COVID-19 Science Update released: September 24, 2021 Edition 106

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 database, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
The BNT162b2 vaccine effectiveness against new COVID-19 cases and complications of breakthrough cases: A nation-wide retrospective longitudinal multiple cohort analysis using individualised data. Glatman-Freedman et al. EBioMedicine (September 16, 2021).
Key findings:
- Vaccine effectiveness (VE) of BNT162b2 (Comirnaty, Pfizer/BioNTech) for SARS-CoV-2-related outcomes was 54.3% (CI 50.6%-57.8%) for infection, 77.3% (CI 71.2%-82.1%) for severe/critical disease 14–20 days after the 1st dose, and over 95% for all outcomes 15–21 days and 22–28 days after the 2nd dose.
- VE for symptomatic disease increased from 14–20 days after first dose (58.3% CI 54.7%-61.6%) to 8–14 days after second dose (93.6% CI 92.7%-94.3%) to 15–21 days after second dose (98.1% CI 97.7%-98.5%).
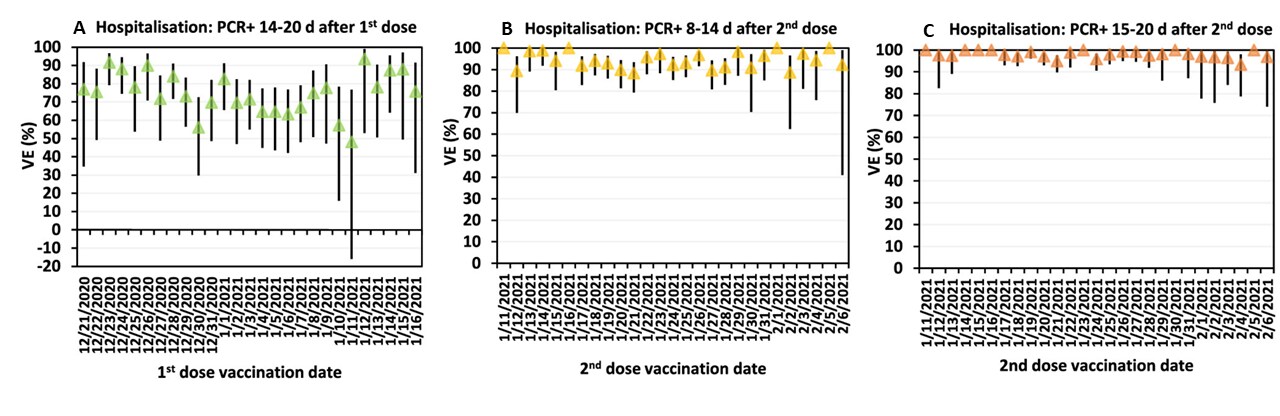
- VE for hospitalization increased from 14–20 days after first dose (74.5% CI 69.1%-79.0%) to 8–14 days after 2nd dose (93.8% CI 91.9%-95.2%) to 15–21 days after 2nd dose (98.0% CI 97.1%-98.6%) (Figure).
- VE was slightly lower among persons ≥80 years but reached levels similar to other age groups by 22–28 days after 2nd dose.
Methods: Retrospective longitudinal cohort study from Israel Ministry of Health national databases analyzed 5 outcomes (infection, symptomatic disease, hospitalization, severe/critical disease, and death) for 27 consecutive cohorts (≥16 years) by vaccination day of 1st dose from December 21, 2020–January 16, 2021 and 2nd dose from January 11, 2021–February 6, 2021. Limitations: Information on co-morbidities was not available; vaccine effectiveness against Delta variant (B.1.617.2) was not studied.
Implications: Comirnaty provided a high level of protection 2 weeks after 2nd dose; however reevaluation of vaccine effectiveness against other variants is needed.
Figure:
Note: Adapted from Glatman-Freedman et al. Adjusted vaccine effectiveness and 95% CI of 27 cohorts against SARS-CoV-2-associated hospitalizations. Panel A represents outcomes that occurred on days 14–20 after the 1st vaccine dose; panel B represents outcomes 8–14 after the 2nd dose; panel C represents outcomes 15–21 after the 2nd vaccine dose. Statistical adjustment was performed for age and sex. Licensed under CC-BY-NC-ND.
PREPRINTS (NOT PEER-REVIEWED)
Duration of protection of COVID-19 vaccines against clinical disease. Public Health England. (September 9, 2021).
Key findings:
- Vaccine effectiveness (VE) against symptomatic disease at ≥20 weeks after 2nd dose was higher for BNT162b2 (Comirnaty, Pfizer/BioNTech) (~70%) compared with AZD1222 (Vaxzevria, AstraZeneca) (~50%).
