COVID-19 Science Update released: December 17, 2021 Edition 117

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 database, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
BNT162b2 vaccine booster and mortality due to COVID-19. Arbel et al. NEJM (December 8, 2021).
Key findings:
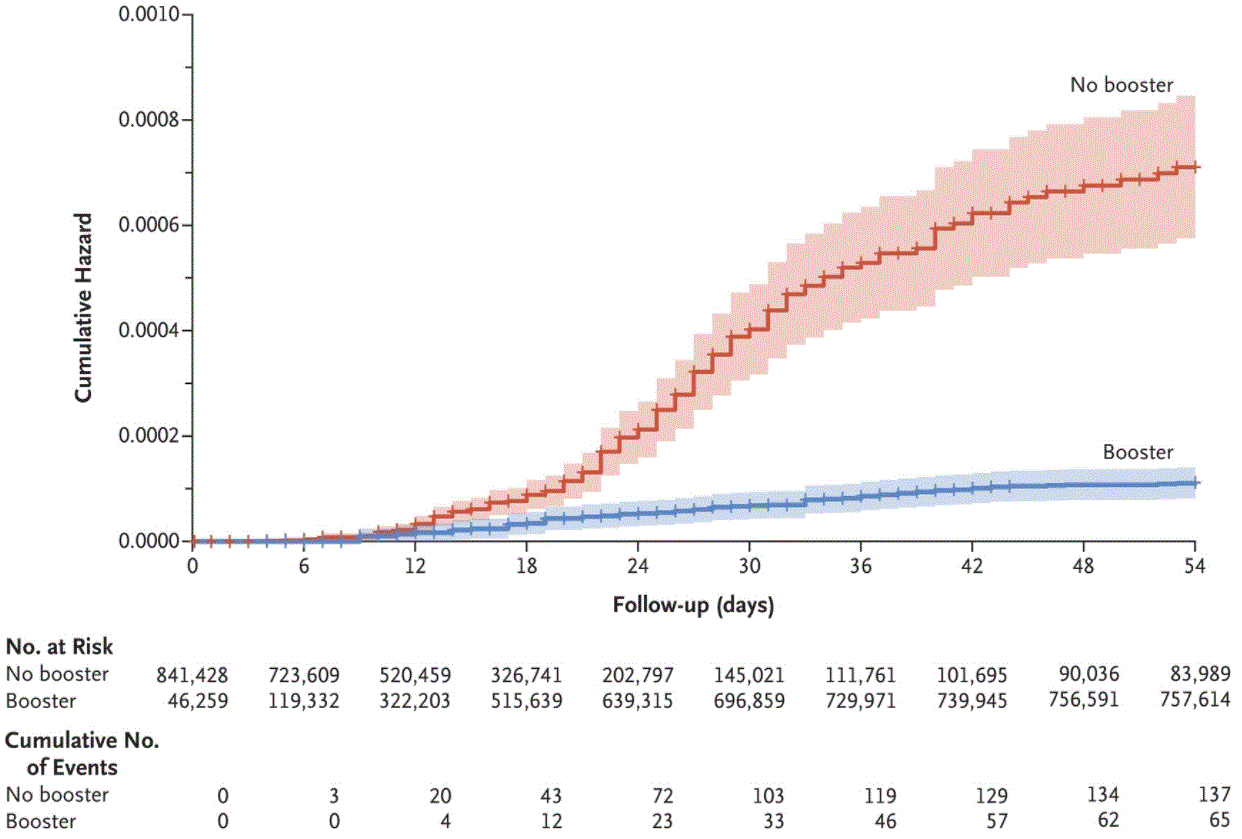
- Among adults aged ≥50 years, COVID-19 mortality was 90% lower among those who received a 3rd(booster) dose of BNT162b2 (Comirnaty, Pfizer/BioNTech) compared with those who received only 2 doses (adjusted hazard ratio [aHR]0.10, 95% CI 0.07-0.14) (Figure).
- Mortality was 0.16 per 100,000 persons/day in those who received a 3rd(booster) dose and 2.98 per 100,000 persons/day in those who did not.
- The aHR for SARS-CoV-2 infection in the booster group, as compared with the no booster group, was 0.17 (95% CI 0.16-0.18).
Methods: Analysis of electronic health record data for 843,208 adults aged ≥50 years in Israel who were eligible for 3rd dose of BNT162b2 (had received 2 doses ≥5 months previously) during August–September 2021 and had no prior history of SARS-CoV-2 infection. HRs were assessed using a Cox proportional-hazards regression model and were adjusted for coexisting conditions and demographics. Limitations: Brief follow-up period (54 days); participants aged >60 years were eligible for boosters in the first 2 weeks of the study period, and younger participants were eligible thereafter, leading to possible overestimation of mortality in the booster group; findings are limited to the BNT162b2 vaccine.
Implications: A BNT162b2 booster was associated with reduced risk of SARS-CoV-2 infection and death due to COVID-19 among adults ≥50 years of age. These findings support COVID-19 booster recommendations for persons in this age group.
Figure:
Note: Adapted from Arbel et al. Cumulative HR for death due to COVID-19. The shaded area indicates the 95% CI. Individuals were assigned to the no booster group from the start of the study (August 6, 2021) until 7 days after receiving a booster dose, at which point they were re-assigned to the booster group for the remainder of the follow up period. From the New England Journal of Medicine, Arbel et al., BNT162b2 vaccine booster and mortality due to COVID-19. December 8, 2021, online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Nursing home staff vaccination and COVID-19 outcomes. McGarry et al. NEJM (December 8, 2021).
