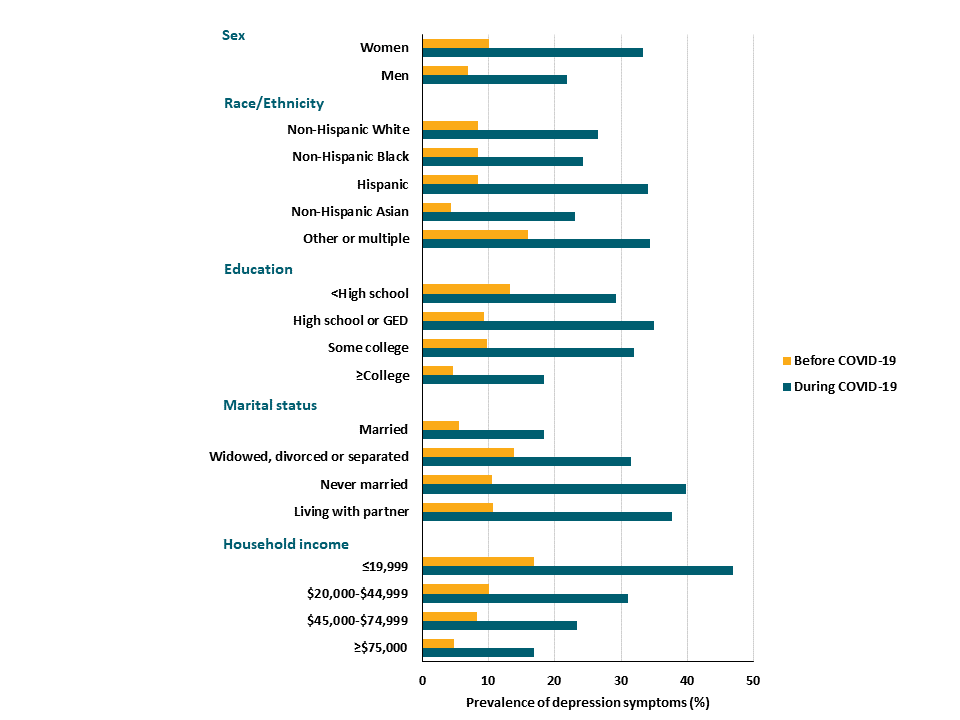COVID-19 Science Update released: September 11, 2020 Edition 47

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
Below we present three randomized clinical trials (RCTs) and a meta-analysis that contributed to the inclusion of corticosteroids into standard of care for COVID-19-related ARDS.
PEER-REVIEWED
A. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trialexternal icon. Tomazini et al. JAMA (September 2, 2020).
Key findings:
- Patients in the dexamethasone group plus standard care had significantly more ventilator-free days in the first 28 days, 6.6 days (95% CI 5.0-8.2) than the standard care alone group, 4.0 days (95% CI 2.9-5.4), (p = 0.04).
- Mean Sequential Organ Failure Assessment (SOFA) score was significantly lower in the dexamethasone group at 7 days (6.1; 95% CI 5.5-6.7) vs standard care (7.5; 95% CI 6.9-8.1; p = 0.004), indicating lower organ dysfunction in the dexamethasone group.
- There was no significant difference in all-cause mortality at 28 days for the dexamethasone group (56.3%) vs the standard care group (61.5%), hazard ratio 0.97 (95% CI 0.72-1.31).
- Dexamethasone was not associated with increased risk of adverse events.
Methods: Randomized, open-label, multi-center clinical trial of COVID-19 patients with moderate-to-severe ARDS receiving mechanical ventilation in 41 intensive care units in Brazil between April and July 2020, to evaluate the efficacy of intravenous (IV) dexamethasone plus standard care (n = 151) vs standard care only (n = 148). Primary endpoint was ventilator-free days during the first 28 days; secondary endpoints included all-cause mortality at 28 days and SOFA score. Limitations: Small sample size and underpowered for secondary outcomes; study halted early after results of the RECOVERY trialexternal icon were released.
B. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19. A randomized clinical trial.external icon Dequin et al. JAMA (September 2, 2020).
Key findings:
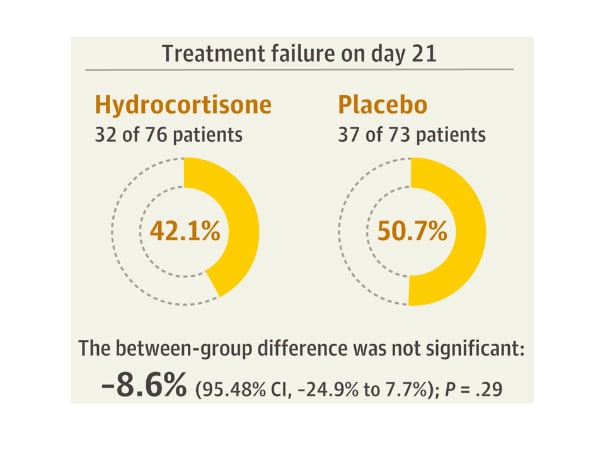
- There was no difference in treatment failure at day 21 in patients who received hydrocortisone vs placebo (Figure).
- There were 11 deaths in the hydrocortisone group and 20 deaths in the placebo group (p = 0.057).
- There was no significant difference in the groups for any secondary outcome and no adverse events were reported.
Methods: The Community-Acquired Pneumonia: Evaluation of Corticosteroids in Coronavirus Disease (CAPE COVID) trial was a multicenter randomized, placebo-controlled trial of low-dose hydrocortisone vs placebo of 149 adult patients admitted to the intensive care unit for COVID-19–related acute respiratory failure in France between March and June 2020. Primary endpoint was treatment failure on day 21, defined as death or continued dependence on mechanical ventilation or high-flow oxygen therapy. Limitations: Study halted early after results of the RECOVERY trialexternal icon were released; sample size was small and underpowered for primary outcomes.
Figure:
Note: Adapted from Dequin et al. Treatment failure on day 21 for hydrocortisone and placebo groups. Reproduced with permission from JAMA. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19. A randomized clinical trial. Published online September 2, 2020. doi:10.1001/jama.2020.16761 Copyright©2020 American Medical Association. All rights reserved.
C. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19. The REMAP-CAP COVID-19 corticosteroid domain randomized clinical trialexternal icon. Writing Committee for REMAP-CAP Investigators. JAMA (September 2, 2020).
Key findings:
- Compared to no hydrocortisone, the fixed-dose regimen, OR = 1.43 (95% CI 0.91-2.27), and the shock-dependent regimen, OR = 1.22 (95% CI 0.76-1.94), resulted in fewer days on organ support.
- For a fixed-dose steroid regimen, there was a 93% probability of an OR >1 for organ-free support days; for the shock-dependent regimen, the probability of an OR >1 was 80%.
- Serious adverse events were reported in 4 (3%), 5 (3%), and 1 (1%) of the patients in the fixed-dose, shock-dependent, and no hydrocortisone groups, respectively.
Methods: A randomized open label trial of multiple interventions at 21 sites in 8 countries. Patients with severe COVID-19 were randomized to fixed-dose (7-days of IV hydrocortisone 50 mg every 6 hours, n = 143), shock-dependent dose (50 mg hydrocortisone every 6 hours while in shock for up to 28 days, n = 152), or no hydrocortisone (n = 108). The primary end point was organ support–free days (i.e., days alive and free of intensive care unit-based respiratory or cardiovascular support) within 21 days. Limitations: Study halted early after results of the RECOVERY trialexternal icon and another trial were released; 15% of the no-hydrocortisone group received systemic corticosteroids.
D. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. A meta-analysisexternal icon. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. JAMA (September 2, 2020)
Key findings:
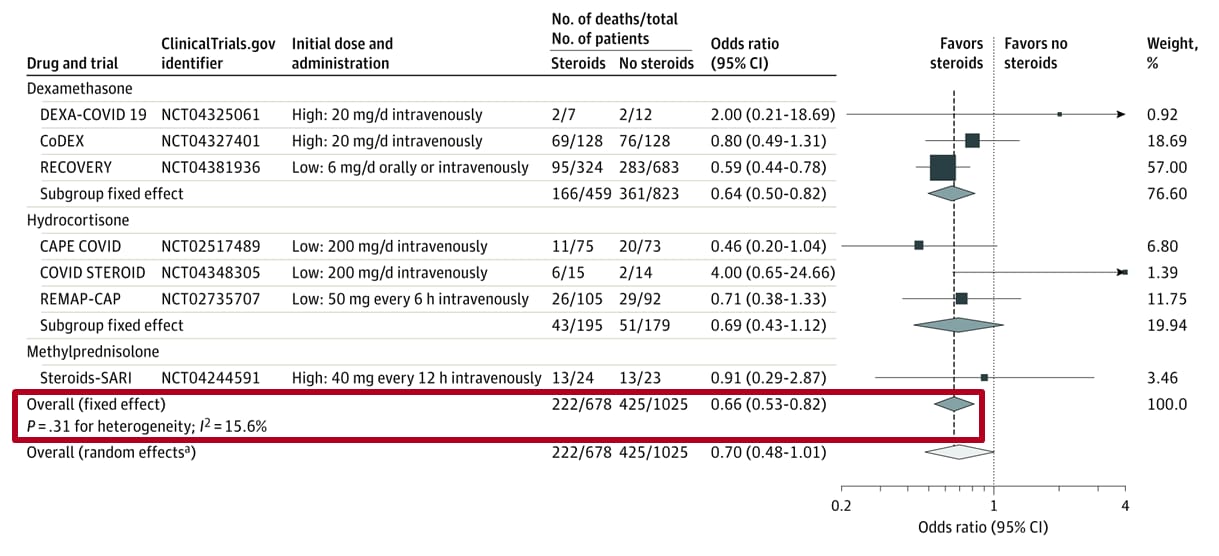
- 28-day all-cause mortality was lower among patients who received corticosteroids compared with those who received usual care or placebo (summary odds ratio, 0.66), (Figure).
Methods: Prospective meta-analysis of pooled data from 7 RCTs (including the above trials: CoDEX trial, CAPE COVID trial and REMAP-CAP trial) to estimate the association between administration of corticosteroids vs usual care or placebo among ciritcally ill patients with COVID-19. Main outcome was 28-day all-cause mortality post randomization. Limitations: Inconsistent definitions and reporting of adverse events across trials; outcomes censored once patients discharged from the hospital; one trial contributed 57% of weight to outcome of all-cause mortality.
Figure:
Note: Adapted from the REACT Working Group. Association between corticosteroids and 28-day all-cause mortality in each trial, overall, and according to corticosteroid drug. The area of the data marker for each trial is proportional to its weight in the fixed-effects meta-analysis. Reproduced with permission from JAMA. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. A meta-analysis. Published online September 2, 2020. doi:10.1001/jama.2020.17023 Copyright©2020 American Medical Association. All rights reserved.
Implications for 4 studies (Tomazini et al., Dequin et al., REACT Working Group & Writing Committee for the REMAP-CAP Investigators): The results of individual studies and the meta-analysis have shifted usual care of persons with COVID-19 to include corticosteroids. An accompanying editorialexternal icon (Prescott, JAMA, 2020) notes actions and collaborations among researchers undertaken to overcome challenges that have been encountered in the context of the pandemic. It describes the importance of analysis of pooled data, especially when ongoing randomized controlled trials were halted early as in the three studies reported here.
PEER-REVIEWED
Humoral immune response to SARS-CoV-2 in Iceland.external icon Gudbjartsson et al. NEJM (September 1, 2020).
