COVID-19 Science Update released: October 30, 2020 Edition 61

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
Cytokine storm syndrome, seen in some COVID-19 patients, results from an excessive inflammatory response that can aggravate respiratory failure and lead to systemic organ failure and death. The cytokine interleukin-6 (IL-6) has appears to be a potentially important mediator of the cytokine storm syndrome. Tocilizumab, a monoclonal antibody that blocks IL-6 and is used to treat rheumatoid arthritis, has been examined as a potential treatment for COVID-19. Below we share summaries of a series of studies evaluating the ability of tocilizumab to prevent clinical worsening and death of COVID-19 patients.
PEER-REVIEWED
A. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19external icon. Guirao et al. Molecular Immunology (October 14, 2020).
Key findings:
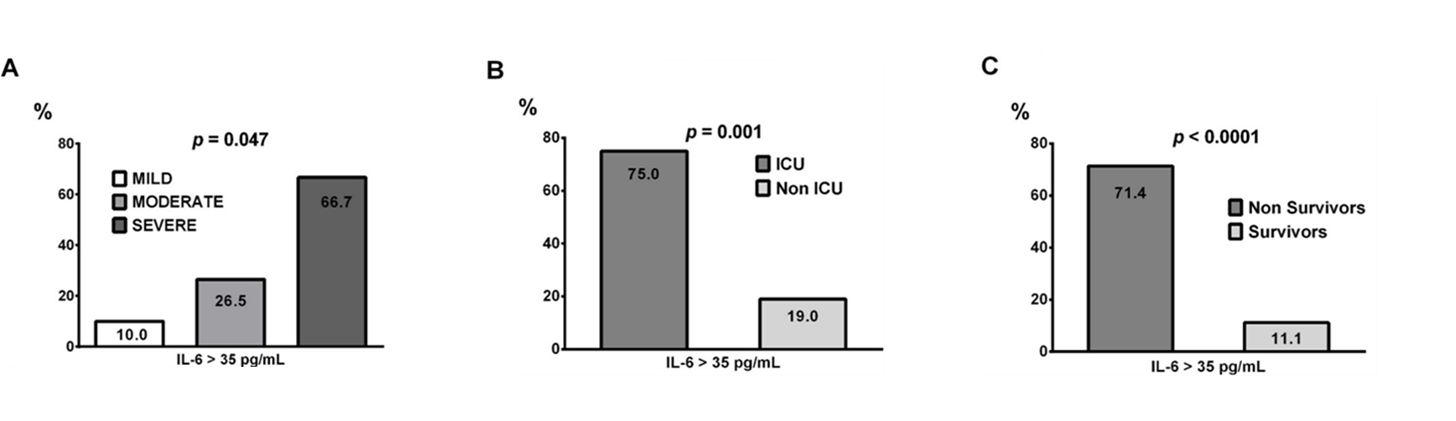
- IL-6 serum concentrations above 35 pg/mL were associated with a higher risk of (Figure):
- More severe pneumonia (OR = 4.47, 95% CI 1.15-17.45, p = 0.031).
- Increased risk of mortality (OR = 20.00, 95% CI 4.21-94.91, p = 0.0001).
- ICU admission (OR = 12.75, 95% CI 2.16-75.33, p = 0.005).
- Of the 27 patients treated with tocilizumab, 8 (28%) died, while 6 (26%) of the 23 patients who were not treated with tocilizumab died.
Methods: Retrospective cohort study of 50 patients hospitalized with mild (n = 10), moderate (n = 34), or severe (n = 6) SARS-CoV-2 pneumonia in Spain, between April 1 and April 30, 2020. IL-6 serum concentrations were evaluated against clinical parameters to establish a predictive indicator of clinical outcomes. Some patients were given tocilizumab. Limitations: Small sample size; tocilizumab treatment not randomized.
Figure:
Note: From Guirao et al. Percentage of patients with IL-6 serum concentrations above 35 pg/mL (y-axis and numbers within bars) depending on the pneumonia severity, (A), ICU admission (B) and mortality (C). Reprinted from Molecular Immunology. Guirao et al., High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. DOI: https://doi.org/10.1016/j.molimm.2020.10.006external icon. Copyright (2020), with permission from Elsevier.
B. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial.external icon Hermine et al. JAMA Internal Medicine (October 20, 2020).
Key findings:
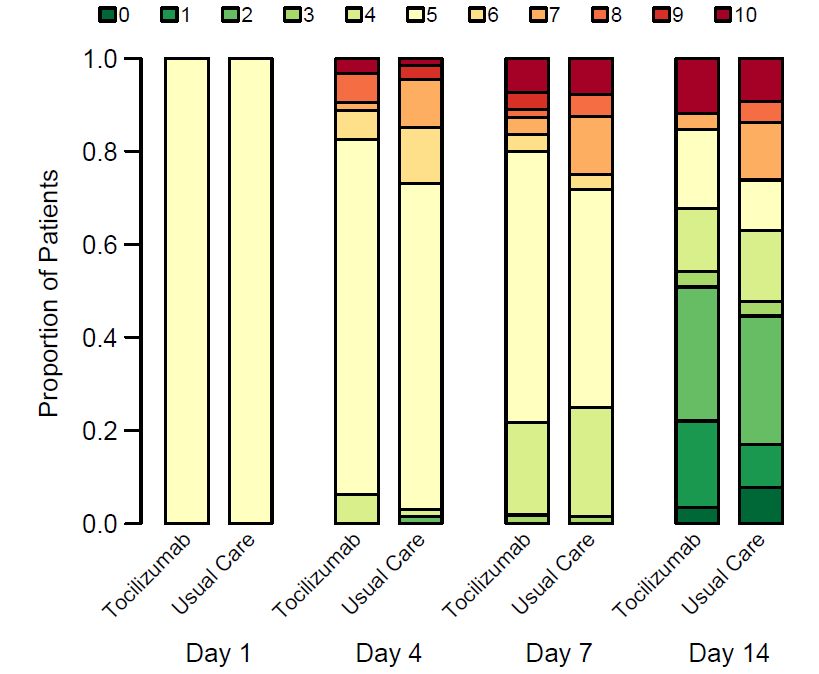
- Patients receiving tocilizumab had similar scores to the usual care [UC] group on the World Health Organization 10-point Clinical Progression Scale (WHO-CPS) on day 4 (Figure).