Key findings:
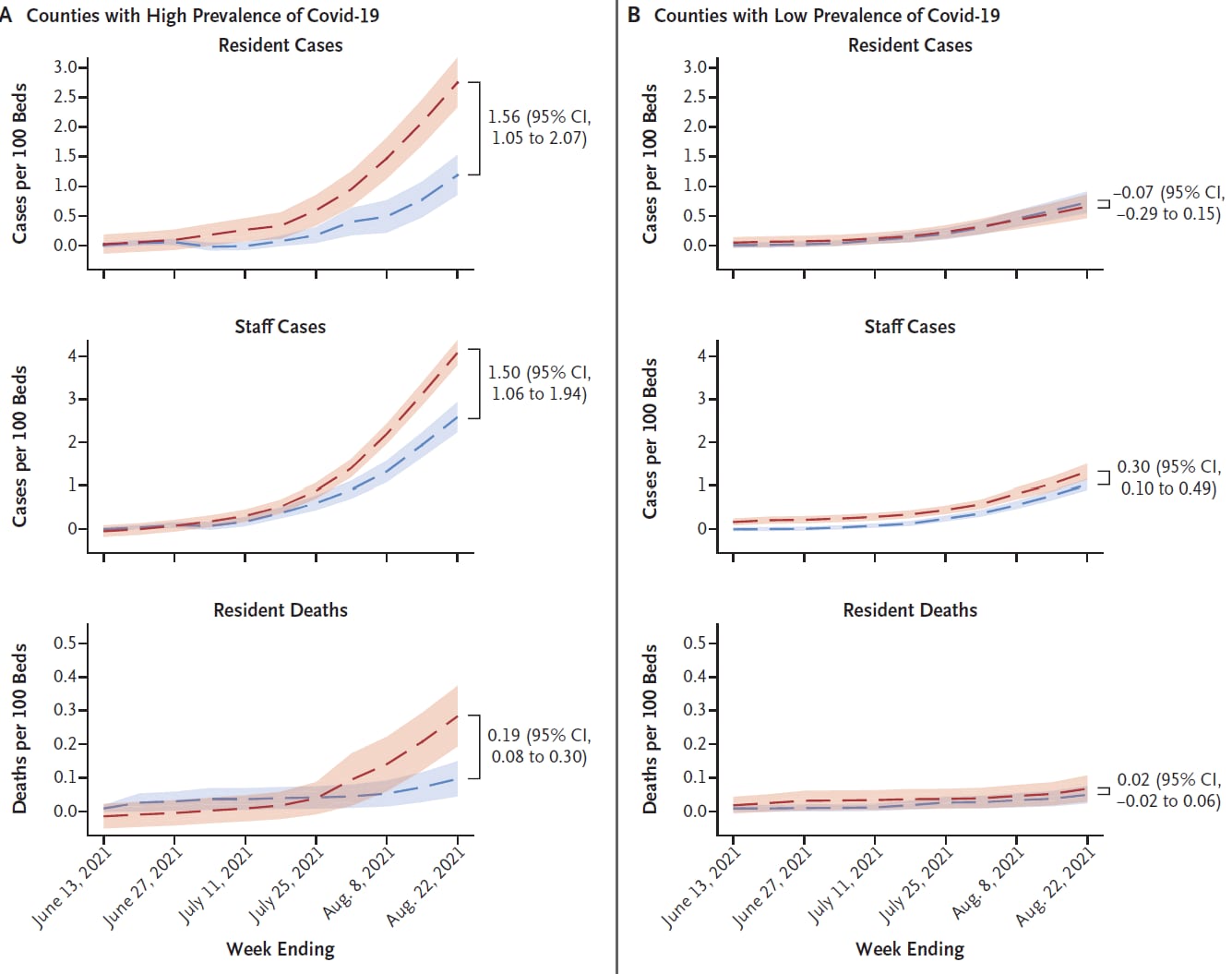
- In counties with a high prevalence of COVID-19, nursing homes with low staff vaccination coverage had higher rates of cases (1.56 additional resident cases/100 beds, 95% CI 1.05-2.07) and deaths (0.19 additional deaths/100 beds, 95% CI 0.08-0.30) compared with nursing homes with high staff vaccination coverage (Figure).
- Resident case and death rates did not significantly differ by staff vaccination coverage in counties with the lowest prevalence of COVID-19.
- Modeling suggests 4,775 (29%) resident cases and 703 (48%) resident deaths may have been prevented If staff vaccination coverage had matched the highest quartile (82.7% average) in all nursing homes.
Methods: Using U.S. national COVID-19 nursing home data, nursing homes (n = 12,364, 81% of all US nursing homes) were classified into quartiles by staff vaccination coverage and county prevalence of COVID-19 from June-August 2021. Outcomes among the lowest and highest quartiles were compared using multivariate regression models with adjustment for resident vaccination rates, previous COVID-19 infection rates among staff and residents, facility characteristics, and county as a fixed effect. Limitations: Data limited to nursing homes that reported vaccination rates for the week ending June 13 and reported outcomes in each week of the study period.
Implications: High vaccination coverage among nursing home staff can prevent COVID-19 cases and deaths among nursing home residents in communities with high prevalence of disease. These findings emphasize the importance of vaccination among staff in nursing homes and other long-term care facilities.
Figure:
Note: Adapted from McGarry et al. Cumulative adjusted COVID-19 outcomes, according to nursing home staff vaccination coverage (facilities in lowest quartile for staff vaccination coverage and facilities in highest quartile for staff vaccination coverage) and county-level prevalence (A. High prevalence; B. Low prevalence). Shaded areas indicate 95% confidence intervals. From the New England Journal of Medicine, McGarry et al., Nursing home staff vaccination and COVID-19 outcomes. December 8, 2021, online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
PREPRINTS (NOT PEER-REVIEWED)
Protection and waning of natural and hybrid COVID-19 immunity. Goldberg et al. medRxiv (December 5, 2021). Published in NEJM (June 9, 2022).