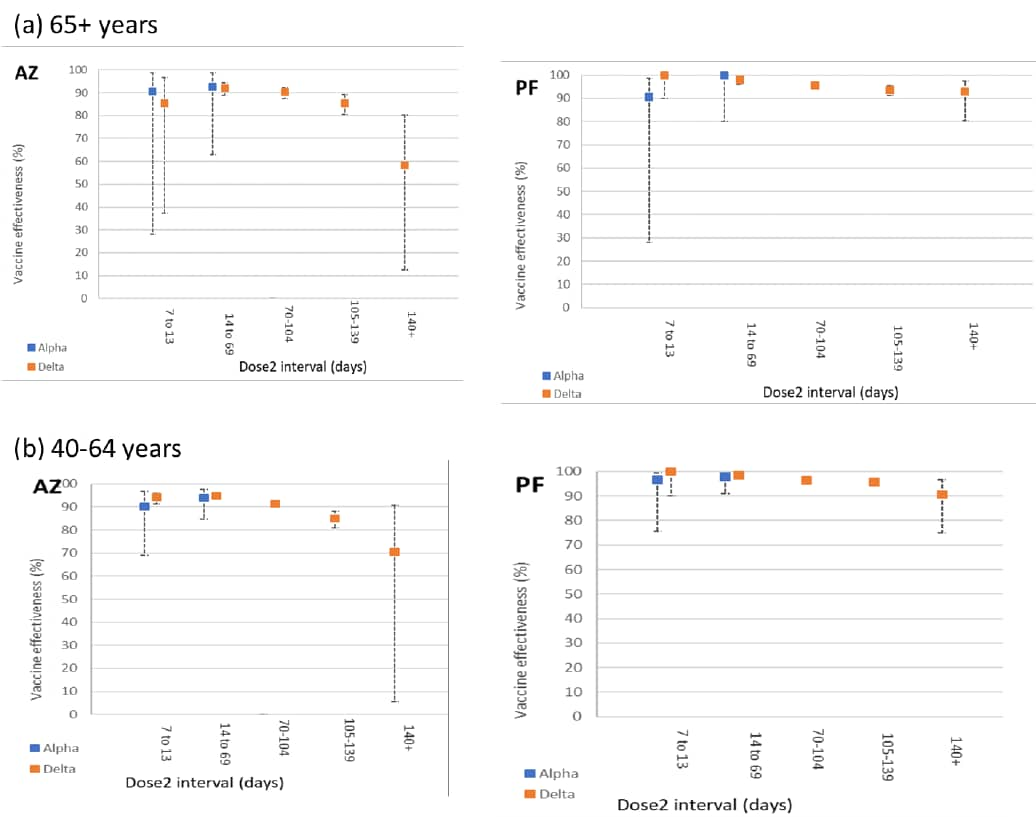
- VE against hospitalization remained high after 20 weeks, although VE was lower for Vaxzevria (~60%, persons ≥65 years; ~70%, persons ages 40–64 years) compared with Comirnaty (~90% for persons ≥65 years and 40–64 years) (Figure).
- Among persons ≥80 years, Comirnaty effectiveness against hospitalization might have declined, but results may be confounded by different spacing of doses for sub-groups.
- VE against death remained high after 20 weeks for Vaxzevria and Comirnaty.
Methods: Vaccine effectiveness (VE) of AZD1222 (Vaxzevria, AstraZeneca), BNT162b2 (Comirnaty, Pfizer/BioNTech), and mRNA-1273 (Moderna, Spikevax) was measured by comparing the vaccination status of symptomatic persons in the UK who tested positive for SARS-CoV-2 with those who tested negative during December 8, 2020–August 20, 2021. VE against symptomatic disease, hospitalization, and death for Alpha (B.1.1.7) and Delta (B.1.617.2) variants were analyzed by number of days since 2nd dose. Limitations: Data from persons 16–39 years of age were excluded due to limited follow-up period; Moderna VE was limited to symptomatic disease for 10-week follow-up only; unclear timing of vaccination; intervals between doses varied over time.
Implications: Vaccine effectiveness of 2 doses of Vaxzevria and Comirnaty against hospitalization and death due to the Delta variant remained high 20 weeks after vaccination.
Note: Adapted from Public Health England. Vaccine effectiveness against hospitalization for Vaxzevria (AZ) and Comirnaty (PF) for a) ages ≥65 years and b) ages 40–64 by days since 2nd dose. Blue indicates Alpha variant; orange indicates Delta variant. Used via the UK Open Government Licence for public sector information.
PREPRINTS (NOT PEER-REVIEWED)
Reduced magnitude and durability of humoral immune responses by COVID-19 mRNA vaccines among older adults. Brockman et al. medRxiv (September 12, 2021). Published in Journal of the Infectious Diseases (December 9, 2021).
Key findings:
- After 2 doses of mRNA vaccine, SARS-CoV-2 wild-type strain and the Delta (B.1.351) variant elicited similar antibody production, although antibody response was functionally impaired by the Delta variant.
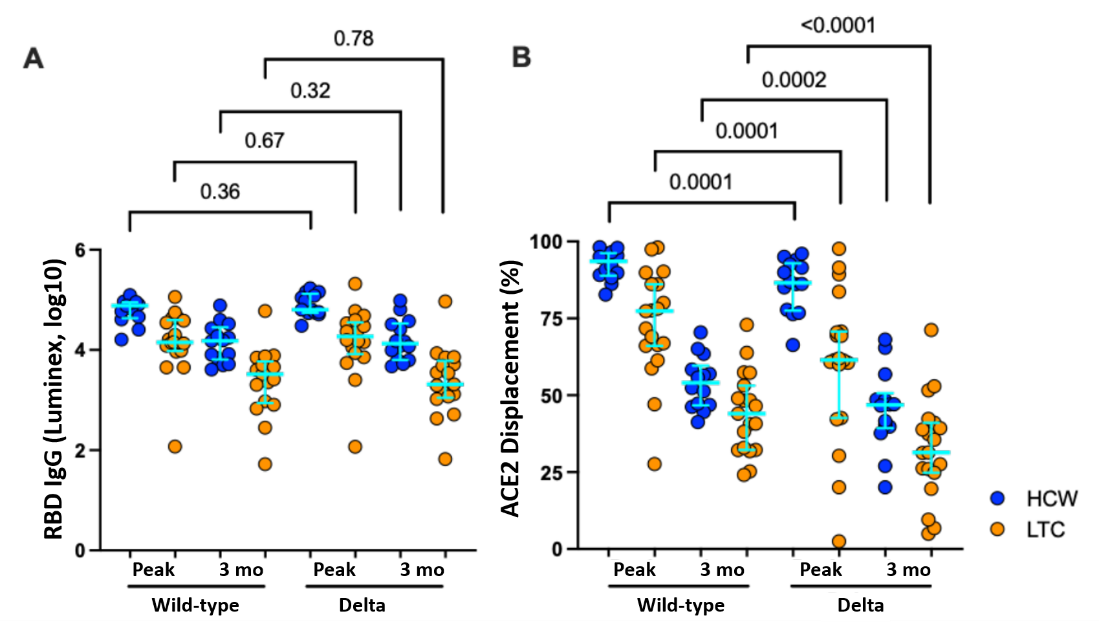
- Post-vaccination anti-spike receptor binding domain (RBD) antibody levels were similar between the wild-type strain and the Delta variant. Antibody levels were lower among older adults and decreased over time in both groups (Figure 1A).
