COVID-19 Science Update released: August 21, 2020 Edition 41

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
As the global COVID-19 pandemic matures, population-level antibody prevalence estimates and as well as those in healthcare workers (HCW) are needed to gauge priority prevention needs and assess potential herd immunity. Below, we summarize three papers, two focusing upon national seroprevalence estimation and one upon seroprevalence in HCW.
PEER-REVIEWED
A. Usability and acceptability of home-based self-testing for SARS-CoV-2 antibodies for population surveillance.external icon Atchison et al. Clinical Infectious Diseases (August 12, 2020).
Key findings:
- Most volunteers (86.5%) in the pilot study managed to complete the self-test. Reported difficulties included using the pipette and lancet, creating a drop of blood, and applying the blood to the sample well.
- For the usability study that included improvements based on the pilot test, 97% of users completed the two different lateral flow immunoassay (LFIA) self-tests
- Acceptability and usability were high for both LFIA self-tests.
- Few invalid tests were reported, (LFIA1 7.4%, LFIA2 4.8%).
- High concordance between physician and participant interpretation of results with kappas 0.72 (95% CI 0.71-0.73) and 0.89 (95% CI 0.88-0.92) for two tests used, both p <0.001.
Methods: A pilot study of self-testing for SARS-CoV-2 antibodies among 315 volunteers followed by a usability study of 14,400 persons using one of two LFIA for SARS-CoV-2 antibody self-tests, England, May, 2020. Limitations: Study participants might not be representative of the population.
B. Prevalence of SARS-CoV-2 antibodies in health care personnel in the greater New York area.external icon Moscola, et al. JAMA (August 6, 2020).
Key findings:
- 13.7% of healthcare workers (HCW) tested antibody-positive; with prevalence comparable to that of randomly sampled adults in New York State (14.0%).
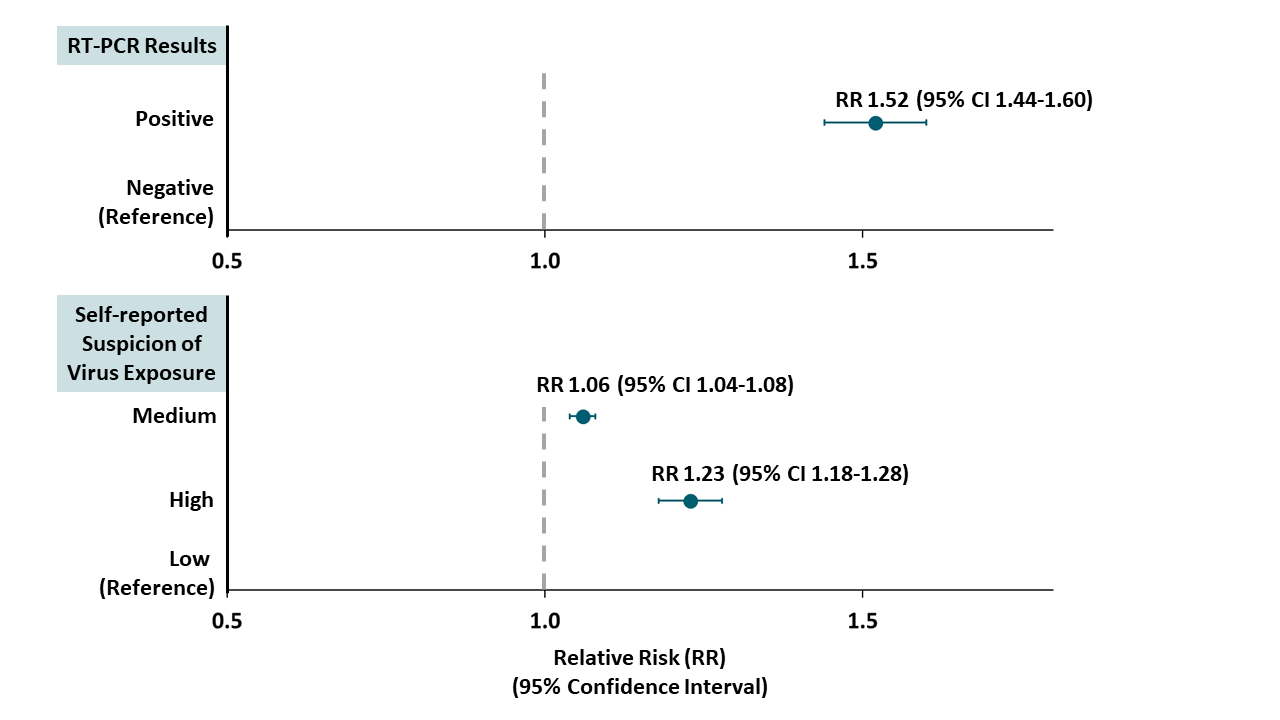
- Previous positive RT-PCR test and high suspicion of infection were associated with seropositivity (Table).
- There were no significant differences in seropositivity rates across age, race, job function, work location, direct patient care, or COVID-19 patient care.
Methods: Antibody testing performed in 40,329 HCW, between April 20 and June 23, 2020, greater New York City area. Limitations: Only 56% of HCW offered testing accepted; multiple assays used; potential selection bias; high percentage of missing data for some variables; multiple possible sources of exposure (home, community, work).
Figure:
Note: Adapted from Moscola et al. Adjusted relative risk (RR) for detection of antibodies in HCW reporting a prior positive RT-PCR test (top panel) and reporting medium or high suspicion of virus exposure (bottom panel). Dots represent point estimates; bars represent 95% CI. Reproduced with permission from JAMA. doi:10.1001/jama.2020.14765. Copyright©2020 American Medical Association. All rights reserved.