Key findings:
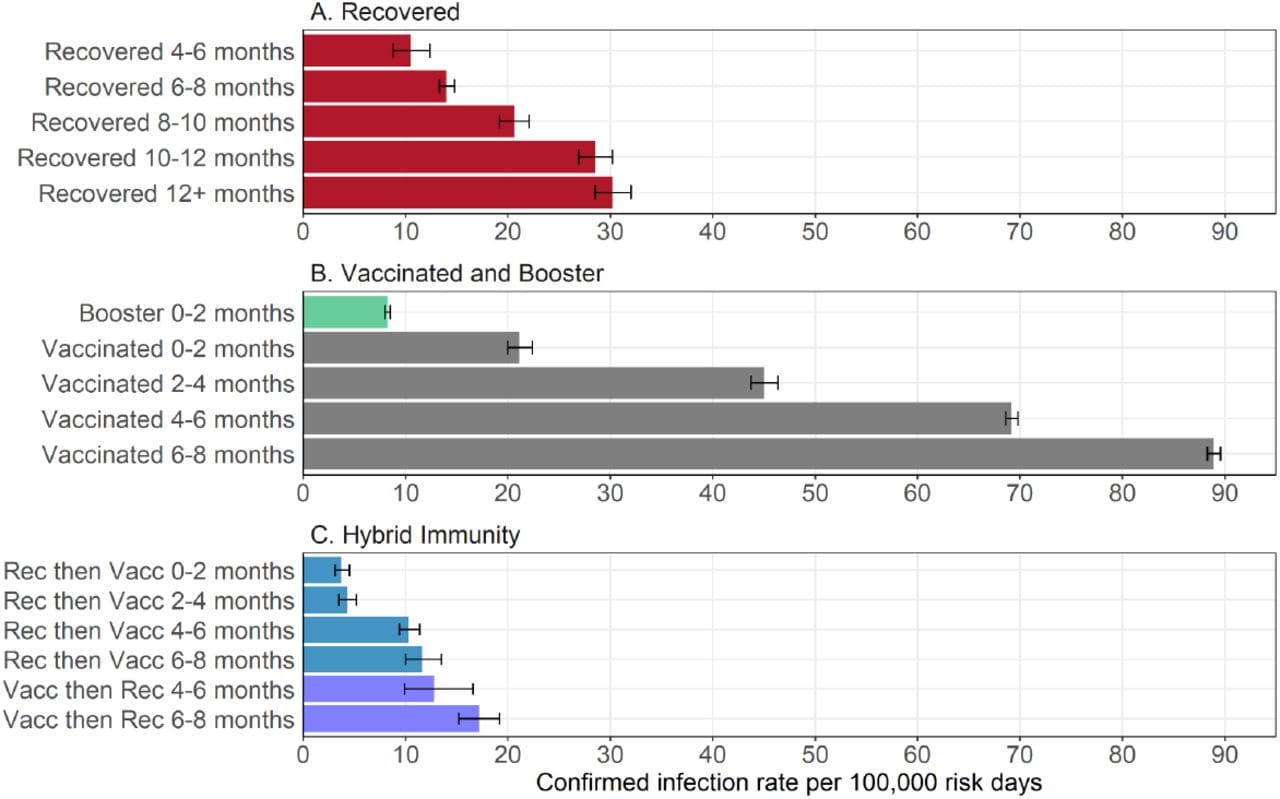
- Among persons aged ≥16 years, persons with a history of SARS-CoV-2 infection (regardless of vaccination status) had longer duration of immunity than persons with no history of infection who had received 2 doses of BNT162b2 (Comirnaty, Pfizer/BioNTech) (Figure).
- A booster (3rd) dose of vaccine given to a vaccinated person restored protection to levels in the early months following recovery or vaccination.
Methods: Rates of infection in Israel per 100,000 person-days were calculated using Poisson regression analysis using data from the Israel Ministry of Health from August–September 2021 (n > 5.7 million). Limitations: Study measures observed rate of infection which could be affected by detection bias; effect could be confounded by different variants circulating over time; duration of immunity from booster is not known.
Implications: Immunity from SARS CoV-2 infection appears to wane more slowly than immunity conferred from a 2-dose vaccination series, findings similar to Kim et al. A booster dose of vaccine appears to restore at least short-term protection in previously vaccinated people.
Figure:
Note: Adapted from Goldberg et al. Estimated covariate-adjusted rates of confirmed SARS-CoV-2 infections per 100,000 person-days stratified by immunity-inducing event and time since most recent event (vaccine or infection) for (A) recovered (unvaccinated, previously infected individuals), (B) vaccinated (individuals who received 2 vaccine doses and had no history of infection) and booster (individuals who received 3 doses of vaccine and had no history of infection), and (C) hybrid immunity individuals (previously infected individuals who subsequently received a single vaccine dose [Rec then Vacc] and individuals who received 1 or 2 vaccine doses and were subsequently infected [Vacc then Rec]). Permission request in process.
PREPRINTS (NOT PEER-REVIEWED)
Two studies evaluated neutralization of SARS-CoV-2 Omicron variant in sera from vaccinated persons.
A. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. Wilhelm et al. medRxiv (December 11, 2021).
Key findings:
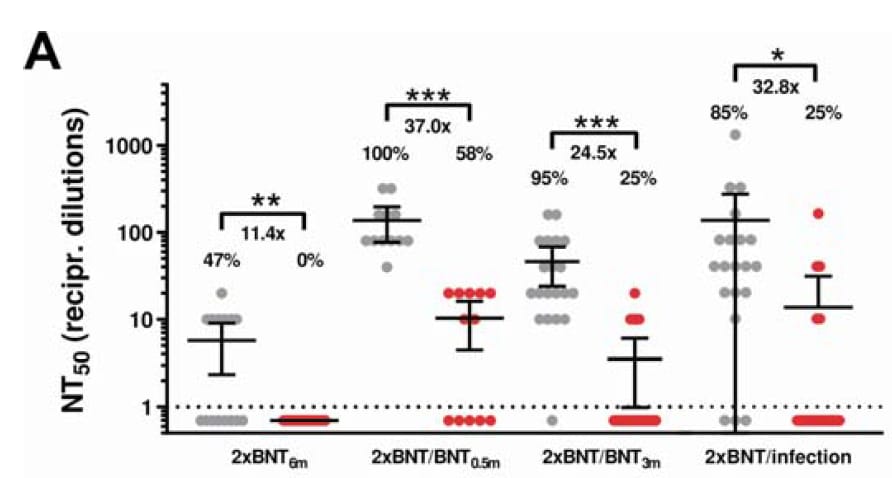
- Sera from persons vaccinated with BNT162b2 (Comirnaty, Pfizer/BioNTech) or mRNA-1273 (Moderna) had 11.4–37.0-fold lower neutralization against the SARS-CoV-2 Omicron (B.1.1.529) variant compared with Delta (B.1.617.2) (Figure).
- Among participants who received a mRNA vaccine primary series, sera collected 0.5 or 3 months after a BNT162b2 booster and sera collected from participants with a previous history of infection had higher neutralization than sera collected 6 months after the primary series (prior to booster) from participants with no history of infection.
Methods: Neutralization efficacy against live Omicron and Delta isolates was assessed using sera collected from 117 participants of previous studies who were vaccinated and had sera collected at 0.5, 3, or 6 months after most recent vaccine dose. Limitations: Small sample size; single isolate of Omicron variant used.