- 12 of 63 (19%) patients randomized to receive tocilizumab had a WHO-CPS score higher than 5 vs 19 of 667 (28%) in the UC group (absolute difference -9%, 90% credible interval -21.0-3.1).
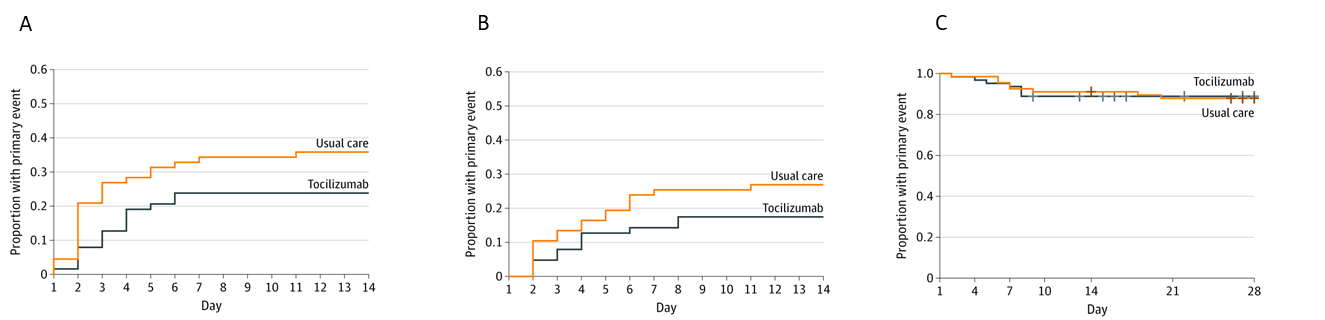
- On day 14, the proportion of patients with noninvasive ventilation (NIV), high flow oxygen, mechanical ventilation (MV) or death was lower in the tocilizumab group: 24% (95% CI 13%-35%) compared with 36% (95% CI 33%-58%) in the UC group.
- No difference in mortality over 28 days was found between the two groups.
Methods: A randomized clinical trial of UC (n = 67) vs UC plus tocilizumab (n = 64) in patients with COVID-19 and moderate or severe pneumonia conducted from March 31 to April 18, 2020 in 9 hospitals in France. Primary outcomes were scores >5 (death or needing NIV or MV) on the WHO-CPS on day 4, and survival without need of ventilation at day 14, with follow up through 28 days. Limitations: Trial was not blinded; UC could have differed among centers over time; may not be generalizable to COVID-19 patients without pneumonia.
Figure 1
Note: Adapted from Hermine et al. WHO-CPS scores during 14-day follow up. WHO-CPS scores (0 = uninfected; 1-3 = ambulatory; 4 = hospitalized with mild disease and no oxygen requirement; 5 = hospitalized with mild disease and supplemental oxygen; 6–9 = hospitalized with severe disease requiring ventilation; 10 = death). Reproduced with permission from JAMA Internal Medicine. Hermine et al., Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial. DOI: 10.1001/jamainternmed.2020.6820. Copyright©2020 American Medical Association. All rights reserved.
Note: Adapted from Hermine et al. A: Probability of primary outcome death, mechanical ventilation, high-flow oxygen or non-invasive ventilation at day 14. B: Probability of death or mechanical ventilation at day 14. C: Probability of overall survival at day 28 in usual care or tocilizumab groups. Reproduced with permission from JAMA Internal Medicine. Hermine et al., Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial. DOI: 10.1001/jamainternmed.2020.6820. Copyright©2020 American Medical Association. All rights reserved.
C. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19.external icon Gupta et al. JAMA Internal Medicine (October 20, 2020).
Key findings:
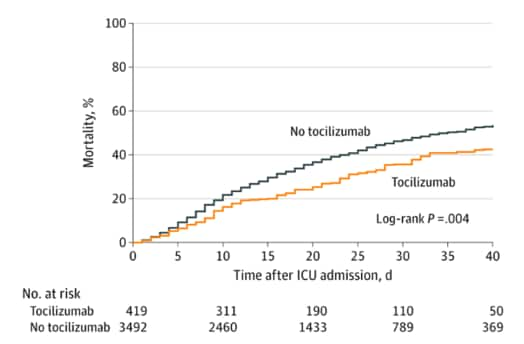
- Patients treated within 2 days of ICU admission with tocilizumab (n = 433) had a lower risk of death compared with those not treated with tocilizumab (n = 3,491) (hazard ratio [HR] 0.71, 95% CI 0.56-0.92) (Figure).