Key findings:
- Estimated seroprevalence in Icelanders was 0.9% (95% CI 0.8-0.9), and the estimated infection fatality risk was 0.3% (95% CI 0.2-0.6).
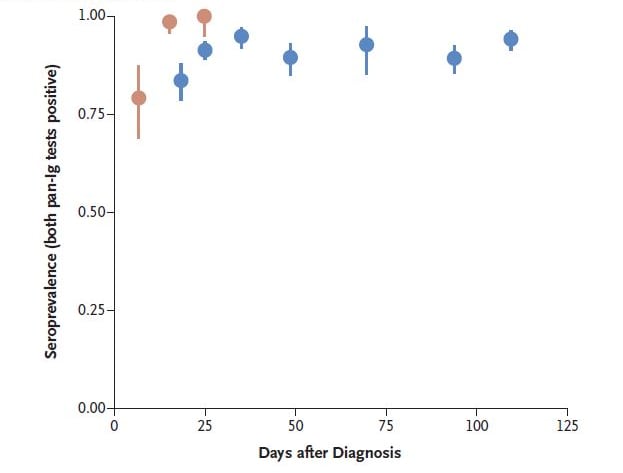
- 91.1% (95% CI 89.4-92.6) of 1,215 people who had recovered from infection had detectable antibodies to both nucleocapsid (N) and spike (S1) proteins (Figure).
- 95.1% (95% CI 93.8-96.3) had detectable antibodies to at least one protein.
- Antibody response was stable up to 4 months after infection (Figure).
Methods: Antibody serum testing in 30,576 people, Iceland, between February 18 and July 8, 2020. Specimens collected in 2017 served as negative controls. Seroprevalence estimates were weighted by region, sex and age. Limitations: Numbers of persons tested with SARS-CoV-2 were inconsistent in methods and article Figure 1 and Table 1; findings might not be externally generalizable.
Implications: Seroprevalence surveys may be useful to estimate prevalence of SARS-CoV-2 infection in populations, as discussed in Alter et al., The power of antibody-based surveillanceexternal icon. Future research should examine the duration of detectable antibodies, risk of reinfection, and thereby potential serologic correlates of immunity.
Figure:
Note: From Gudbjartsson et al. Dots are seroprevalence estimates post SARS-CoV-2 diagnosis in hospitalized patients and recovered people. Vertical lines represent 95% CI. From NEJM. Humoral immune response to SARS-CoV-2 in Iceland. Gudbjartsson et al. DOI: 10.1056/NEJMoa2026116. Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
What protective health measures are Americans taking in response to COVID-19? Results from the COVID impact survey.external icon Qeadan et al. International Journal of Environmental Research and Public Health (August 29, 2020).
Key findings:
- The three most common protective behaviors taken were washing hands (94.7%), keeping 6 feet away from persons outside the household (90.1%), and wearing a face mask (86.2%).
- Persons with a close friend or family member who died of COVID-19 were more likely to have taken protective measures than those who did not: adjusted incidence rate ratio (aIRR) in April = 1.04 (95% CI 1.00-1.08); May = 1.02 (95% CI 0.99-1.06); June = 1.16 (95% CI 1.11-1.20).
- Females, persons of non-White race, persons living in larger households, older adults, and persons with comorbidities took more protective measures.
Methods: Randomly selected households in 10 states participated in three independent cross-sectional surveys to assess use of individual protective measures to safeguard against SARS-CoV-2 infection during April, May, and June 2020. One member per household (totaling 25,269 adults) was asked about protective measures, demographic information, and comorbidities. Limitations: Information was self-reported and subject to recall bias; survey didn’t have national coverage and thus potentially limited generalizability.
Implications: Most Americans were aware of and reported following recommended personal protective measures, particularly hand washing, physical distancing, and wearing a face mask.