Figure:
Note: Adapted from Wilhelm et al. Antibody-mediated neutralization efficacy against authentic SARS-CoV-2 variants Delta and Omicron among persons vaccinated with BNT162b2 (A) or mRNA-1273 (B). Values represent reciprocal dilutions of SARS-CoV-2 variant microneutralization titers resulting in 50% virus neutralization (NT50). Percentages are the percentage of samples that achieved a measurable titer. Numbers over brackets are fold-differences in NT50 values for Delta vs. Omicron. Black dotted line represents limit of detection. 2x BNT (6m): 2 doses of BNT162b2, 6 months after vaccination (n = 15). 2xBNT/BNT (0.5m): 3 doses of BNT162b2, 0.5 months after 3rd dose (n = 12). 2xBNT/BNT (3m): 3 doses of BNT162b2, 3 months after 3rd dose (n = 20). 2xBNT/infection: 2 doses of BNT162b2 and a previous SARS CoV-2 infection (n = 20). 2xMOD (6m): 2 doses of mRNA-1273, 6 months after vaccination (n = 14). 2xMOD/BNT (0.5m): 2 doses of mRNA-1273 with a booster dose of BNT162b2, 0.5 months after booster (n = 9). Licensed under CC-BY-ND 4.0.
B. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. Cele et al. medRxiv (December 7, 2021). Published in Nature as Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization (December 23, 2021).
Key findings:
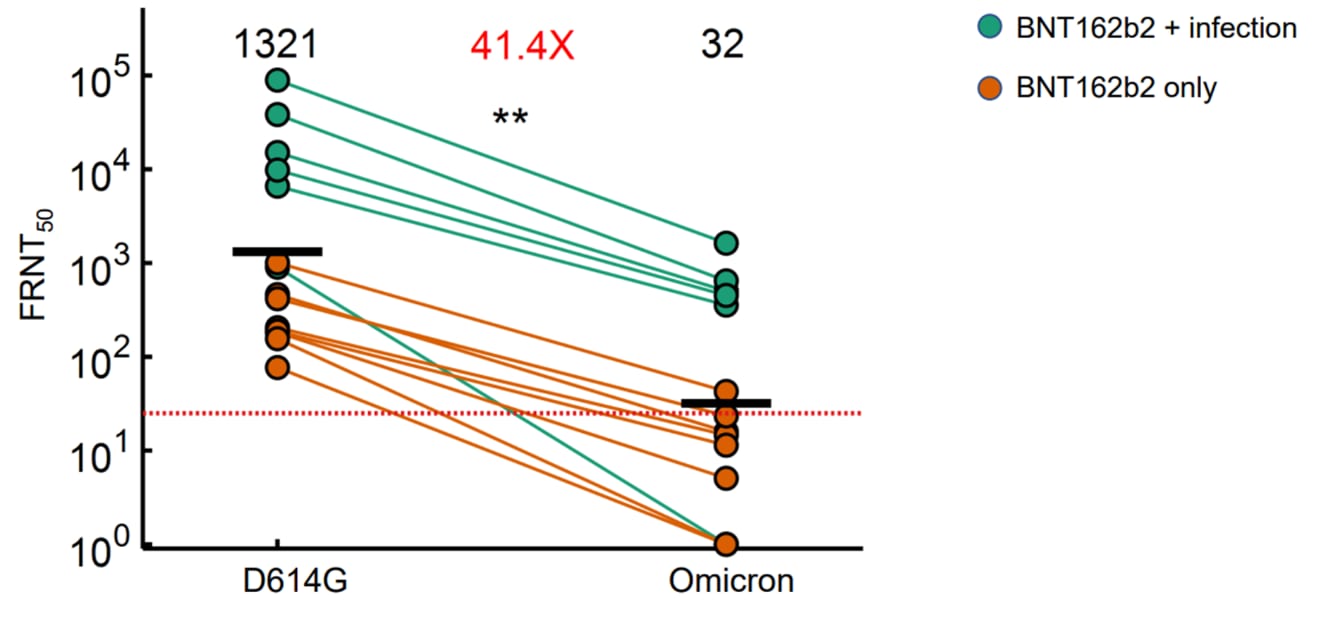
- Plasma from individuals vaccinated with BNT162b2 (Comirnaty, Pfizer/BioNTech) had a 41-fold decrease in virus neutralization against Omicron (B.1.1.529) compared with ancestral D614G virus (Figure).
- Among vaccinated persons with a history of infection, neutralization against Omicron was lower compared with D614G, but was still higher than Omicron neutralization among vaccinated persons with no history of infection.
Methods: Plasma collected a median of 24 days post-vaccination from individuals in South Africa with (n = 6) or without (n = 6) previous infection were tested for neutralization activity against Omicron and D614G using live virus neutralization assay. Limitations: Small sample size; single Omicron isolate tested; findings are limited to the BNT162b2 vaccine.
Figure:
Note: Adapted from Cele et al. Neutralization of the Omicron variant compared to D614G ancestral virus among BNT162b2-vaccinated participants with or without history of prior infection with D614G SARS-CoV-2. Red dashed horizontal line denotes most concentrated plasma tested. Numbers in black above each virus strain are geometric mean titers (GMT) of the focus reduction neutralization test (FRNT50), which is the inverse of the plasma dilution required for 50% reduction in infection focus number. Number in red denotes fold-change in GMT between D614G and Omicron. **p=0.0018 as determined by the Wilcoxon rank sum test. Licensed under CC-BY-ND 4.0.
Implications for Wilhelm et al. and Cele et al.: The Omicron variant appears to be less susceptible to current COVID-19 vaccine- and infection-induced neutralizing antibodies. This effect appears to be attenuated in vaccinated persons with a previous history of infection and in persons who recently received a 3rd (booster) dose of vaccine.
PEER-REVIEWED
An upper bound on one-to-one exposure to infectious human respiratory particles. Bagheri et al. PNAS (December 7, 2021).