- 125 (28.9%) of patients treated with tocilizumab and 1,419 (40.6%) of patients not treated with tocilizumab died.
- The estimated 30-day mortality was 27.5% (95% CI 21.2%-33.8%) in the tocilizumab-treated patients and 37.1% (95% CI 35.5%-38.7%) in the non-tocilizumab-treated patients (risk difference, 9.6%, 95% CI 3.1%-16.0%).
- Compared to patients who did not receive tocilizumab, tocilizumab-treated patients:
- Were younger (median age, 58 vs 63 years).
- Had fewer comorbidities (hypertension, 54.0% vs 62.6%, coronary artery disease, 9.0% vs 14.4%, or congestive heart failure, 5.3% vs 11.1%).
- Were more likely to have severe hypoxemia (47.3% vs 37.9%).
- Were more likely to receive corticosteroids on ICU admission (18.7% vs 12.6%).
Methods: Multicenter cohort study of 3,924 COVID-19 ICU patients at 68 hospitals in the US from March 4 to May 10, 2020. Patients were followed until hospital discharge, death, or June 12, 2020. The primary outcome was in-hospital death. Limitations: No details of other medications given; not randomized.
Figure:
Note: From Gupta et al. Mortality in tocilizumab-treated vs non-tocilizumab-treated patients. Reproduced with permission from JAMA Internal Medicine. Gupta et al., Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. DOI: 10.1001/jamainternmed.2020.6252. Copyright©2020 American Medical Association. All rights reserved.
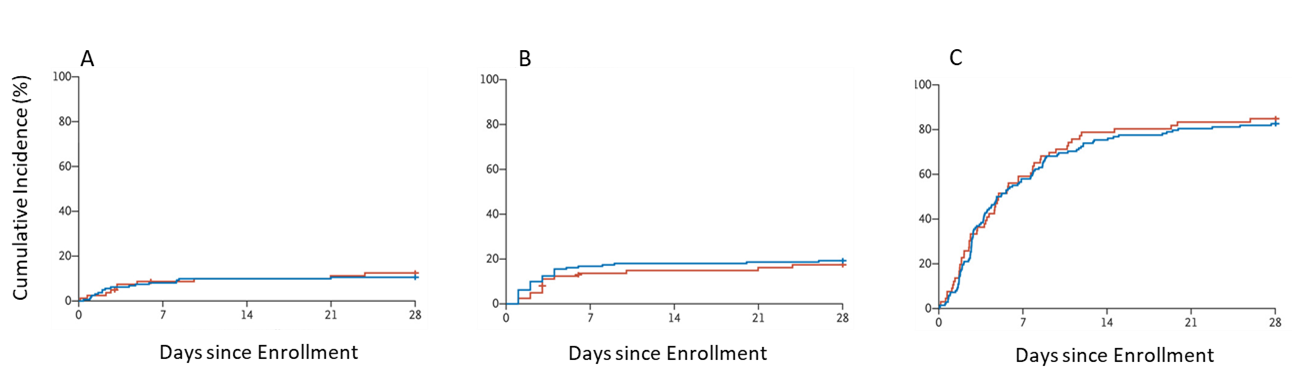
D. Efficacy of tocilizumab in patients hospitalized with COVID-19. external iconStone et al. NEJM. (October 21, 2020).
Key findings:
- Compared to placebo, early administration of tocilizumab in hospitalized COVID-19 patients did not (Figure):
- Prevent progression to intubation or death (hazard ratio [HR] 0.83, 95% CI 0.38-1.81, p = 0.64).
- Prevent clinical worsening (HR 1.11, 95% CI 0.59-2.10, p = 0.73).
- Reduce duration of supplemental oxygen (HR 0.94, 95% CI 0.67-1.30, p = 0.69).
Methods: Randomized (2:1), double-blind placebo-controlled trial among 243 hospitalized COVID-19 patients at 7 Boston hospitals between April 20 and June 15, 2020. Enrolled patients had pulmonary infiltrates and/or a need for supplemental oxygen and laboratory evidence of a hyperinflammatory response. Limitations: Observed primary event rate was lower than anticipated; wide confidence intervals.