- Post-vaccination antibody blocking of angiotensin-converting enzyme 2 (ACE2) receptor binding was 7% lower against the Delta variant than wild-type strains among healthcare workers (HCWs) and 15% lower among older adults (Figure 1B).
Methods: Anti-spike RBD antibody titers and ACE2 competitions were measured in serum and plasma 1 and 3 months after the 2nd dose of mRNA vaccines from 89 HCWs (median age 41 years old) and 62 older adults (median age 79 years old). Limitations: Due to small sample sizes, differences in responses between mRNA vaccines could not be assessed; correlation between vaccine immunogenicity and clinical outcome not established.
Implications: Older adults might be more susceptible to infection by the Delta variant even after 2 doses of mRNA vaccine, supporting consideration of a 3rd dose.
Figure:
Note: Adapted from Brockman et al. A) Binding IgG antibody responses to spike RBD from wild-type strain and Delta variant 1 month (peak) and 3 months after the 2nd dose of mRNA vaccines. B) ACE2 competition results. Blue circles indicate healthcare workers (HCW) and orange circles indicate older adults (seniors and long-term care [LTC] facilities residents). Bars represent median and interquartile range. Licensed under CC-BY-ND 4.0.
PEER-REVIEWED
Equity and the uneven distribution of federal COVID-19 relief funds to US hospitals. Buxbaum et al. Health Affairs (September 8, 2021).
Key findings:
- Hospitals serving areas with a higher percentage of Black residents received 45.2% higher provider relief funding in June 2020, and 70.4% higher funding in October 2020 and February 2021 than hospitals serving other areas.
- Hospitals serving areas with a higher percentage of Hispanic residents received lower provider relief funding (40.3% lower in June 2020, 31.5% lower in October 2020, and 31.2% lower February 2021) than hospitals serving other areas.
Methods: Provider Relief Fund distribution records were matched to hospitals (n = 2,709); modeling correlated provider relief funding with community and hospital characteristics, controlling for hospital beds and hospital wages. Limitations: Excluded hospitals that returned the funding awards (e.g., HCA Healthcare and nearly all Kaiser hospitals), 1,350 rural critical access hospitals, and long-term care facilities.
Implications: Decisions about pandemic relief allocation to hospitals should consider community need to improve equitable distribution.
COVID-19 mortality at the neighborhood level: racial and ethnic inequalities deepened in Minnesota in 2020. Wrigley-Field et al. Health Affairs (October 2021).
Key findings:
- Total mortality increased 41% among non-White BIPOC (Black, Indigenous, People of Color) populations and 14% among non-Hispanic White populations from 2017–2019 to 2020 in Minnesota.
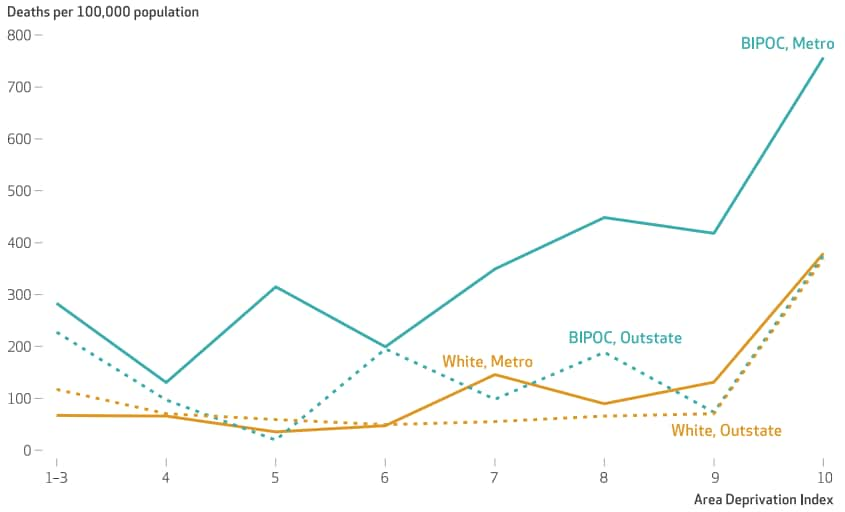
- Excess deaths directly attributed to COVID-19 were over 3 times higher in neighborhoods with the most disadvantage (Area Deprivation Index [ADI] of 10; 75/100,000) compared to those with the least disadvantage (ADI of 1; 23/100,000) (Figure).
Methods: Total mortality and COVID-19 mortality were examined over time by race/ethnicity and census tract. Area Deprivation Index (a composite of poverty, housing, employment, and education measures collected by the Census Bureau American Community Survey) and geography (metro/non-metro). Limitations: Possible cause of death misclassification may have resulted in an undercount of total COVID-19 mortality, and differences by race and geography.
Implications: Interventions to reduce COVID-19 mortality and address health disparities may need to consider neighborhood contexts.
Figure:
Note: Adapted from Wrigley-Field et al. Excess mortality in Minnesota, by race and ethnicity, region, and area deprivation, 2020. Results are adjusted for age and sex. White is non-Hispanic White. Metro is the 7-county Minneapolis/St. Paul Twin Cities Metro area. Outstate is all other census tracts outside that area. Trends in gold for Whites, blue for BIPOC; solid lines for Metro residents, dashed lines for non-Metro or “outstate.” Permission request in process.
PREPRINTS (NOT PEER-REVIEWED)
Comprehensive evaluation of COVID-19 patient short- and long-term outcomes: disparities in healthcare utilization and post-hospitalization outcomes. Salerno et al. medRxiv (September 12, 2021). Published in PLOS One (October 6, 2021).