Key findings:
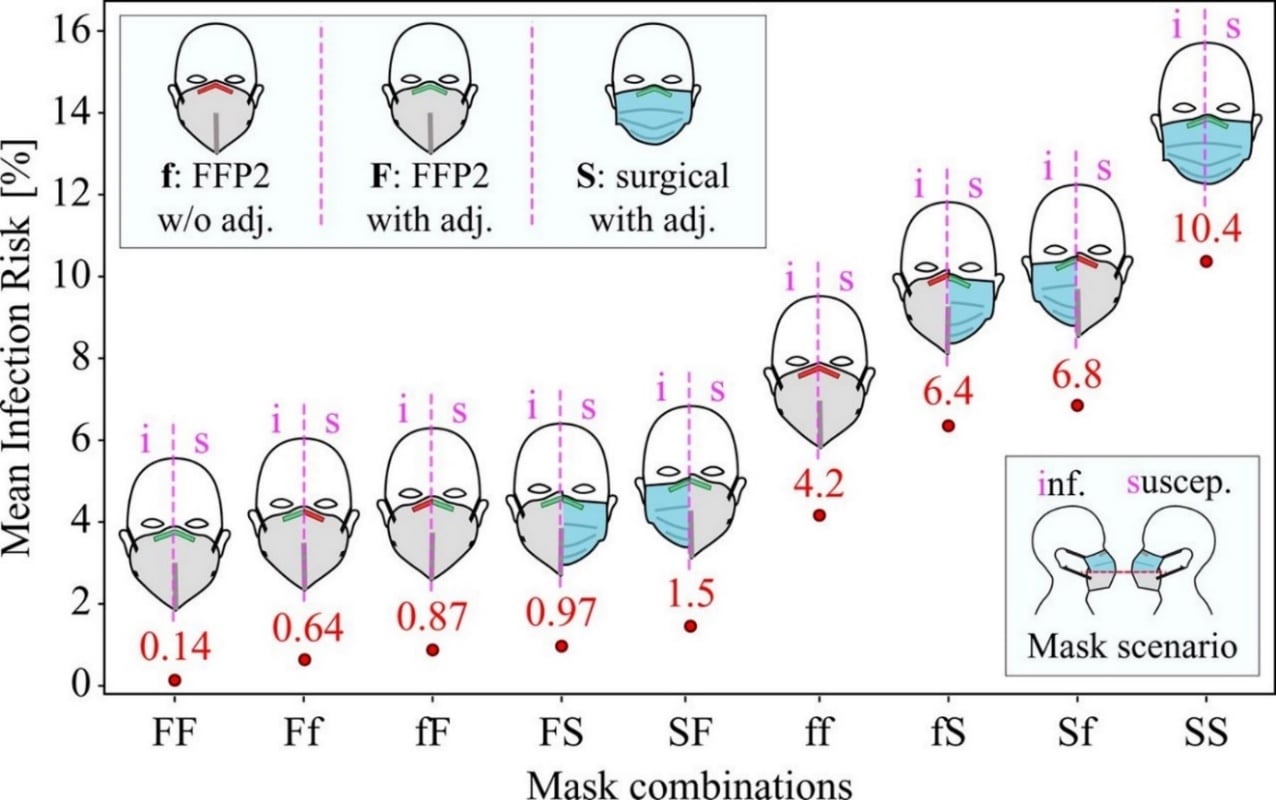
- A model of face-to-face interactions between an infectious person and susceptible person found lower estimated risk of SARS-CoV-2 transmission in scenarios where at least 1 individual wore a face mask compared with physical distancing alone.
- Estimated risk was lowest when both individuals wore FFP2 masks (approximately comparable to N95 masks) with nose-piece adjustments (Figure).
- FFP2 masks without nose-piece adjustments were associated with decreased risk compared to surgical masks with nose-piece adjustments.
Methods: Mathematical modeling to determine upper bound of transmission risk from an infectious to susceptible individual under various masking and distancing conditions. Modeling calculations included respiratory particle size and dynamics, mask penetration and leakage, pathogen concentration produced by an infectious individual, and susceptibility of the uninfected individual. Limitations: Upper bound risk assessment is conservative and may overestimate actual infection risk; does not assess risk in real-world conditions.
Implications: These findings indicate that face mask use among infectious and susceptible individuals significantly reduces SARS-CoV-2 infection risk compared with physical distancing alone; level of protection varies by type of mask worn and the fit by both the infectious and susceptible individual.
Figure:
Note: Adapted from Bagheri et al. Mean risk of infection when face-to-face for 20 minutes for different mask use combinations. Infectious individual (i) shown on left and susceptible individual (s) shown on right for each mask pair. f = FFP2 mask without nose-piece adjustment, F = FFP2 mask with nose-piece adjustment, S = surgical mask with nose-piece adjustment. Licensed under CC-BY 4.0.
PREPRINTS (NOT PEER-REVIEWED)
Racial and ethnic disparities in COVID-19 disease incidence independent of comorbidities, among people with HIV in the US. Ignacio et al. medRxiv (December 8, 2021).
Key findings:
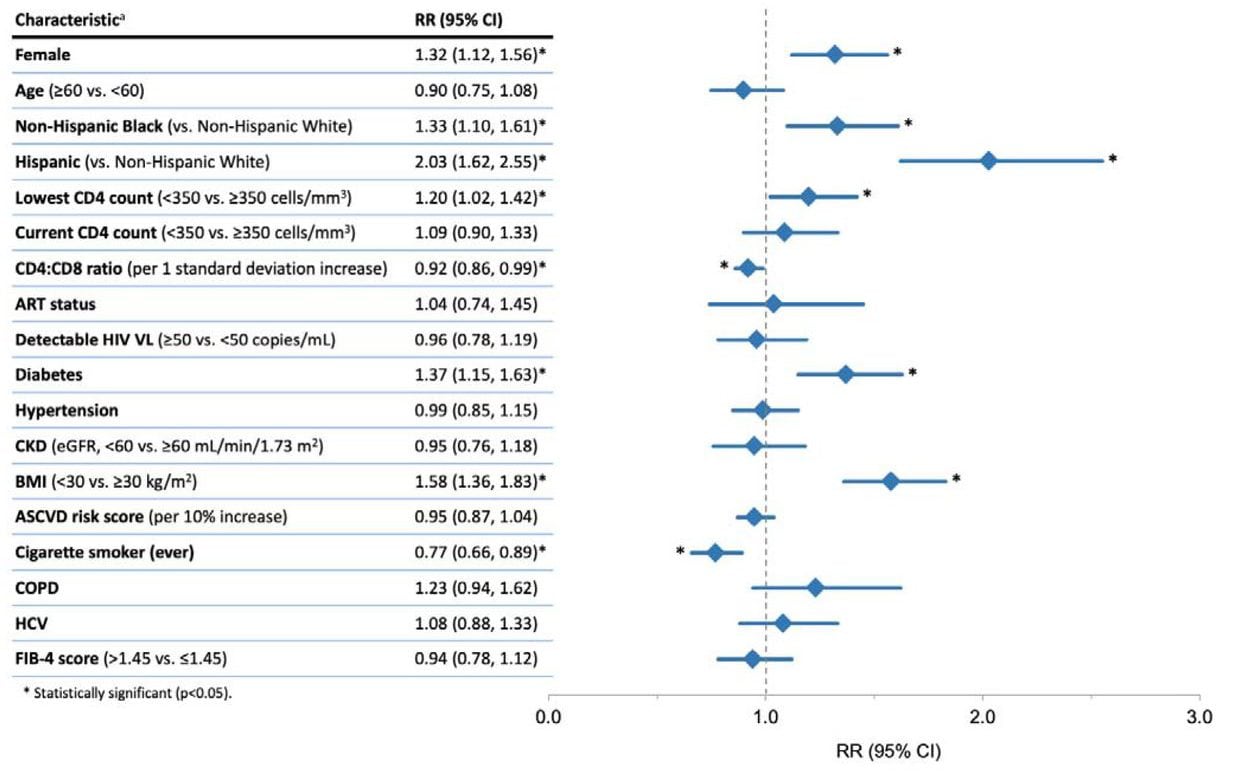
- Among persons with HIV (PWH) receiving routine care, Hispanic or Latino (aRR 2.03, 95% CI 1.62-2.55), Black or African American (aRR 1.33, 95% CI 1.10-1.61), PWH had higher incidence of COVID-19 compared with non-Hispanic White PWH (Figure).