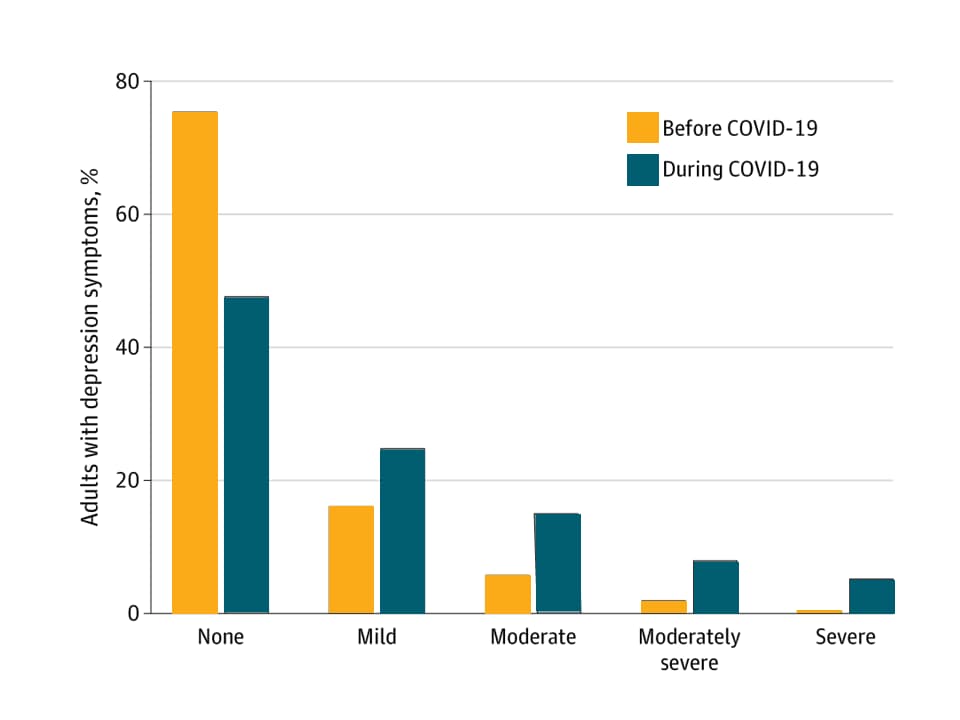
Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic.external icon Ettman et al. JAMA (September 2, 2020).
Key findings:
- During the COVID-19 pandemic period (March-April), depression symptoms were reported three times more often across all demographic groups than during a comparison pre-pandemic period (Figure 1).
- 27.8% of persons had depression symptoms during the pandemic period, compared with 8.5% who had symptoms before the pandemic.
- Severity of depression symptoms was also greater during the pandemic period compared with before (Figure 2).
- Lower income, <$5,000 in savings, or experiencing COVID-19-related stressors (e.g., losing a job, knowing someone who died of COVID-19, having financial problems) were all associated with increased depression.
Methods: Data were collected about depression symptoms during a nationally representative survey of 1,441 US adults conducted from March 31 to April 13, 2020 and were compared with data collected during the nationally representative 2017-2018 National Health and Nutrition Examination Survey. Both surveys used the Patient Health Questionnaire-9 in to assess for depression symptoms. Limitations: Two cross-sectional data sources compared that might lead to between-group differences; small sample size for certain demographic groups.
Implications: Prevalence of depression symptoms has increased three-fold since prior to the pandemic. Mental health resources should be allocated for persons at higher risk of depression, particularly those with lower social and economic resources.
Figure 1
Note: Adapted from Ettman et al. Prevalence of depression symptoms by demographic characteristic Before and During the COVID-19 pandemic. Licensed under CC-BY.
Figure 2
Note: Adapted from Ettman et al. Depression symptoms in US adults Before and During the COVID-19 pandemic.