Key findings:
- After adjusting for comorbidities and other demographic conditions, Black or African American patients with COVID-19 had higher hospitalization (adjusted hazard ratio [aHR] 1.89, 95% CI 1.61-2.17) and mortality (aHR 1.52, 95% CI 1.02-2.22) rates compared to other populations with COVID-19.
- Patients with fluid and electrolyte disorders (aHR 5.50, 95% CI 3.27-9.23) and with blood loss anemia (aHR 2.85, 95% CI 1.84-4.40) had higher mortality compared to those without those conditions.
Methods: A retrospective study of medical encounters, hospitalization, readmission, and mortality among patients with COVID-19 (N = 6,731) treated at a large Midwest university medical center (March 2020–March 2021). Limitations: Excluded patients admitted by transfer because of missing health histories; single hospital in 1 state may have biases in the patient mix and lack generalizability to other geographic areas.
Implications: Greater hospitalization and mortality risk among Black or African American patients likely represent the impact of structural determinants of health. Addressing structural inequities should be considered in efforts to prevent and control COVID-19.
PEER-REVIEWED
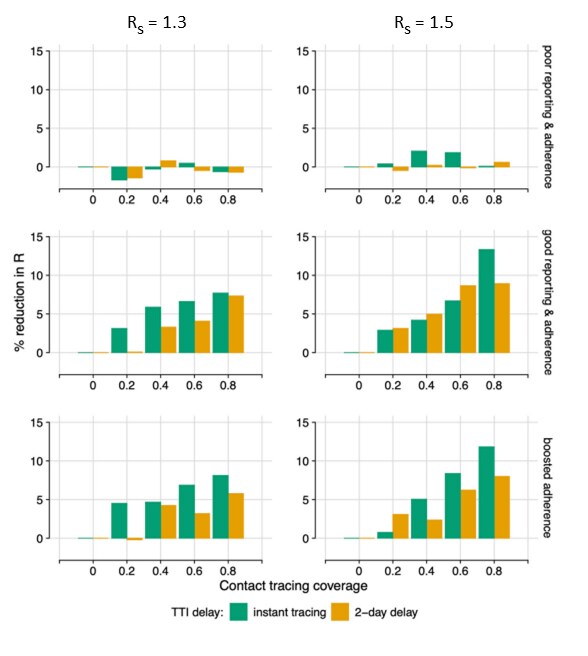
Contact tracing is an imperfect tool for controlling COVID-19 transmission and relies on population adherence. Davis et al. Nature (September 13, 2021).
Key findings:
- A 6–13% reduction in R (reproduction number) was observed when testing and contact tracing identifies and traces 80% of contacts with good self-reporting and adherence to isolation (Figure).
- Immediate contact tracing compared to 2-day delay modestly reduced the probability of a large outbreak (>2000 cases).
- Difference in probability of a large outbreak between instant testing with a 65% sensitive test and a 2-day delay with a 95% sensitive test is relatively small.
Methods: Modeling study exploring the effects of contact tracing using 5,000 simulations with estimated parameters for incubation period, range of self-reported adherence to isolation, tracing rates, and sensitivity of diagnostic tests occurring in the United Kingdom. Limitations: Temporal variation in testing was not assessed, resulting in overestimation of test sensitivity in some cases.
Implications: Contact tracing is an important tool to control SARS-CoV-2 infection transmission and to prevent outbreaks. However, contact tracing alone is unlikely to prevent outbreaks and needs to be combined with other interventions, such as physical distancing and increased vaccination.
Figure:
Note: Adapted Davis et al. Contact tracing efficacy. Percentage reduction in the effective reproductive number (R) for testing, tracking, and isolation (TTI) scenarios: instant tracing or a 2-day delay, assuming 95% test sensitivity. Poor reporting & adherence (top); Good reporting & adherence (middle); Good reporting & high adherence (bottom): Combined results of 5,000 simulations. Licensed under CC BY.
PREPRINTS (NOT PEER-REVIEWED)
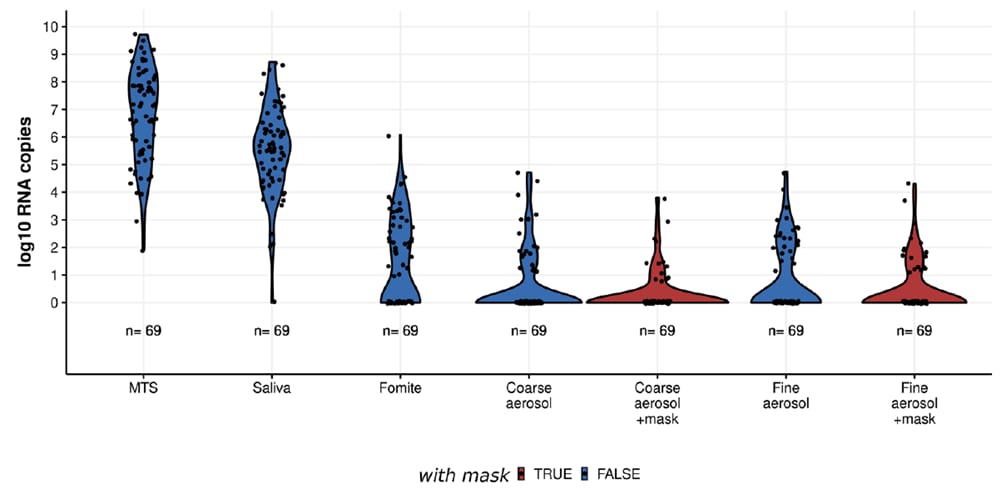
Infectious SARS-CoV-2 in exhaled aerosols and efficacy of masks during early mild infection. Adenaiye et al. Clinical Infectious Diseases (September 14, 2021).
Key findings:
- Among 45 participant cases with mild infection, SARS-CoV-2 RNA was detected in all respiratory (mid-turbinate) swabs, 99% of saliva, 49% of fomite (cell phone swabs), 19% of coarse (>5 µm), and 31% of fine aerosol (≤5 µm) samples.