Figure:
Note: Adapted from Stone et al. Cumulative incidence of mechanical ventilation or death (A), clinical worsening (B), or discontinuation of supplemental oxygen (C) in the tocilizumab group and standard care group. From NEJM. Stone et al., Efficacy of tocilizumab in patients hospitalized with COVID-19. DOI: 10.1056/NEJMoa2028836. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
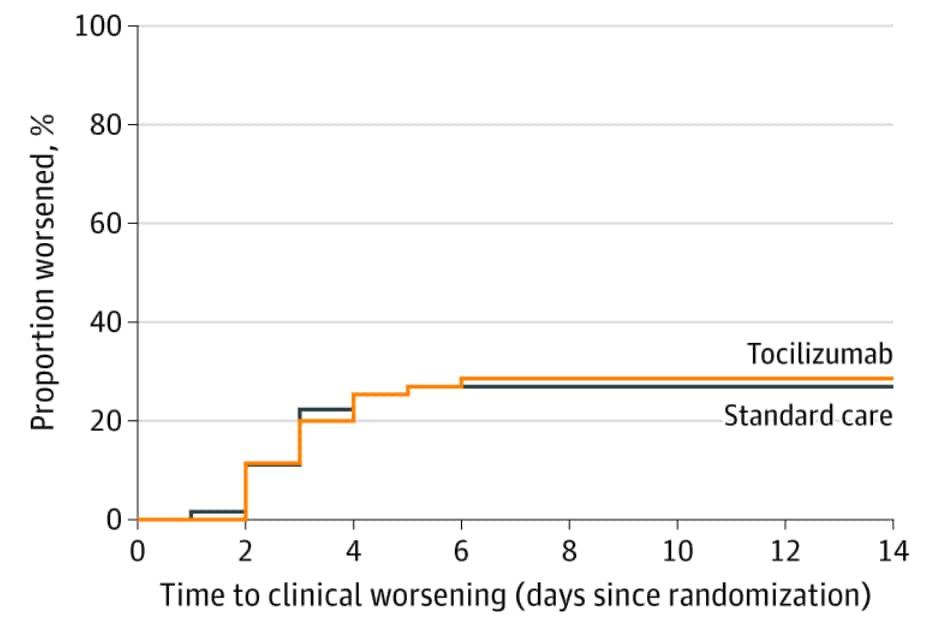
E. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: A randomized clinical trial.external icon Salvarani et al. JAMA Internal Medicine (October 20, 2020).
Key findings:
- There was no difference in rates of clinical worsening (admission to ICU with mechanical ventilation, respiratory distress, or death) in patients who received tocilizumab, n = 17, 28.3%, compared with those who received standard care, n = 17; 27.0% (rate ratio = 1.05, 95% CI 0.59-1.86, p = 0.87) (Figure 1).
- Groups did not differ in time to clinical worsening.
- Patients who received tocilizumab did not differ from control patients in ICU admission rate (10.0% vs 7.9%) or in rate of hospital discharge at 14 (56.7% vs 57.1%) or 30 days (90.0% vs 92.1%).
Methods: 24-center, open-label, randomized clinical trial (RCT) conducted from March 31 to June 11, 2020 in Italy comparing tocilizumab treatment to standard care in hospitalized patients with COVID-19 pneumonia. Patients were randomized to receive either tocilizumab (n = 60) or standard care (n = 63). Primary outcome was clinical worsening. Patients were clinically assessed for 14 days and followed for ≥30 days to determine ICU admission and mortality. Limitations: Open-label trial; patients not matched on baseline characteristics; no data for patients with more severe disease.
Figure:
Note: From Salvarini et al. Cumulative clinical worsening in tocilizumab and standard care groups. Reproduced with permission from JAMA Internal Medicine. Salvarini et al., Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. DOI: 10.1001/jamainternmed.2020.6615. Copyright©2020 American Medical Association. All rights reserved.
Implications from Guirao et al., Hermine et al., Gupta et al., Stone et al., & Salvarini et al.: High serum concentrations of IL-6 are strongly associated with severe COVID-19 and serve as the biologic basis for early off-label use of tocilizumab for COVID-19. While the two cohort studies (Gupta & Hermine) did see a benefit of tocilizumab, the RCTs presented did not show that tocilizumab shortened the COVID-19 clinical course or decreased mortality (Stone & Salvarini). A commentary on these studies by Parr elaborates on the confounders associated with observational studies and why high-quality RCTs should guide decisions about tocilizumab use in COVID-19 patients. The studies presented here support current National Institutes of Health and Infectious Disease Society of America guidelines that do not recommend routine tocilizumab use for treatment of COVID-19.
PEER-REVIEWED
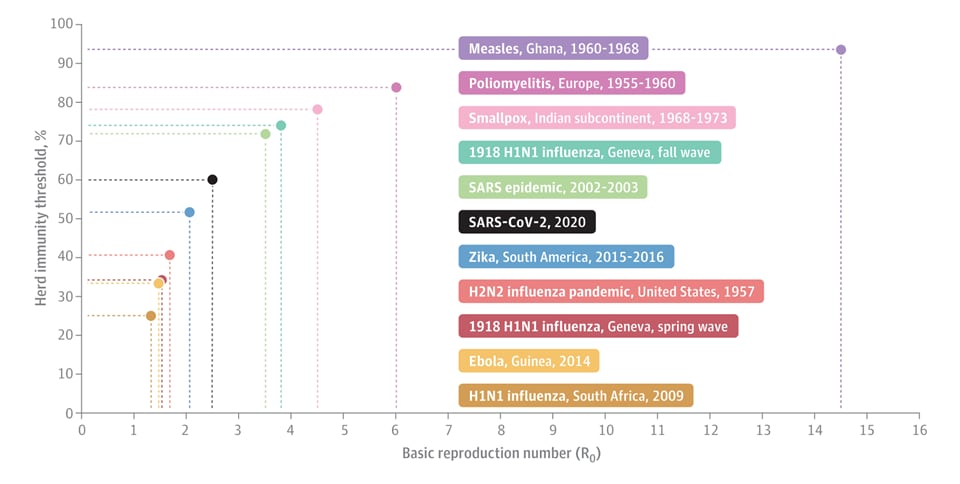
Herd immunity and implications for SARS-CoV-2 controlexternal icon. Omer et al. JAMA Insights (October 19, 2020).