PEER-REVIEWED
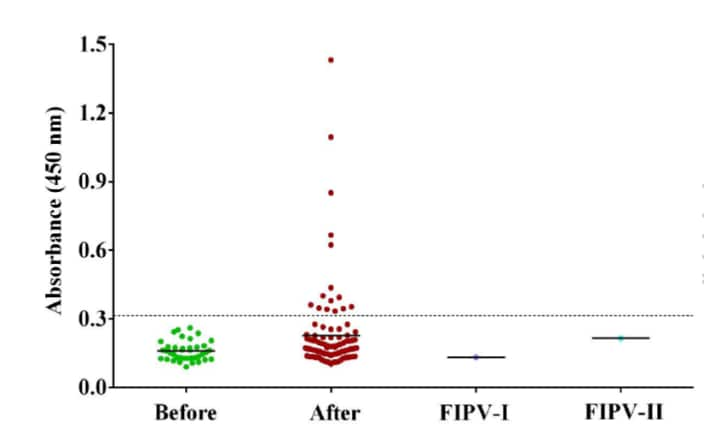
A serological survey of SARS-CoV-2 in cats in Wuhan.external icon Zhang et al. Emerging Microbes & Infections (September 1, 2020).
Key findings:
- A serological survey of cats found that 15/102 (14.7%) had antibodies specific to SARS-CoV-2, and 11/102 (10.8%) had neutralizing antibodies (Figure).
- None of the 15 positive samples cross-reacted with type I or II feline infectious peritonitis virus, a feline coronavirus.
- Samples from 39 cats collected in 2019 were negative for SARS-CoV-2 antibodies.
- The three cats with the highest antibody levels to the SARS-CoV-2 S protein receptor binding domain (RBD) lived with individuals with COVID-19; neutralizing antibodies were found in 2 of the 3 cats.
- Two cats whose owners were positive for COVID-19 had antibody levels that decreased to undetectable levels by 110 days after sampling began, a similar pattern was seen for neutralizing antibodies.
Methods: Between January and March 2020, 102 cats were sampled from animal shelters (n = 46), pet hospitals (n = 41), and families with COVID-19 (n = 15), Wuhan, China. Each cat had blood, NP swabs, and anal swabs collected. Sera collected from 39 cats from March to May 2019 were used as negative controls. Antibodies against the recombinant receptor binding domain (RBD) of SARS-CoV-2 spike protein were measured with an immunoassay and antibody-positive samples were tested for virus neutralization.
Implications: SARS-CoV-2 infection in some animals such as cats appears to have been introduced by humans in close proximity. Further study of zoonotic transmission is an important area for surveillance to identify potential reservoirs and determine risk factors for people and animals.
Figure:
Note: From Zhang et al. Immunoassay of cat serum samples. The x-axis demonstrates sera from 2019 (Before), sera from January-March 2020 (After), and activity against Feline Infectious Peritonitis Virus I (FIPV-1), and II (FIPV-2). Dotted line represents the cutoff for positive samples. The y-axis is the optical density measurement at 450 nm and represents antibody concentration. Licensed under CC-BY.
PEER-REVIEWED
Acute arterial thromboembolism in patients with COVID-19 in the New York City areaexternal icon. Etkin et al. Annals of Vascular Surgery (August 27, 2020).
Key findings:
- 49 COVID-19 patients had arterial thromboembolism diagnosed during hospitalization (median age 67 years [IQR 58-75]).
- 22 (45%) COVID-19 patients had acute arterial ischemia on presentation and were subsequently diagnosed with COVID-19.
- The most common preexisting conditions were hypertension (53%) and diabetes (35%); 18% were current or former smokers.
- 21 (46%) patients died in the hospital.
- Mortality in 35 patients with lower limb ischemia was 50% and in 2 patients with mesenteric ischemia was 100%.
- 9 (18%) patients experienced limb loss.
Methods: Retrospective multisite study of 12,630 hospitalized patients with confirmed COVID-19 over 11 weeks (March 1, 2020 to May 15, 2020) in New York State. Limitations: Not randomized, (no control group); limited clinical laboratory information; descriptive analyses only; study cohort might have had underlying risks for thromboembolism prior to hospital admission.