- Female PWH had higher incidence of COVID-19 compared to male PWH (aRR 1.32, 95% CI 1.12-1.56).
- Lowest historical CD4 count <350 and lower current CD4:CD8 ratio were associated with increased incidence of COVID-19, but current CD4 count, antiretroviral treatment status, and presence or absence of detectable HIV viral load were not.
Methods: Cumulative incidence of COVID-19 during March–December 2020 among 16,056 PWH in routine care was calculated using Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) data. Risk factors were assessed using relative risk regression models adjusted for disease risk scores. Limitations: Lack of systematic testing, resulting in potential misclassification and potential ascertainment bias.
Implications: These findings indicate that structural racial and gender inequities, in addition to medical comorbidities, might increase the risk of COVID-19 among PWH.
Figure:
Note: Adapted from Ignacio et al. Relative risk of COVID-19 among people with HIV (PWH) by key characteristics. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis-4 scoring system for liver fibrosis; HCV, hepatitis C virus. aRelative risk regression models adjusted for demographic and clinical characteristics using disease risk scores, except for the ASCVD risk score analysis which is unadjusted. Licensed under CC-BY-NC-ND 4.0.
Vaccines
- Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2 experienced individuals. Samanovic et al. Science Translational Medicine (December 7, 2021). Among 15 adults with a history of SARS-CoV-2 infection, BNT162b2 vaccination was associated with strong humoral and antigen-specific antibody-secreting cell (ASC) responses following the 1st dose but not the 2nd dose. In contrast, 21 infection-naïve adults demonstrated progressive increases in humoral and ASC responses following both the 1st and 2nd doses. Both groups exhibited robust cytotoxic CD8+ T cell responses following the 2nd dose.
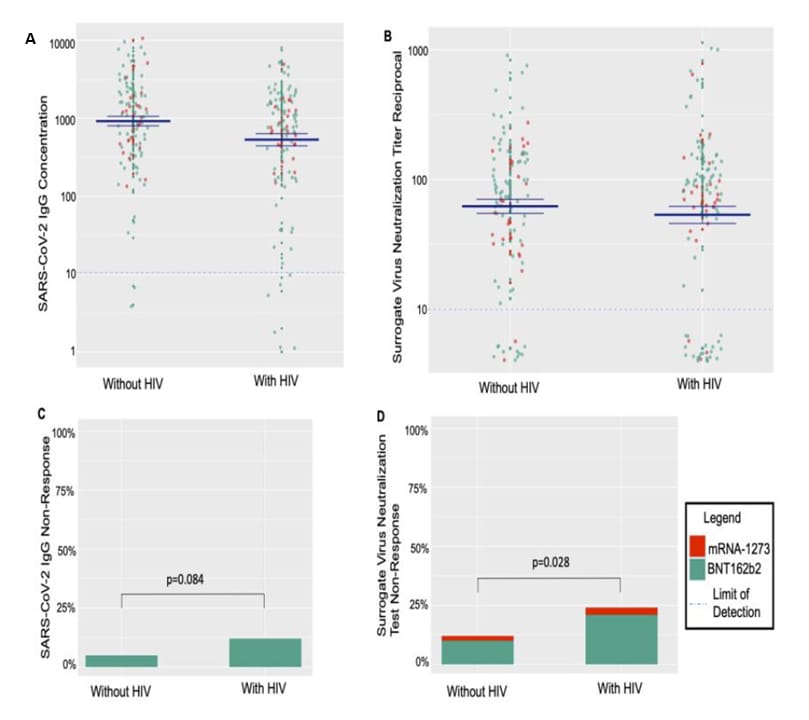
- Differences in post-mRNA vaccination SARS-CoV-2 IgG concentrations and surrogate virus neutralization test response by HIV status and type of vaccine: A matched case-control observational study. Spinelli et al. Clinical Infectious Diseases (December 5, 2021). Following mRNA COVID-19 vaccination, people with HIV (PWH, n = 100) had higher odds of surrogate virus neutralization test non-response (adjusted odds ratio [aOR] 2.43, 95% CI 1.09-5.39, p = 0.03) and a trend towards higher odds of IgG non-response (aOR 2.74, 95% 0.87-8.61, p = 0.08) compared with matched HIV-negative controls. Among PWH, lower CD4+ T-cell count and receipt of BNT162b2 (vs. mRNA-1273) were associated with non-response.
Note: Adapted from Spinelli et al. Serologic responses to mRNA-based SARS-CoV-2 vaccination (BNT162b2 and mRNA-1273) by HIV status. For A and B, the dotted lines show lower limits of detection, and the solid blue lines indicate the mean +/- 1 standard error. A) SARS-CoV-2 IgG anti-receptor binding domain (RBD) concentrations in relative fluorescent units by HIV status. B) SARS-CoV-2 surrogate virus neutralization antibody titer reciprocals by HIV status. C) Proportion of participants demonstrating lack of SARS-CoV-2 anti-RBD IgG response (<10 relative fluorescent units). D) Proportion of participants demonstrating SARS-CoV-2 surrogate virus neutralization non-response (<10 reciprocal titer). Used by permission of Oxford University Press for the Infectious Diseases Society of America.