Key findings:
- Highly communicable pathogens have a large reproductive number (R0); therefore, a greater proportion of the population must be immune to decrease sustained transmission and achieve herd immunity (Figure).
- Herd immunity threshold (~60% for SARS-CoV-2) in the US without vaccination would require 198 million infections (Figure).
- With a 0.5% fatality rate, up to 1 million deaths could result.
- Currently, it is estimated that <10% of the population has been infected based on antibody testing.
- There is little precedent for achieving herd immunity without vaccination.
- While a SARS-CoV-2 vaccine could help achieve herd immunity, effectiveness and potential coverage of a future vaccine are not yet known.
Methods: Discussion contextualizing herd immunity in relation to COVID-19 and other major communicable diseases. Limitations: Assumptions for herd immunity threshold for SARS-CoV-2 include no underlying population immunity and equal susceptibility and infectiousness for all individuals.
Implications: Without an effective vaccine that is widely available and broadly adopted, the US will likely not achieve protection against SARS-CoV-2 through herd immunity.
Figure:
Note: Adapted from Omer et al. Herd immunity thresholds by disease in locations where the threshold was measured. SARS-CoV-2 threshold is estimated. Reproduced with permission from JAMA Insights. Omer et al., Herd immunity and implications for SARS-CoV-2 control. DOI: 10.1001/jama.2020.20892. Copyright©2020 American Medical Association. All rights reserved.
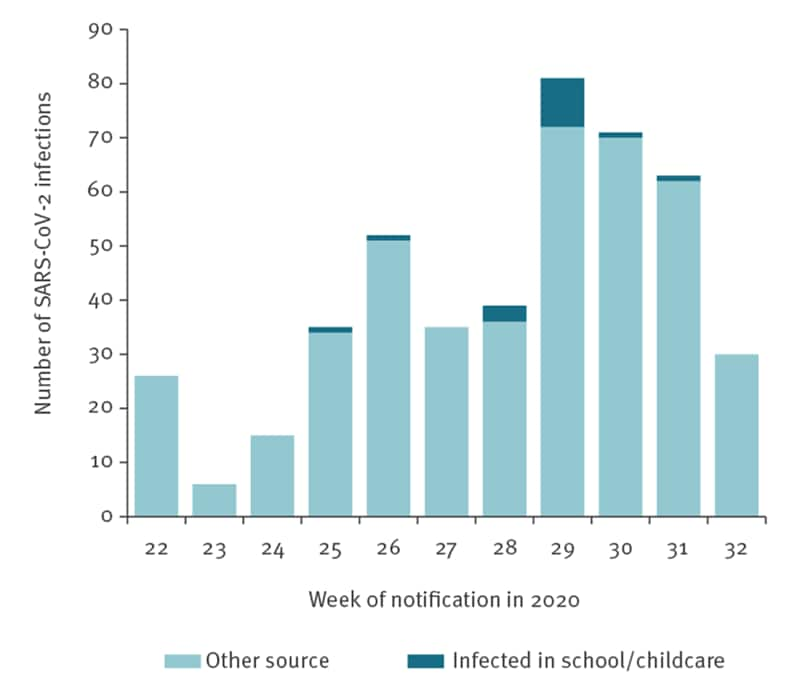
Transmission of SARS-CoV-2 in children aged 0 to 19 years in childcare facilities and schools after their reopening in May 2020, Baden-Württemberg, Germanyexternal icon. Ehrhardt et al. Eurosurveillance (September 10, 2020).
Key findings:
- Child-to-child transmission in schools and childcare facilities was not common and was not the primary cause of SARS-CoV-2 infection in children.
- 137 (30%) of 453 COVID-19 cases among children 0–9 years of age attended school or childcare settings for at least 1 day in their infectious period, 316 (70%) were at home during their entire infectious period.
- 6/137 (4.4%) infected a total of 11 additional students, no secondary infections were detected for the remaining cases.
- Four students were infected by two teachers.
- All remaining cases (n = 437) were infected by sources outside of school and childcare facilities (Figure).
- Infection prevention and control measures were not uniformly implemented in childcare facilities and schools.
Methods: Epidemiological investigation of 453 COVID-19 cases in 0–19-year-olds who attended schools/childcare facilities between May 25 and August 5, 2020, in Baden-Württemberg, Germany, to assess their role in transmission. Limitations: Inconsistent infection control measures across locations; study was conducted in one federal state in Germany and may not be generalizable.
Implications: Child-to-child transmission may not be an important driver of transmission of SARS-CoV-2 in school and childcare settings.
Figure:
Note: Adapted from Ehrhardt et al. Weekly number of SARS-CoV-2 infections in 0–19-year-olds by source of infection. Cases infected in school/childcare facility and cases infected by other sources. Licensed under CC 4.0.
