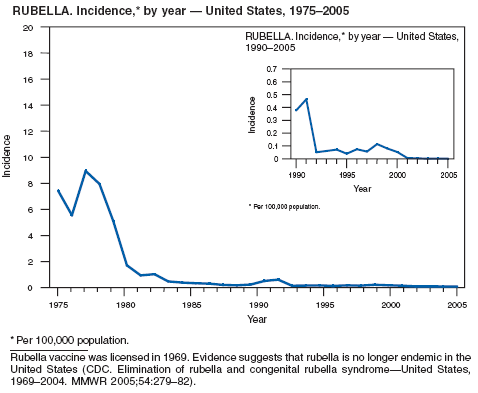
Danovaro-Holliday MC, Gordon E, Woernle C, et al. Identifying risk factors for rubella susceptibility in a population at risk in the United States. Am J Public Health 2003;93:289--91.
Reef SE, Frey TK, Theall K, et al. The changing epidemiology of rubella in the 1990s: on the verge of elimination and new challenges for control and prevention. JAMA 2002;287;464--72.
Reef S, Plotkin S, Cordero J, et al. Preparing for congenital rubella syndrome elimination: summary of the Workshop on Congenital Rubella Elimination in the United States. Clin Infect Dis 2000;31:85--95.
Braden CR. Salmonella enterica serotype Enteritidis and eggs: a national epidemic in the United States. Clin Infect Dis 2006;43:512--7.
Olsen SJ, Bishop R, Brenner FW, et al. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987--1997. J Infect Dis 2001;183:756--61.
Voetsch AC, Van Gilder TJ, Angulo FJ, et al. FoodNet estimate of burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis 2004;38(Suppl 3):S127--34.
Shane A, Crump J, Tucker N, Painter J, Mintz E. Sharing Shigella: risk factors and costs of a multi-community outbreak of shigellosis. Arch Pediatr Adolesc Med 2003;157:601--3.
Gupta A, Polyak CS, Bishop RD, Sobel J, Mintz ED. Laboratory-confirmed shigellosis in the United States, 1989--2002: epidemiologic trends and patterns. Clin Infect Dis 2004;38:1372--7.
Sivapalasingam S, Nelson JM, Joyce K, Hoekstra M, Angulo FJ, Mintz ED. A high prevalence of antimicrobial resistance among Shigella isolates in the United States, 1999--2002. Antimicrob Agents Chemother 2006;50:49--54.
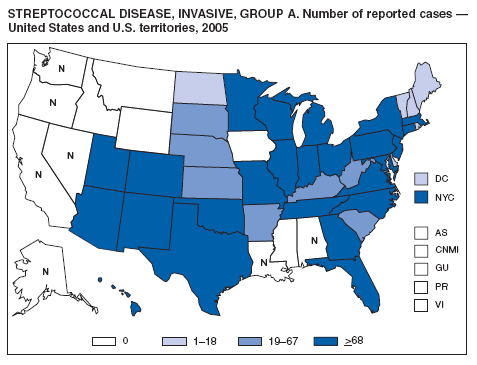
CDC. Active Bacterial Core Surveillance report. Emerging Infections Program Network. Group A streptococcus, 2005-provisional. Atlanta, GA: US Department of Health and Human Services, CDC; 2006. Available at http://www.cdc.gov/ncidod/dbmd/abcs/survreports/gas05prelim.pdf.
CDC. Investigating clusters of group A streptococcal disease. Atlanta, GA: US Department of Health and Human Services, CDC; 2005. Available at http://www2.cdc.gov/ncidod/dbmd/abcs/calc/calc_new/new_page.htm.
Bisno AL, Rubin FA, Cleary PP, Dale JB. National Institute of Allergy and Infectious Diseases. Prospects for a group A streptococcal vaccine: rationale, feasibility, and obstacles: report of a National Institute of Allergy and Infectious Diseases workshop. Clin Infect Dis 2005;41:1150--6.
O'Brien KL, Beall B, Barrett NL, et al. Epidemiology of invasive group A streptococcus disease in the United States, 1995--1999. Clin Infect Dis 2002;35:268--76.
The Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002;35:950--9.
Bisno AL. Brito MO. Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis 2003;3:191--200.
O'Brien KL, Beall B, Barrett NL, et al. Epidemiology of invasive group A streptococcus disease in the United States, 1995--1999. Clin Infect Dis 2002;35:268--76.
Stevens DL. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu Rev Med 2000;51:271--88.
The Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002;35:950--9.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 15th informational supplement [No. M100-S15]. Wayne, PA: National Committee for Clinical Laboratory Standards; 2005.
Flannery B, Schrag S, Bennett NM, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA 2004;291:2197--203.
Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006;354:1455--63.
Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 2006;295:1668--74.
Ray GT, Whitney CG, Fireman BH, Ciuryla V, Black SB. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J 2006;25: 494--501.