- Among 4 additional Alpha (B.1.1.7) variant cases, SARS-CoV-2 RNA was detected in all respiratory, saliva, fomite, and aerosol samples.
- Alpha variant samples had higher viral RNA concentrations than other samples.
- Face masks (cloth or surgical) reduced exhaled viral RNA by 48% (95% CI 3%-72%) in fine aerosols and by 77% (95% CI 51%-89%) in coarse aerosols (Figure 1).
- There was no difference in reduction of viral RNA between cloth and surgical masks.
- Mask performance was not significantly different for Alpha variant infections than for other infections.
Methods: Quantitative SARS-CoV-2 RNA measured from respiratory swabs, saliva, and aerosols from participants with q-RT-PCR-positive saliva samples, recently diagnosed infections, and positive contacts. To test mask effectiveness, participants provided paired breath samples at 2 visits, 2 days apart, first while wearing a mask and then without (May 2020–April 2021). Limitations: Cases were mild or asymptomatic and may not represent viral shedding during the most infective phase; quality of masks varied; study done before emergence of the Delta (B.1617.2) variant.
Implications: Face masks effectively reduced aerosol viral shedding of Alpha variant infections. With suboptimal vaccination rates in many U.S. locations, tight-fitting face masks are an important mitigation strategy for COVID-19, especially with the emergence of more infectious SARS-CoV-2 variant strains.
Figure:
Note: Adapted from Adenaiye et al. Viral RNA shedding in paired with and without face mask samples. Viral RNA measured during 69 same-day paired sampling events with and without mask from 46 seronegative cases. Samples with no detected viral RNA assigned a copy number value of 1. Exhaled breath aerosols obtained in 30-minute sampling durations. “+mask” = sample collected while wearing face mask. MTS = nasal mid-turbinate (respiratory) swab, Fomite = swab of participant’s mobile phone. Licensed under CC BY.
Two articles this week provide findings from studies on SARS-CoV-2 testing among students; one involved daily lateral flow device testing at school, the other implemented weekly rapid diagnostic testing at home.
PEER-REVIEWED
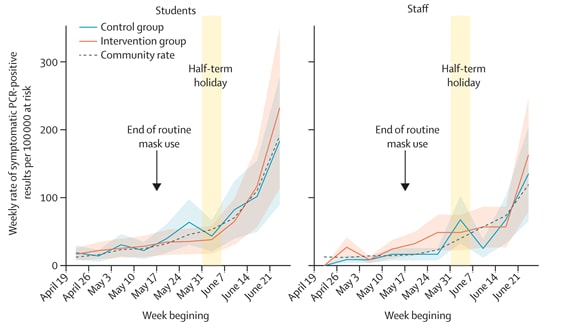
Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV transmission in English secondary schools and colleges: An open-label, cluster-randomised trial. Young et al. Lancet (September 14, 2021).
Key findings:
- COVID-19 rates among students or staff were equivalent in schools in which school-based contacts continued school attendance with daily testing (intervention group) and those with self-isolation of school-based contacts (control group) (Figure).
- Rates of symptomatic PCR-confirmed infections were similar across groups (adjusted Relative Risk [aRR] 0.96 [95% CI 0.75-1.22]; p = 0.72).
- COVID-19-related absences were 20% lower in the intervention group than the control group, but the difference was not statistically significant.
Methods: Cluster-randomized controlled trial in secondary and post-secondary schools in England (April 19–June 27, 2021); schools 1:1 assigned to intervention (n = 102; voluntary daily lateral flow device (LFD) testing for 7 days; LFD-negative contacts remaining at school) or control (n = 99; self-isolation of school-based COVID-19 contacts for 10 days) groups. Randomization was stratified by school type and size and by socio-economic markers. Limitations: Participation varied leading to possible missing data for PCR-positive cases and contacts; study conducted during period of low incidence; LFD testing may not be as reliable in the face of new variants.
Figure:
Note: Adapted from Young et al. Incidence of symptomatic PCR-positive results (co-primary outcome) by study group (Control group, Intervention group); Community rate is the dashed line. Incidence of symptomatic PCR-positive results in students and staff by study group. Weekly incidence is shown per 100,000 at risk. The shaded area is the mean rate plus or minus 1 SE using a negative binomial model to account for overdispersion (θ = 0.28). Reprinted from The Lancet Infectious Diseases, September 14, 2021, Young et al., Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV transmission in English secondary schools and colleges: An open-label, cluster-randomised trial, online ahead of print. Copyright 2021, with permission from Elsevier.
PREPRINTS (NOT PEER-REVIEWED)
“Like brushing teeth” – Implementation experiences with opt-in, at-home screening for SARS-CoV-2 among schoolchildren in Germany.Wachinger et al. medRXiv (September 15, 2021). Published in BMJ Paediatrics Open as Experiences with opt-in, at-home screening for SARS-CoV-2 at a primary school in Germany: An implementation study (October 20, 2021).
Key findings:
- 21 (62%) staff and 109 (59%) students and parents opted to participate in rapid diagnostic testing (RDT) at-home screening.
- No positive results were detected during the 4-week study period.
- Participants were motivated by a sense of increased safety through screening and by a sense that screening did not increase the risk to oneself or to others.
- RDT was not burdensome and was gradually integrated into daily routines, just “like brushing teeth.”
- Screening did not appear to increase risk-taking behaviors among participants.
Methods: Prospective quantitative and qualitative implementation study among primary school staff and students in southwestern Germany of experiences and perceptions of starting in-home weekly screening by RDT of anterio-nasal swabs for COVID-19 starting March 2021; compulsory screening was introduced April 2021 after 4 weeks of study testing. Limitations: Participation was voluntary and may have led to self-selection bias in the perceptions around testing; may not be generalizable to areas where RDTs are less commonly available and in use.
Implications for Young et al. and Wachinger et al.: School-based and home-based rapid testing methods were broadly accepted in 2 countries. Daily contact testing reduced isolation time without substantially increasing transmission of COVID-19. At-home self-testing appears to be accepted even by primary school aged students.