PEER-REVIEWED
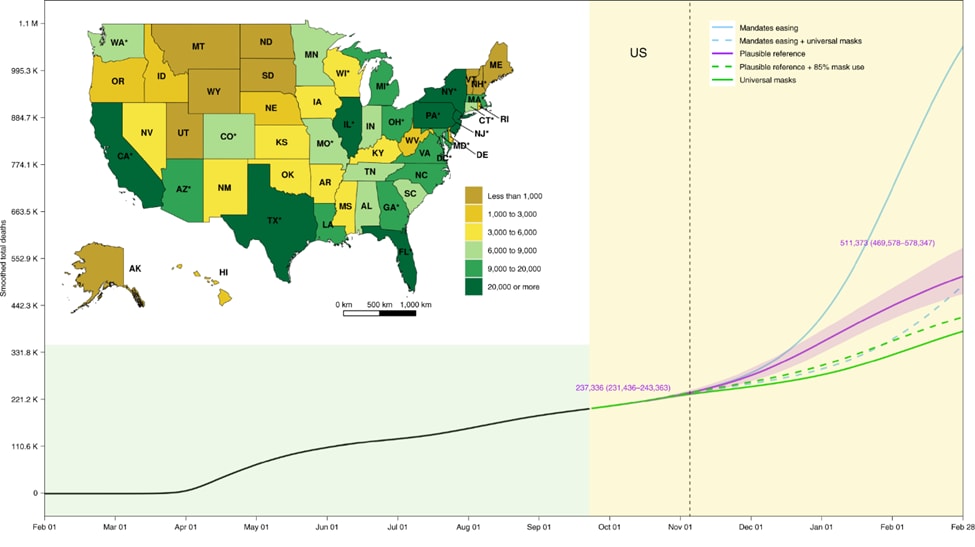
Modeling COVID-19 scenarios for the United States.external icon IHME COVID-19 Forecasting Team, Nature Medicine (Oct. 23, 2020).
Key findings:
- Projected cumulative lives lost to COVID-19 between September 2020 and February 2021:
- Reference scenario: 511,373 lives lost (uncertainty Interval [UI] 496,578–578,347).
- Mandate easing scenario: 1,053,206 lives lost (UI 759,693–1,452,397).
- Projected lives saved with non-pharmaceutical interventions:
- Universal, 95%, mask wearing scenario and social distancing: 129,574 lives saved (UI 85,284─170,867).
- 85% mask wearing and social distancing: 95,814 lives saved (UI 60,731─133,077).
Methods: A hybrid, compartmental model was used to examine potential state-level effects of non-pharmaceutical interventions (NPI) from September 2020 to February 2021. Three boundary scenarios were modeled: “mandate easing”, states continue to ease social distancing mandates with no new mandates (worst case scenario), “plausible reference” in which states may re-implement mandates in response to rising infections, and “universal (95%) mask use” (best case scenario) in which mandates may also be reinstated. Two derivative scenarios to add nuance to the boundary scenarios were modeled: “plausible reference + 85% mask use” and “mandate easing + universal (95%) mask use” in the absence of NPI. Key model assumption: SARS-CoV-2 seasonality similar to pneumonia. Limitations: Large number of simplifying assumptions; uncertainty around transmission parameters; mixing by location not incorporated; limited sensitivity analyses.
Implications: Under all scenarios the COVID-19 pandemic in the US is projected to accelerate through February 2021 resulting in significant loss of life. Universal mask wearing combined with social distancing mandates when daily deaths reach threshold values is projected to save the most lives.
Figure:
Note: From IHME COVID-19 Forecasting Team. Projected cumulative US COVID-19 Deaths, February 2020 to February 2021. Inset map displays the cumulative deaths by state under the plausible reference scenario on 28 February 2021. The observed part of the time series before September 22, 2020, and the predicted part after that date, are shaded. The dashed vertical line is 3 November 2020. Solid lines represent boundary scenarios and dashed lines represent derivative scenarios. Numbers are the means and UIs for the plausible reference scenario on the highlighted dates. An asterisk indicates states with population centers exceeding 2 million persons. Licensed under CC-BY.
PEER-REVIEWED
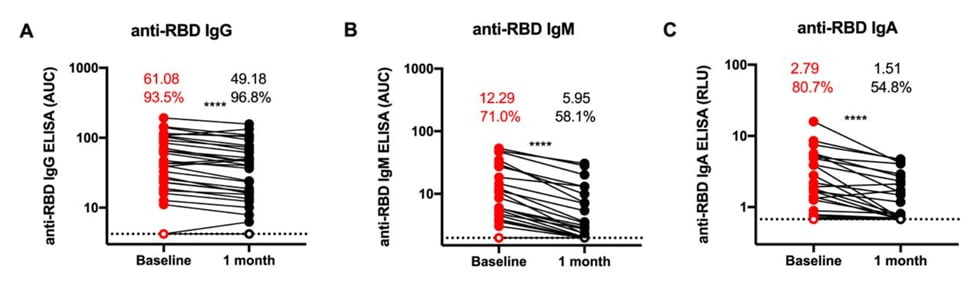
Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals.external icon Beaudoin-Bussières et al. mBio (October 16, 2020).
Key findings:
- Levels of spike protein receptor binding domain (RBD)-specific IgG, IgA, and IgM antibodies in convalescent plasma (CP) significantly decreased from baseline (median 6 weeks after onset of symptoms) to 1-month follow-up (p <0.0001) (Figure).
- The proportion of individuals with detectable anti-RBD IgM and IgA decreased from baseline to 1 month by 13% and 25%, respectively.
- 8% of CP had antibody that bound to both SARS-CoV-2 RBD forms at baseline and 1 month.
- The levels of antibodies binding to both forms decreased from baseline to 1 month.
- Neutralizing activity against both SARS-CoV-2 forms was detected in 71% of CP at baseline; the proportion decreased at 1 month to 41.9% (VSV-G) and 51.6% (D614G).