Implications: In persons with COVID-19, morbidity and mortality were greater in the presence of arterial thromboembolism, particularly for patients with chronic medical comorbidities. Efforts are warranted to identify the most effective preventive and treatment strategies to reduce thromboembolic risk among persons with COVID-19 and to rapidly detect and address arterial thromboembolism.
PEER-REVIEWED
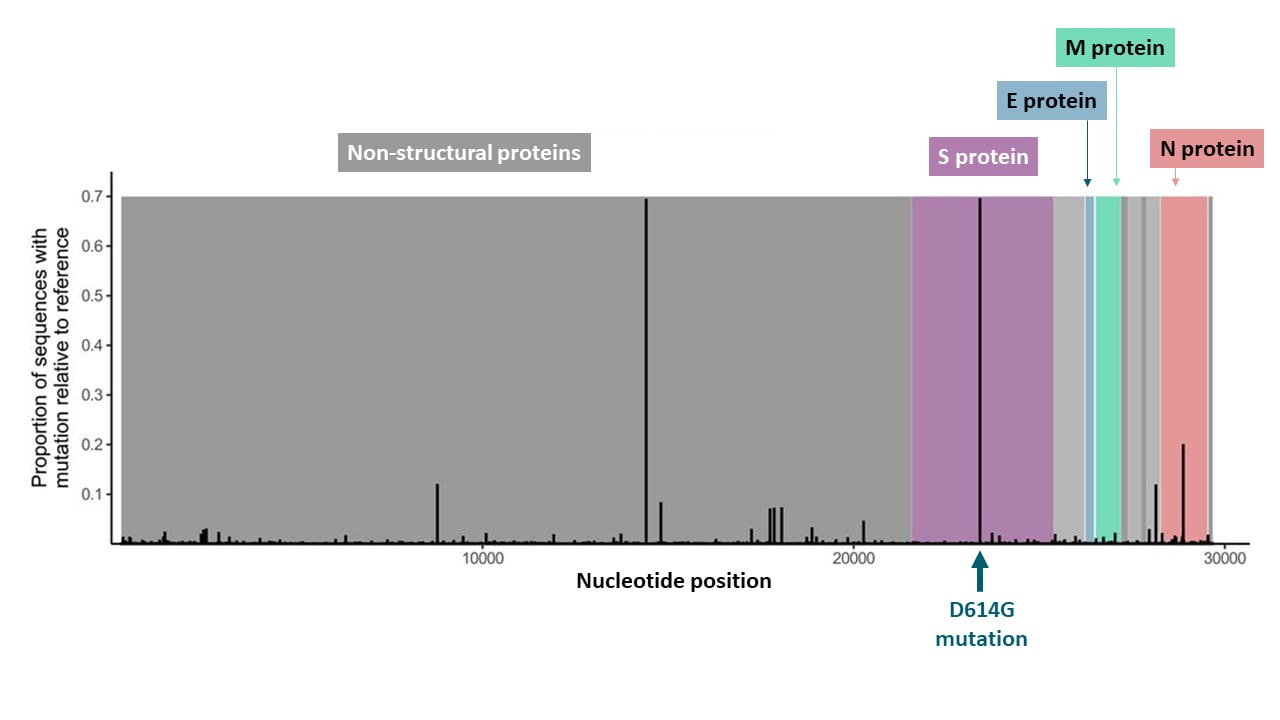
A SARS-CoV-2 vaccine candidate would likely match all currently circulating variantsexternal icon. Dearlove et al. Proceeding of National Academy of Sciences (August 31, 2020).
Key findings:
- An analysis of 18,514 SARS-CoV-2 genome sequences observed limited viral diversity.
- 7,559 polymorphisms were detected (Figure).
- 95% of sequences had 11 mutations or fewer.
- The D614G mutation (found in 69.4% of sequences) in the spike (S) protein became dominant due to its presence in the early European cases (i.e., a founder effect), rather than adaption to host responses.