Vaccines
- Disparities in SARS-CoV-2 vaccination-to-infection risk during the COVID-19 pandemic in Massachusetts. Dryden-Peterson et al. JAMA Health Forum (September 17, 2021). State health department data for 6.8 million Massachusetts residents showed lower vaccine coverage to infection risk ratios (indicating less equitable vaccination relative to infection risk), in areas of high socioeconomic vulnerability and with larger proportions of Black and Latinx individuals (Gini coefficient 0.47 by race and ethnicity). To achieve equity, ~810,000 full vaccination courses would need to be administered in under-vaccinated communities.
Note: Adapted from Dryden-Peterson et al. Cumulative proportion of vaccination and SARS-CoV-2 infection by community and individual residents in Massachusetts. Asian, Black, Latinx residents, and residents of multiple or other races and ethnicities accounted for 62% of confirmed infections and 30% of full vaccinations (January 29, 2020–June 24, 2021). Socioeconomic vulnerability was estimated using the Socioeconomic Status domain, CDC Social Vulnerability Index. Licensed under CC BY.
- Association of nursing home characteristics with staff and resident COVID-19 vaccination coverage. McGarry et al. JAMA Internal Medicine (September 16, 2021). As of July 18, 2021, across 14,900 nursing homes, 60.0% of staff and 81.4% of residents were fully vaccinated, with lowest coverage (49.2%) in certified nursing assistants. Increased coverage was associated with nonprofit facilities, higher Medicare quality rating, and long tenured employees; lower coverage was associated with higher percentage non-White staff and residents, and lower county-level vaccination coverage.
Variants
- Exponential growth, high prevalence of SARS-CoV-2 and vaccine effectiveness associated with Delta variant in England during May to July 2021. Elliott et al. medRxiv (Preprint; September 10, 2021). Published in Science as Exponential growth, high prevalence of SARS-CoV-2, and vaccine effectiveness associated with the Delta variant (November 2, 2021). Analysis of RT-PCR swab-positivity data from round 12 (May 20–June 7, 2021) and round 13 (June 24–July 12, 2021) of the REal-time Assessment of Community Transmission-1 (REACT-1) study (ages 5 years and older) in England, showed that the average prevalence of SARS-CoV-2 infection increased from 0.15% (95% CI 0.12%-0.18%) in round 12 to 0.63% (95% CI 0.57%-0.18%) in round 13, with a 9-fold growth among youths 13–17 years. The increase was attributed to the Delta variant. In round 13, the prevalence of infection was 3-fold higher among unvaccinated persons compared with fully vaccinated persons; however, 44% of infections were among those who were fully vaccinated. Vaccinated effectiveness against symptomatic infection was 59%.
Note: Adapted from Elliott et al. Comparison of the exponential model fit to round 12 and 13 and the exponential model fit to round 13 only. Licensed under CC-BY-NC-ND 4.0.
Health Equity
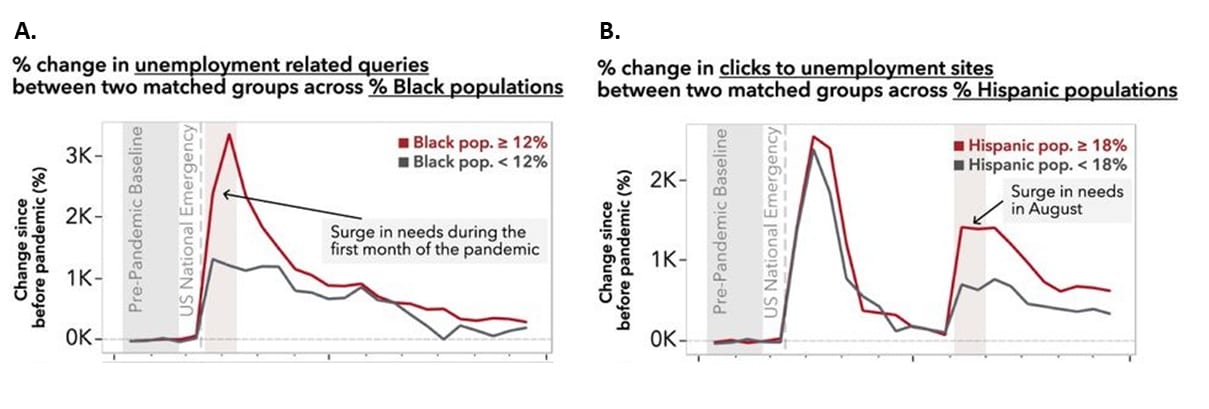
- Widening disparities in online information access during the COVID-19 pandemic. Suh et al. medRxiv (Preprint; September 17, 2021). A study of anonymized web search logs from a large search engine provider in the United States from January 2020–January 2021 showed ZIP code level differences by socioeconomic status and race/ethnicity in digital access to health information, economic assistance, and use of online education or food services.
Note: Adapted from Suh et al. Disparities in online economic assistance access search queries to state unemployment sites and comparison of ZIP codes with high and low A) Black populations, and B) Hispanic populations. Licensed under CC-BY-NC-ND 4.0.