CDC. Congenital syphilis---United States, 2002. MMWR 2004;53:716--9.
CDC. The National Plan to Eliminate Syphilis from the United States. Atlanta, GA: US Department of Health and Human Services, CDC; 1999.
CDC. The National Plan to Eliminate Syphilis from the United States. Atlanta, GA: US Department of Health and Human Services, CDC; 2006.
CDC. Primary and secondary syphilis---United States, 2003--2004. MMWR 2006;55:269--73.
CDC Sexually transmitted disease surveillance supplement 2005; syphilis surveillance report. Atlanta, GA: US Department of Health and Human Services, CDC. In press.
CDC. Tetanus---Puerto Rico, 2002. MMWR 2002;51:613--5.
McQuillan GM, Kruszon-Moran D, Deforest A, Chu SY, Wharton M. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med 2002;136:660--6.
CDC. Trichinellosis associated with bear meat---New York and Tennessee, 2003. MMWR 2004;53:606--10.
Moorhead A, Grunenwald PE, Dietz VJ, Schantz PM. Trichinellosis in the United States, 1991--1996: declining but not gone. Am J Trop Med Hyg 1999;60:66--9.
CDC. Reported tuberculosis in the United States, 2003. Atlanta, GA: US Department of Health and Human Services, CDC; 2004. Available at http://www.cdc.gov/nchstp/tb.
CDC. Trends in tuberculosis---United States, 2004. MMWR 2005;54:245--9.
Saraiya M, Cookson ST, Tribble P, et al. Tuberculosis screening among foreign-born persons applying for permanent US residence. Am J Public Health 2002;92:826--9.
Talbot EA, Moore M, McCray E, Binkin NJ. Tuberculosis among foreign-born persons in the United States, 1993--1998. JAMA 2000;284:2894--900.
CDC. Outbreak of tularemia among commercially distributed prairie dogs, 2002. MMWR 2002;51:688, 699.
CDC. Tularemia---United States, 1990--2000. MMWR 2002;51:182--4.
Dennis DT, Inglesby TV, Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. JAMA 2001;285:2763--73.
Feldman KA, Enscore RE, Lathrop SL, et al. Outbreak of primary pneumonic tularemia on Martha's Vineyard. N Engl J Med 2001;345:1219--26.
Petersen JM, Schriefer ME. Tularemia: emergence/ re-emergence. Vet Res 2005;36:455--67.
Crump J, Barrett TJ, Nelson JT, Angulo FJ. Reevaluating fluoroquinolone breakpoints for Salmonella enterica serotype Typhi and for non-Typhi Salmonellae. Clin Infect Dis 2003;37:75--81.
Kubota K, Barrett TJ, Hunter S et al. Analysis of Salmonella serotype Typhi pulsed-field gel electrophoresis patterns associated with international travel. J Clin Micro 2005;43:1205--9.
Olsen SJ, Bleasdale SC, Magnano AR, et al. Outbreaks of typhoid fever in the United States, 1960--1999. Epidemiol Infect 2003;130:13--21.
Reller M, Olsen S, Kressel A. Sexual transmission of typhoid fever: a multi-state outbreak among men who have sex with men. Clin Infect Dis 2003;37:141--4.
Steinberg EB, Bishop RB, Dempsey AF, et al. Typhoid fever in travelers: who should be targeted for prevention? Clin Infect Dis 2004;39:186--91.
CDC. Public health response to varicella outbreaks---United States, 2003--2004. MMWR 2006;55:993--5.
CDC. Prevention of varicella: provisional ACIP recommendations for prevention of varicella. Atlanta, GA: US Department of Health and Human Services, CDC; 2006. Available at http://www.cdc.gov/nip/vaccine/varicella/varicella_acip_recs_prov_june_2006.pdf.
CDC. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. In press.
CDC. Varicella surveillance practices, United States, 2004. MMWR. In press.
Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA 2004;292:704--8.
Fridkin SK, Hageman J, McDougal LK, et al. Vancomycin-Intermediate Staphylococcus aureus Epidemiology Study Group. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997--2001. Clin Infect Dis 2003;36:429--39.
Chang S, Sievert DM, Hageman JC, et al. Vancomycin-Resistant Staphylococcus aureus Investigative Team. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med 2003;348:1342--7.
Whitener CJ, Park SY, Browne FA, et al. Vancomycin-resistant Staphylococcus aureus in the absence of vancomycin exposure. Clin Infect Dis 2004;38:1049--55.
Weigel LM, Clewell DB, Gill SR, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 2003;302:1569--71.
McDonald LC, Hageman JC. Vancomycin intermediate and resistant Staphylococcus aureus: what the nephrologist needs to know. Nephrol News Issues 2004;8:63--4, 66--7, 71--2.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 2/23/2007
|
|
|||||
|
HOME |
ABOUT MMWR |
MMWR SEARCH |
DOWNLOADS |
RSS
|
CONTACT
|
|||||
|
|
|||||
|