- Level of neutralizing activity also decreased from baseline to 1 month (p <0.0001 for both forms).
Methods: Plasma was collected from 31 convalescent COVID-19 donors at a median of 6 weeks after symptom onset and again 1 month later, and tested for RBD-specific antibodies (IgG, IgM, and IgA), binding to full-length SARS-CoV-2 spike protein, and ability to neutralize wildtype (WT) SARS-CoV-2 S and a variant (with the D614G mutation). Limitations: Large range in days from symptom onset to collection of first sample; lack of clinical and demographic characteristics on donors.
Implications: These data support previous work indicating a consistent decline in humoral responses following a post-symptom peak in antibody levels. If neutralizing activity is important for the beneficial effects of CP transfusion, then these data highlight the need for rapid collection of plasma following donor recovery.
Figure:
Note: From Beaudoin-Bussières et al. Change in antibody levels and percent of samples showing antibody response at baseline and one month later. White circles indicate undetectable levels. Numbers indicate mean level and percentages indicate proportion showing response (**** p <0.0001). Licensed under CC-BY.
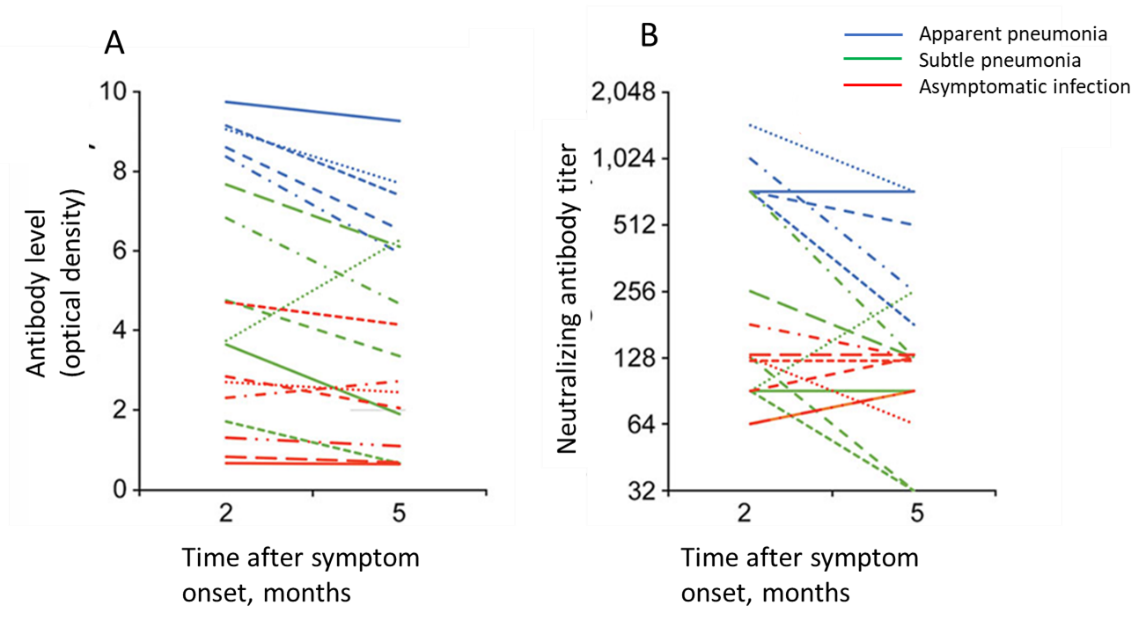
Waning antibody responses in asymptomatic and symptomatic SARS-CoV-2 infection. Choe et al. Emerging Infectious Diseases (August 26, 2020).
Key findings:
- Antibody titers correlated with disease severity.
- The geometric mean titer was 105 among asymptomatic persons, 161 among patients with subtle pneumonia, and 891 among patients with apparent pneumonia.
- The geometric mean titer of neutralizing antibodies declined from 219.4 at 2 months after infection to 143.7 at 5 months after infection.
- 71% of asymptomatic patients had detectable antibodies at 2 months after infection which declined to 57% at 5 months after infection.
- 100% of patients with pneumonia had detectable antibodies at 2 months after infection.
- At 5 months, 83% of patients with subtle pneumonia and 100% of patients with apparent pneumonia had detectable antibodies.
Methods: SARS-CoV-2 antibodies and virus neutralization was evaluated in 7 asymptomatic and 11 symptomatic patients with pneumonia (classified as subtle [n = 6] or apparent [n = 5]) in a hospital setting in South Korea. A ≥4 -fold reduction in antibody titer was considered a decrease. Limitations: Symptomatic patients with pneumonia were older and increasing age is associated with a stronger antibody response to SARS-CoV-2; small sample size.
Implications: Naturally acquired humoral immunity against SARS-CoV-2 might not be long-lasting. Disease severity may affect antibody response.
Figure:
Note: From Choe et al. A: Optical density measurements (p = 0.01). B: Neutralizing antibody titers (p = 0.03). Each line indicates data from a single patient with apparent pneumonia, subtle pneumonia, or asymptomatic infection. Open access journal; all content freely available.
- Labgold et al. Measuring the missing: Greater racial and ethnic disparities in COVID-19 burden after accounting for missing race/ethnicity dataexternal icon. medRxiv Using a combination of race/ethnicity imputation and quantitative bias-adjustment methods for misclassification, the magnitude of disparity in infection rates increased 1.3 and 1.6-fold for Black and Hispanic persons, respectively, compared to classified White persons.