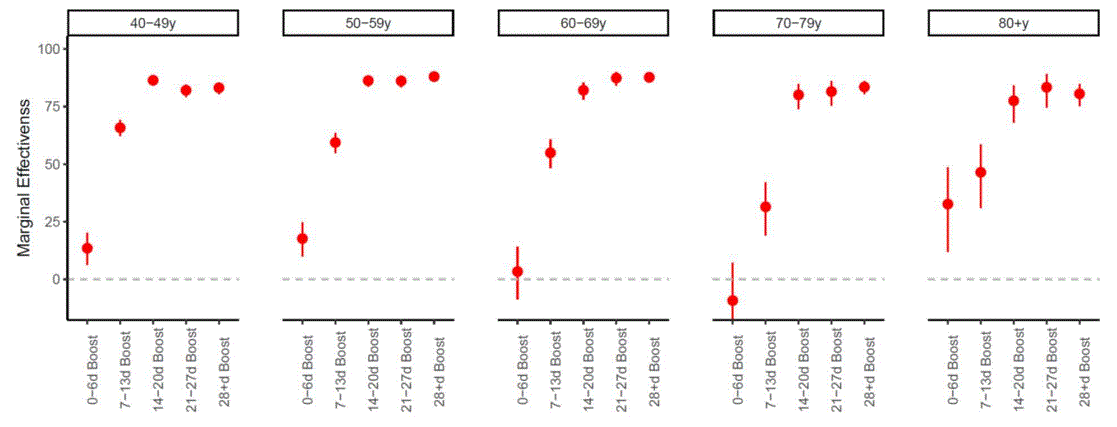
- Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. Patalon et al. JAMA Internal Medicine (November 30, 2021). Among 306,710 Israelis aged >40 years who received either 2 or 3 doses of the BNT162b2 vaccine and had no history of infection, those who received a booster (3rd dose) had 86% (95% CI 85%-87%) reduction in odds of testing positive for SARS-CoV-2 and 97% (95% CI 95%-98%) reduction in odds of COVID-19 hospitalization at 28–65 days post-booster compared with those who received only 2 doses. Marginal effectiveness of a booster dose against infection was highest from 14–20 days post-booster onward in all age groups.
Note: Adapted from Patalon et al. Percent reduction in the odds of testing positive for SARS-CoV-2 in 3rd-dose vaccinees compared with those who received 2 doses (marginal effectiveness of booster) by days since booster, stratified by age group. Reproduced with permission from JAMA Internal Medicine, 2021. Published online November 30, 2021: https://doi.org/10.1001/jamainternmed.2021.7382. Copyright© 2021 American Medical Association. All rights reserved.
Variants
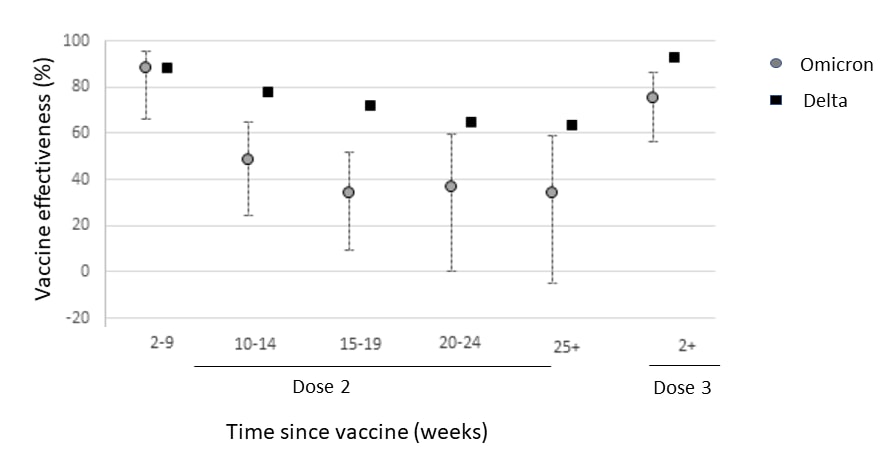
- SARS-CoV-2 variants of concern and variants under investigation in England. UK Health Security Agency (December 10, 2021). Analysis of surveillance data in the United Kingdom found that, compared with Delta, the SARS-CoV-2 Omicron variant was associated with a higher risk of household transmission (aOR 3.2, 95% CI 2.0-5.0), transmission to a close contact (aOR 2.09, 95% CI 1.54-2.79), and re-infection (aRR 5.2, 95% CI 3.4-7.6). Early estimates of BNT162b2 vaccine effectiveness (VE) were significantly lower against symptomatic Omicron infection compared with Delta infection; estimated VE against symptomatic Omicron infection was 76% in the early period (≥2 weeks) following a booster dose (further details available in Andrews et al.).
Note: Adapted from UK Health Security Agency. Estimated vaccine effectiveness of BNT162b2 against symptomatic SARS-CoV-2 infection with Delta (black squares) and Omicron (gray circles) variants by period after dose 2 and at ≥2 weeks after dose 3. Contains public sector information licensed under the UK Open Government Licence v3.0.
- Minimal cross-over between mutations associated with Omicron variant of SARS-CoV-2 and CD8+ T cell epitopes identified in COVID-19 convalescent individuals. Redd et al. bioRxiv (Preprint; December 9, 2021). Published in mBio (March 1, 2022). Analysis of samples collected in April and May 2020 from 30 individuals who had recovered from SARS-CoV-2 infection identified 52 viral epitopes that were targeted by the CD8+ T-cell response. When mutations associated with the Omicron variant were mapped to these epitopes, only 1 low-prevalence epitope from the spike protein had an amino acid change associated with Omicron. These data suggest that, in individuals with existing anti-SARS-CoV-2 CD8+ T-cell responses, these responses will likely recognize Omicron.