- The D614G mutation was not predicted to interfere with vaccine-induced antibody binding.
Methods: Investigators analyzed SARS-CoV-2 genome sequences from infected persons in 84 countries as part of the Global Initiative on Sharing All Influenza Data to assess naturally occurring viral diversity compared with the sequence on which most vaccine candidates are based (reference sequence).
Implications: Current SARS-CoV-2 vaccine candidates are designed to elicit neutralizing antibodies against the S protein and are based on viral sequences derived early in the pandemic. Emergence of the D614G mutation raised concerns that SARS-CoV-2 strains may evolve and escape vaccine-induced immunity. The limited evolution of viral diversity observed thus far suggests these vaccines should provide broad coverage for current and future SARS-CoV-2 strains. A news feature from Callaway et al.external icon further describes research on viral mutations, particularly D614G, and how the slow mutation rate affects future vaccines.
Figure:
Note: Adapted from Dearlove et al. Location and proportion of polymorphisms across the genome. Proportion of sequences that showed polymorphisms compared to the reference sequence. Nonstructural proteins are shown in gray. Structural proteins are shown in color (Spike; Envelope; Membrane; Nucleocapsid). Licensed under CC-BY 4.0.
SARS-CoV-2 Detection
- Ganguli et al. Rapid isothermal amplification and portable detection system for SARS-CoV-2external icon. Proceedings of the National Academy of Sciences of the United States of America. The authors describe point of care testing for SARS-CoV-2 that has 100% agreement with RT-PCR testing done in a laboratory.
- Regen et al. A simple approach to optimum pool size for pooled SARS-CoV-2 testing.external icon International Journal of Infectious Diseases. Pooling samples for RT-PCR analysis is optimized when the number of samples used is based on the prevalence in the community; a formula is provided to calculate optimum sample size.
- Ehre C. SARS-CoV-2 infection of airway cells.external icon Electron micrographs show bronchial epithelial cells that have been infected by SARS-CoV-2 in a laboratory setting.
Mental Health
- Shim R. Mental health inequities in the context of COVID-19.external icon JAMA Network Open. To mitigate the effects of depression during the pandemic, policy makers and health care providers should address social determinants of mental and physical health.
- Wu et al. Prevalence and risk factors of mental distress in China during the outbreak of COVID-19: A national cross-sectional survey.external icon Brain and Behavior. A survey assessing 24,789 respondents in China found an association between lack of exercise, low income, low education, inadequate supplies, and psychological distress.
Preventing transmission
- Mooney G. “A Menace to the Public Health” — Contact tracing and the limits of persuasion.external icon Contact tracers are more likely to be successful if they have the ability to provide quarantined persons with community assets to support them during quarantine.
- Sehra et al. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US.external icon JAMA Internal Medicine. Counties with reduction in cell phone activity at the workplace, in retail locations, and on public transit and increases in at-home activity had larger reductions in COVID-19 cases 5, 10, and 15 days later.
Vaccine development
- Deming et al. Accelerating development of SARS-CoV-2 vaccines — The role for controlled human infection models.external icon A SARS-CoV-2 controlled human infection model might accelerate later rounds of vaccine development and elucidate immunopathogenesis, duration of vaccine-induced immunity, and correlates of infection.
Other Topics
- Negrini et al. Rehabilitation and COVID-19: the Cochrane Rehabilitation 2020 rapid living systematic review.external icon European Journal of Physical and Rehabilitation Medicine. Update to the first systematic review performed by the authors summarizes 23 papers, noting neurological effects and breathlessness in patients recovering from COVID-19.
- Bicudo et al. Co-infection of SARS-CoV-2 and dengue virus: A clinical challenge.external icon Brazilian Journal of Infectious Diseases. Case of laboratory-confirmed SARS-CoV-2 and dengue coinfection, diseases with overlapping clinical presentations, in a 56-year old female in Brazil.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.