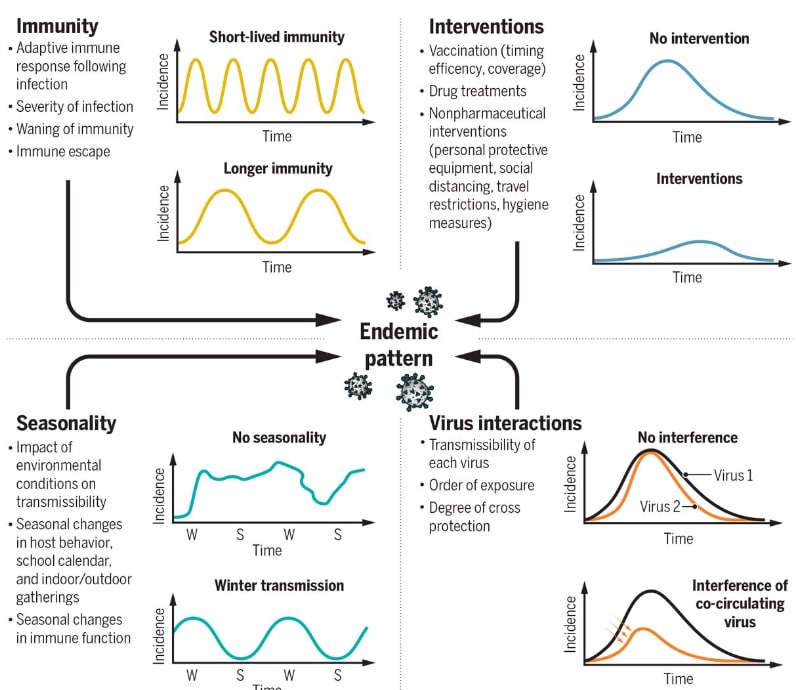
- Shaman & Galanti. Will SARS-CoV-2 become endemic?external icon Rates of repeat infection, factors modulating seasonality, competition with other circulating respiratory viruses, and control measures will influence the endemic pattern of SARS-CoV-2 transmission.
Figure:
Note: From Shaman et al. The combination of immunity, interventions, virus interactions, and virus seasonality come together to establish an endemic pattern for an emerging virus. From Shaman & Galanti SCIENCE 370: 527, 2020 (DOI: 10.1126/science.abe5960). Reprinted with permission from AAAS.
- Villalba et al. Analysis of impact on tissue activity during COVID-19 outbreak: A survey of 8 banks in Spain.external icon Cell and Tissue Banking. Between March and April of 2020, there was a drop in tissue donor number ranging from 33.3% to 78.8% compared to the prior three years in Spain, attributed to the overall decrease in surgical activity during this period.
- Toro et al. Early impact of COVID-19 outbreak on the availability of cornea donors: Warnings and recommendations.external icon Clinical Ophthalmology. While harvested corneal grafts are low risk based on current evidence, there is potential for SARS-CoV-2 transmission through corneal stromal tissue. Post-mortem testing of deceased donors is recommended as is the need for harmonized guidelines and approaches among transplant centers.
- Politis et al. Post-donation information and haemovigilance reporting for COVID-19 in Greece: Information supporting the absence of SARS-CoV-2 possible transmission through blood components.external icon Transfusion Clinique et Biologique. An immunosuppressed patient who was transfused with whole blood-derived platelets from a donor who was later diagnosed with COVID-19 did not develop symptoms of disease and never tested positive for SARS-CoV-2.
- Carbon, C. Wearing face masks strongly confuses counterparts in reading emotions.external icon Frontiers in Psychology. Viewing faces with masks resulted in fewer correctly identified facial emotions and lower confidence by participants in their own assessments of the emotions; all emotional states except for fearful were confused with a neutral state.
Figure:
Note: From Carbon et al. A person showing six different emotions without a mask (A) and wearing a mask (B). Licensed under CC-BY.
- Wolfe et al. Optimizing communication in schools and other settings during COVID-19external icon. The Hearing Journal. Article details the challenges that face masks pose for individuals with hearing loss and presents a number of potential solutions, including clear face masks or shields and hearing-assisted technologies.
- Dyer, O. COVID-19: Eli Lilly pauses antibody trial for safety reasonsexternal icon. Report on the Activ-3 trial of Eli Lilly’s neutralizing antibody LY-CoV555 (bamlanivimab), which was paused for safety reasons.
- Weinheimer et al. Reprocessing N95s with hydrogen peroxide vaporization: A robust system from collection to dispensing.external icon American Journal of Infection Control. Large-scale implementation of a system to reprocess N95 masks at Texas Medical Center is outlined.
- Kuehn, B. COVID-19 halts reproductive care for millions of women.external icon Doctors Without Borders warns that COVID-19 clinic closures, supply chain delays, or travel restrictions have curtailed women’s sexual and reproductive health services in a number of countries.
- Kieran et al. COVID-19 mortality risk in down syndrome: Results from a cohort study of 8 million adults.external icon Annals of Internal Medicine. A project undertaken by the UK government estimated a 4-fold increased risk for COVID-19–related hospitalization and a 10-fold increased risk for COVID-19–related death in persons with Down syndrome, a group that is currently not strategically protected.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.