Natural History, Reinfection, and Health Impact
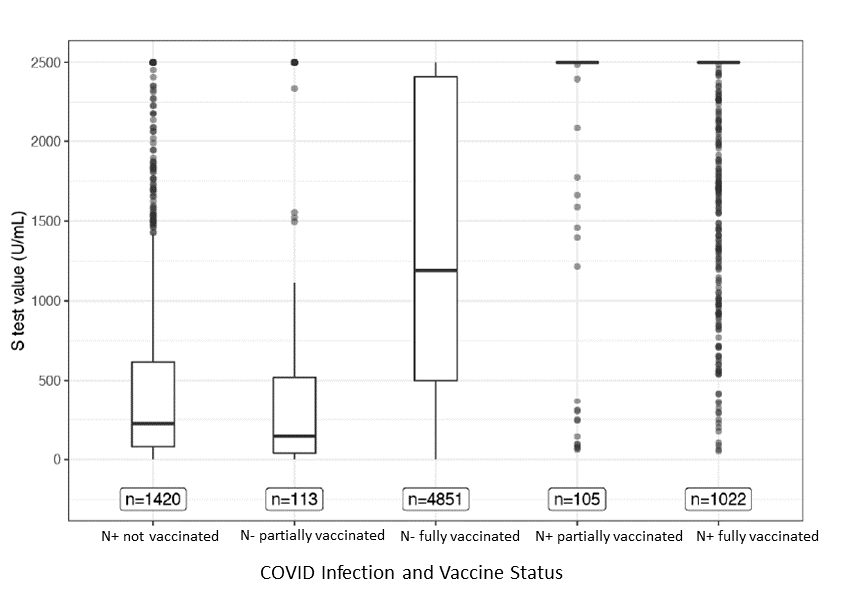
- Combined impact of prior SARS-CoV-2 infection and vaccination on antibody presence. Shuford et al. medRxiv (Preprint; September 12, 2021). Among 8,846 participants of the Texas Coronavirus Antibody REsponse Survey (CARES), persons with prior infection and with either partial or full vaccination had the highest proportion of SARS-CoV-2 spike protein antibody detection at the maximum value (80.95% and 83.07%, respectively). These 2 groups also showed higher antibody median amounts than persons with prior infection and not vaccinated, persons without prior infection and partially vaccinated, and persons without prior infection and fully vaccinated (all p-values <0.0001).
Note: Adapted from Shuford et al. Boxplots of SARS-CoV-2 spike protein antibodies for each infection/vaccination group N+ denotes prior infection; N- denotes no prior infection S-test is Roche Elecsys® Anti-SARS-CoV-2 antibody test. The horizontal bar represents the median. For groups with prior infection and either partial or full vaccination, >80% were ≥ 2,500 U/mL (units per milliliter). Licensed under CC-BY-NC-ND 4.0.
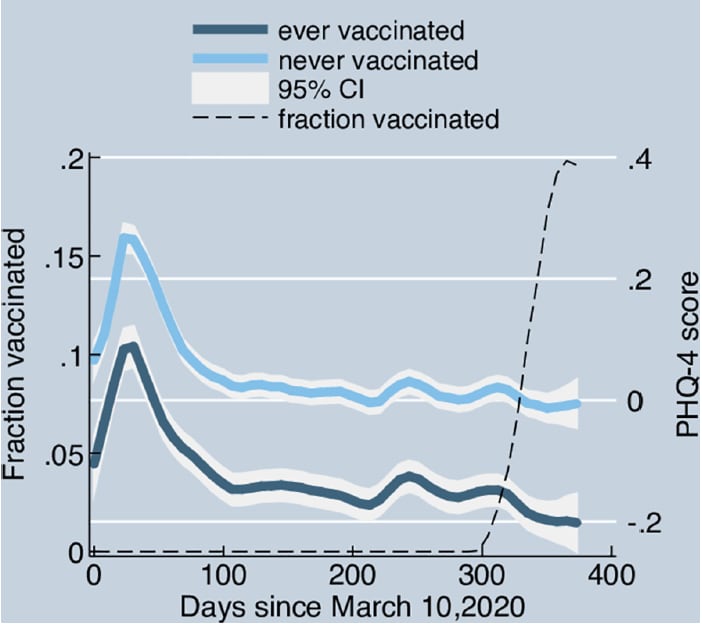
- COVID-19 vaccines and mental distress. Perez-Arce et al. PLOS One (September 8, 2021). Among 8,003 adults (aged 18 years and older) responding, every 14 days by mail or online, to the nationally representative Understanding America Study (March 2020–March 2021), never-vaccinated adults (n = 6,384) reported higher mental distress than the ever-vaccinated adults (n = 1,643) throughout the study period. In both groups, the level of mental distress increased during the first 30 days then declined and remained stable until December 2020, when the vaccines became available. Early in 2021 trajectories diverged, and mental distress rates declined only among ever-vaccinated participants.
Note: Adapted from Perez-Arce et al. Trajectory of mental distress based on the Patient Health Questionnaire (PHQ-4) score over time, by vaccination group. Questionnaire Item responses (0–4) are summed to create a score from 0–16, and standardized to have a mean of 0 and a standard deviation of 1. Ever-vaccinated respondents reported having received at least 1 dose by March 14, 2021; never-vaccinated respondents did not report receiving a vaccine dose by March 14, 2021. Licensed under CC BY 4.0.
- Comparison of characteristics of deaths from drug overdose before vs during the COVID-19 pandemic in Rhode Island. Macmadu et al. JAMA Network Open (September 17, 2021). The rate of overdose deaths in Rhode Island increased by 28.1% (from 29.2 per 100,000 person-years in 2019 to 37.4 per 100,000 person-years in 2020; p = 0.009). Overdose deaths were higher among persons experiencing job loss (8% experienced job loss in 2019 vs. 16% in 2020; p = 0.01) and among male Medicaid beneficiaries with depression (22% vs. 38%; p = 0.008) and anxiety (23% vs. 37%; p = 0.02).
- Mass shootings in the US during the COVID-19 pandemic. Peña et al. JAMA Network Open (September 16, 2021). Publicly available data from the Gun Violence Archive showed an increase in mass shootings in the United States since the start of the pandemic (April 2020). Over 15 months, an estimated 343 more mass shootings occurred than were expected.
Note: Adapted from Peña et al. 28-day, daily mass shootings in the United States. Mass shootings are defined as shootings in which 4 or more people were killed or injured, excluding the perpetrator. The moving mean includes less than 28 days but at least 14 days at the start and the end of the period covered. Licensed under CC BY.