Natural History, Reinfection, and Health Impact
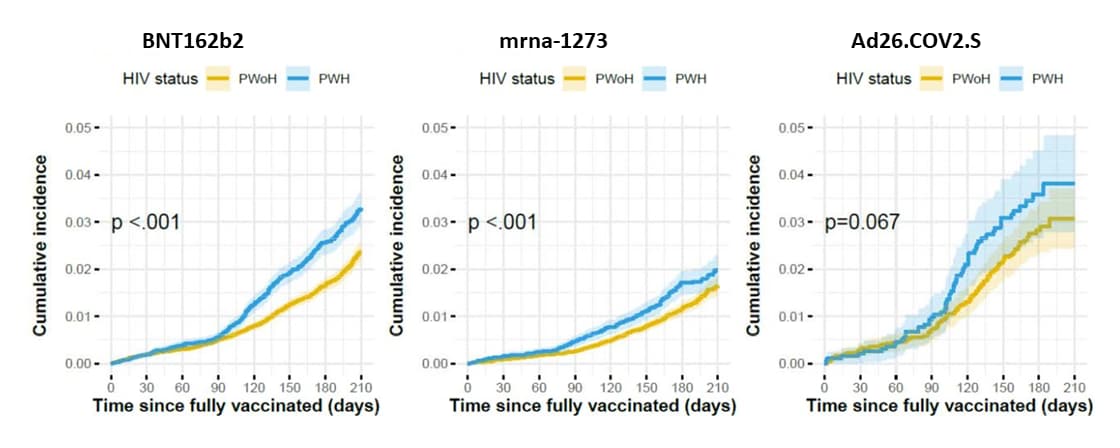
- COVID-19 infections post-vaccination by HIV status in the United States. Coburn et al. medRxiv (Preprint; December 6, 2021). Published in JAMA Network Open as Analysis of postvaccination breakthrough COVID-19 infections among adults with HIV in the United States (June 7, 2022). Among 31,840 people living with HIV (PWH) who were vaccinated December 2020–June 2021, cumulative incidence of breakthrough infection was 2.8% in the 210 days following full vaccination. Risk of breakthrough infection was higher among PWH compared with matched vaccinated controls without HIV (n = 77,759) (aHR 1.41, 95% CI 1.28-1.56). Among vaccinated PWH, younger age, history of COVID-19 prior to full vaccination, and vaccination with Ad26.COV2.S (vs. BNT162b2) were associated with higher risk of breakthrough infection, but HIV viral load suppression and CD4 count were not.
Note: Adapted from Coburn et al. Cumulative incidence of SARS-CoV-2 breakthrough infection (and 95% CI indicated by shading), stratified by HIV status and primary vaccine series type. Maximum follow-up for Ad26.COV2.S was 180 days because it was not authorized until February 2021. PWH: people living with HIV, PWoH: people without HIV. Licensed under CC-BY-NC-ND 4.0.
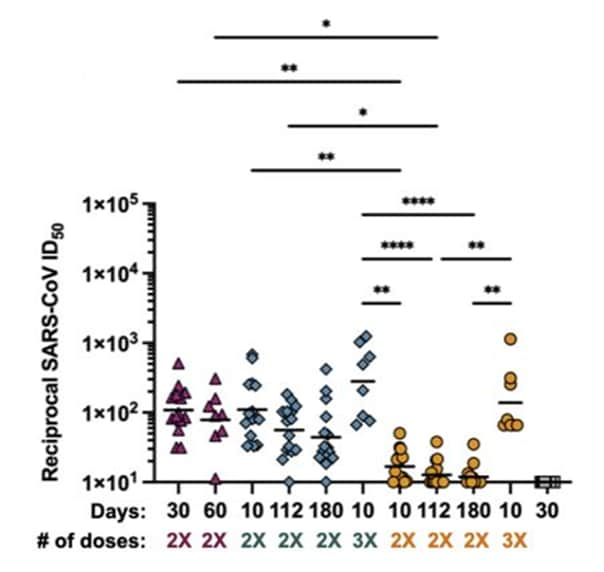
- Delta breakthrough infections elicit potent, broad and durable neutralizing antibody responses. Walls et al. bioRxiv (Preprint; December 9, 2021). Published in Cell as SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses (March 3, 2022). Among individuals with various combinations of COVID-19 vaccination and SARS-CoV-2 infection histories, serum IgG, IgA, and IgM binding titers were higher in vaccinated individuals who had a Delta breakthrough infection, individuals vaccinated after infection, and individuals vaccinated with 3 doses compared with infection-naïve individuals vaccinated with a primary series only and unvaccinated individuals with a history of infection.
Note: Adapted from Walls et al. SARS-CoV-2 spike protein vesicular stomatitis virus (VSV) pseudotype neutralization. Neutralizing antibody levels correspond to the reciprocal of the ID50 (serum dilution that inhibits 50% of the infectivity). Serum samples obtained from individuals who had a Delta breakthrough infection (n = 14, magenta triangles), who were previously infected then vaccinated (n = 15, teal diamonds), who have been vaccinated only (n = 15, orange circles), or who were infected only in 2020 in Washington State (n = 15, gray squares). # of doses: number of vaccine doses received. Statistical significance was determined by Kruskal Wallis and Dunn’s multiple comparisons test and shown only within group or at matched timepoint for ease of viewing when significant. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001. Licensed under CC-BY-NC-ND 4.0.
Health Equity
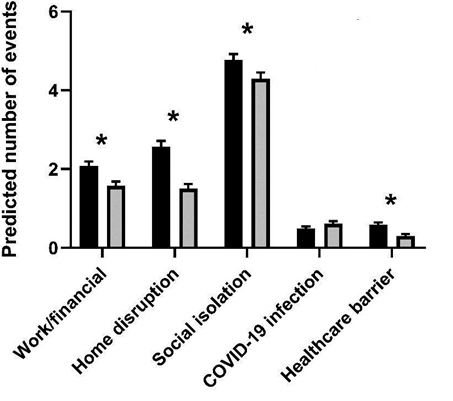
- Disparities in COVID-19–related stressful life events in the United States: Understanding who is most impacted. Thomas et al. Health and Social Care (December 1, 2021). Among 372 participants from 10 study sites in the U.S. who were surveyed about COVID-19-related stressful life events June–August 2020, women reported 31% more work/finance, 64% more home disruption, 13% more social isolation, and 94% more healthcare barrier events compared with men. In addition, individuals with lower socioeconomic status reported more stressful life events related to work/finance, COVID-19 infection, and healthcare barriers.
Note: Adapted from Thomas et al. Counts of events in five COVID-19 related stressful life event domains among women and men, with adjustment for the other sociodemographic factors and covariates. Asterisks signify statistical significance. © 2021 John Wiley & Sons Ltd.
From the Morbidity and Mortality Weekly Report (December 17, 2021).
- Report of Health Care Provider Recommendation for COVID-19 Vaccination Among Adults, by Recipient COVID-19 Vaccination Status and Attitudes — United States, April–September 2021
- SARS-CoV-2 B.1.1.529 (Omicron) Variant — United States, December 1–8, 2021
- Booster and Additional Primary Dose COVID-19 Vaccinations Among Adults Aged ≥65 Years — United States, August 13, 2021–November 19, 2021
- Notes from the Field: Mucormycosis Cases During the COVID-19 Pandemic — Honduras, May–September 2021
- Notes from the Field: COVID-19–Associated Mucormycosis — Arkansas, July–September 2021
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.