- Comparison of RT-PCR cycle threshold values from respiratory specimens in symptomatic and asymptomatic children with SARS-CoV-2 infection. Strutner et al. Clinical Infectious Diseases (September 15, 2021). Respiratory specimens obtained from 728 SARS-CoV-2 positive pediatric patients (ages 0–18 years) from April–August 2020 showed symptomatic children had lower mean Ct values than asymptomatic children, and children aged <5 years had the lowest Ct value compared with children of other age groups.
Prevention Strategies and Non-Pharmaceutical Interventions
- Impact of the fitness of N95 masks with extended use/limited reuse and dry heat decontamination. Zha et al. Journal of Investigative Medicine (September 13, 2021). A study on the impact of substandard use of N95 masks (3M9211 brand) with dry heat decontamination (30 min at 750C of high velocity hot air) determined that limited reuse and modified extended use, when carefully enforced, can preserve the fitness of N95 masks up to 85%. The frequency of mask donning/doffing during a work shift and whether a fit test is performed before decontamination both impact the extended preservation of mask fitness.
- Water shutoff moratoria lowered COVID-19 infection and death across U.S. states. Zhang et al. American Journal of Preventive Medicine (September 10, 2021). SARS-CoV-2 infection and COVID-19 death growth rates, from April 17–December 31, 2020, were lower in states with moratoria on water service shutoffs (infections: 0.235% lower; deaths: 0.135% lower) compared to those with no moratoria, after adjusting for mask mandates and time of pandemic. Uninterrupted access to water may have prevented loss of essential prevention measures in communities at risk of both higher COVID-19 transmission and water disconnection.
Transmission Risk and Dynamics
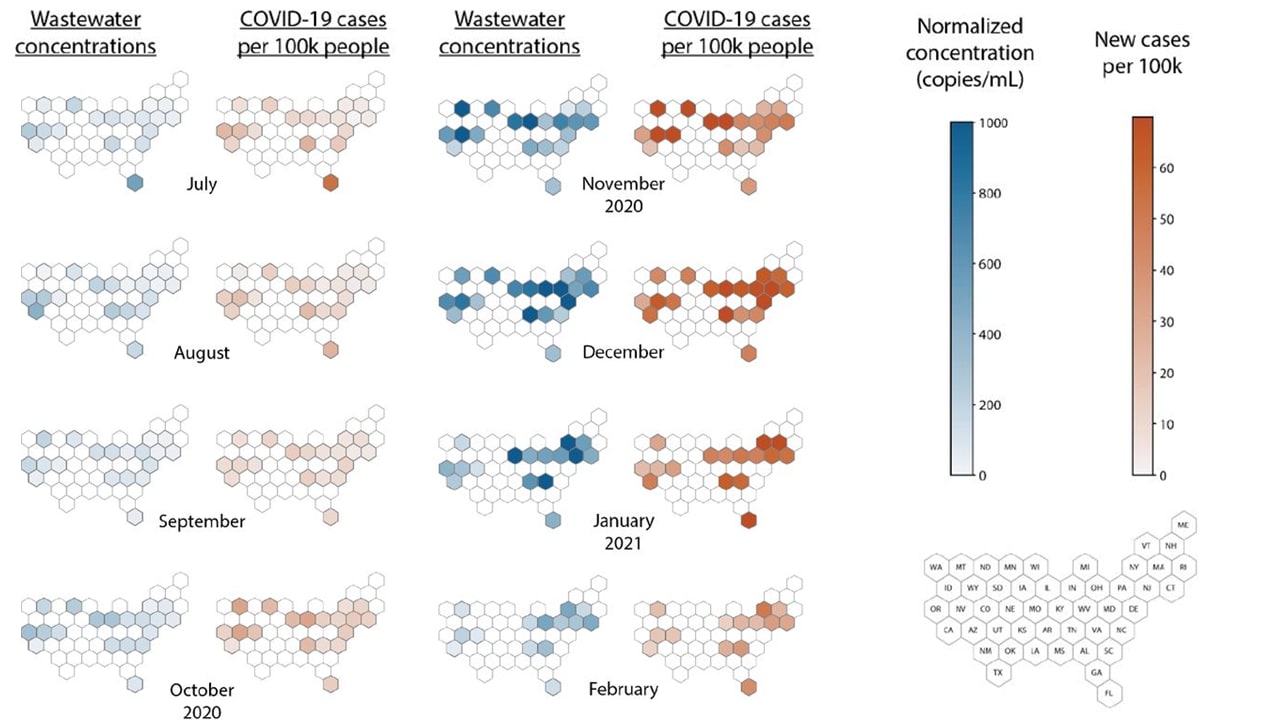
- Nationwide trends in COVID-19 cases and SARS-CoV-2 wastewater concentrations in the United States. Duvallet et al. medRxiv (Preprint; September 12, 2021). In a time-series analysis of wastewater facilities (n = 55) with >1 sample/month for ≥ 6 months across 19 states (June 2020–May 2021), SARS-CoV-2 concentrations in wastewater showed similar relative patterns across states as did clinical cases (median Spearman correlation across states = 0.6; IQR = 0.52 to 0.81). Concentrations closely followed the 7-day average of new clinical cases.
Note: Adapted from Duvallet et al. Monthly geographic trends in wastewater SARS-CoV-2 concentrations and COVID-19 cases. Monthly state-level normalized wastewater SARS-CoV-2 concentrations. Monthly state-level new COVID-19 cases per 100,000 people for counties with at least 1 wastewater sampling location in that month. Licensed under CC-BY-NC 4.0.
From the Morbidity and Mortality Weekly Report (September 24, 2021).
- Decreased Incidence of Infections Caused by Pathogens Transmitted Commonly Through Food During the COVID-19 Pandemic — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2017–2020
- Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults without Immunocompromising Conditions — United States, March–August 2021
- Use of Pfizer-BioNTech COVID-19 Vaccine in Persons Aged ≥16 Years: Recommendations of the Advisory Committee on Immunization Practices — United States, September 2021
- Outbreak of SARS-CoV-2 B.1.617.2 (Delta) Variant Infections Among Incarcerated Persons in a Federal Prison — Texas, July–August 2021
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.