PREPRINTS (NOT PEER-REVIEWED)
Antibody prevalence for SARS-CoV-2 following the peak of the pandemic in England: REACT2 study in 100,000 adultsexternal icon. Ward et al. medRxiv (August 14, 2020).
Key findings:
- 86.6% completed the self-test they were provided; 5.4% reported an invalid or unreadable result.
- Adjusted prevalence was 6.0% (95% CI 5.8-6.1), equivalent to a total 3.36 million persons (95% CI 3.21-3.51) infected with SARS-CoV-2 by end of June 2020 in England.
- Estimated overall infection fatality ratio was 0.90% (95% CI 0.86-0.94).
- Age, type of employment, ethnicity and household size were associated with increased odds of seropositivity (Figure).
- Of those who were antibody-positive, 61.4% (95% CI 60.1-62.7) reported more than one symptom (fever, persistent cough, loss of taste or smell).
- A greater number of symptoms was associated with greater odds of seropositivity.
Methods: Nationally representative seroprevalence study among 129,976 adults who self-tested for SARS-CoV-2 antibodies, England, June 10 to July 13, 2020. Prevalence estimates were adjusted for test performance and sociodemographic sampling weights. Limitations: Response rate varied by ethnicity and socioeconomic status.
Figure:
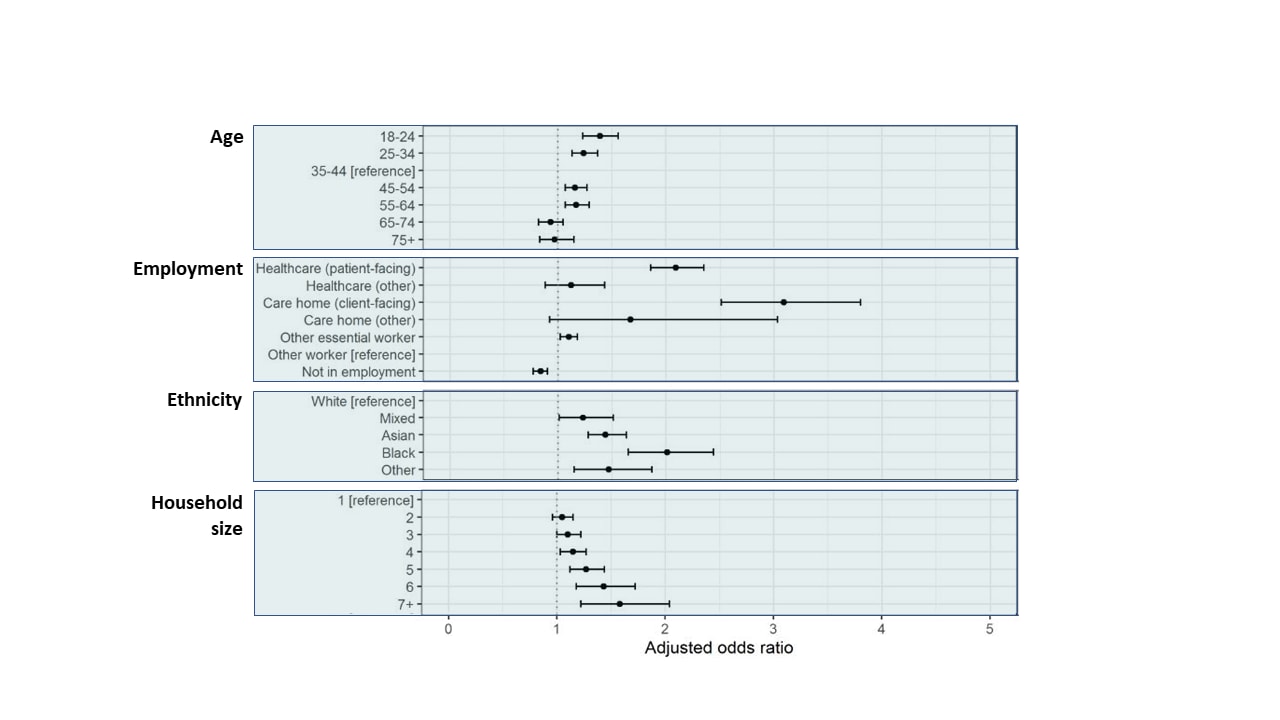
Note: Adapted from Ward et al. Adjusted odds of positive test for SARS-CoV-2 antibodies. Dots represent adjusted OR; bars represent 95% CI. Licensed under CC-BY-NC-ND.
Implications for 3 studies (Atchison et al., Moscola et al. & Ward et al.): National surveys of antibody prevalence provide assessments of populations at greatest risk for previous infection, which may be used to target prevention approaches and assess potential development of herd immunity. Self-testing for SARS-CoV-2 antibodies appears sufficiently acceptable and valid to support large scale serosurveys. Seroprevalence rates in HCW that were comparable to general population suggest availability and appropriate use of PPE can mitigate exposure risk among these essential workers (see Jeremias et al. Prevalence of SARS-CoV-2 among health care workers in a tertiary care hospital.external icon JAMA Internal Medicine).
PEER-REVIEWED
COVID-19 in Americans aboard the Diamond Princess cruise shipexternal icon. Plucinski et al. Clinical Infectious Diseases (August 12, 2020).
Key findings:
- Overall, 26% tested positive for SARS-CoV-2.
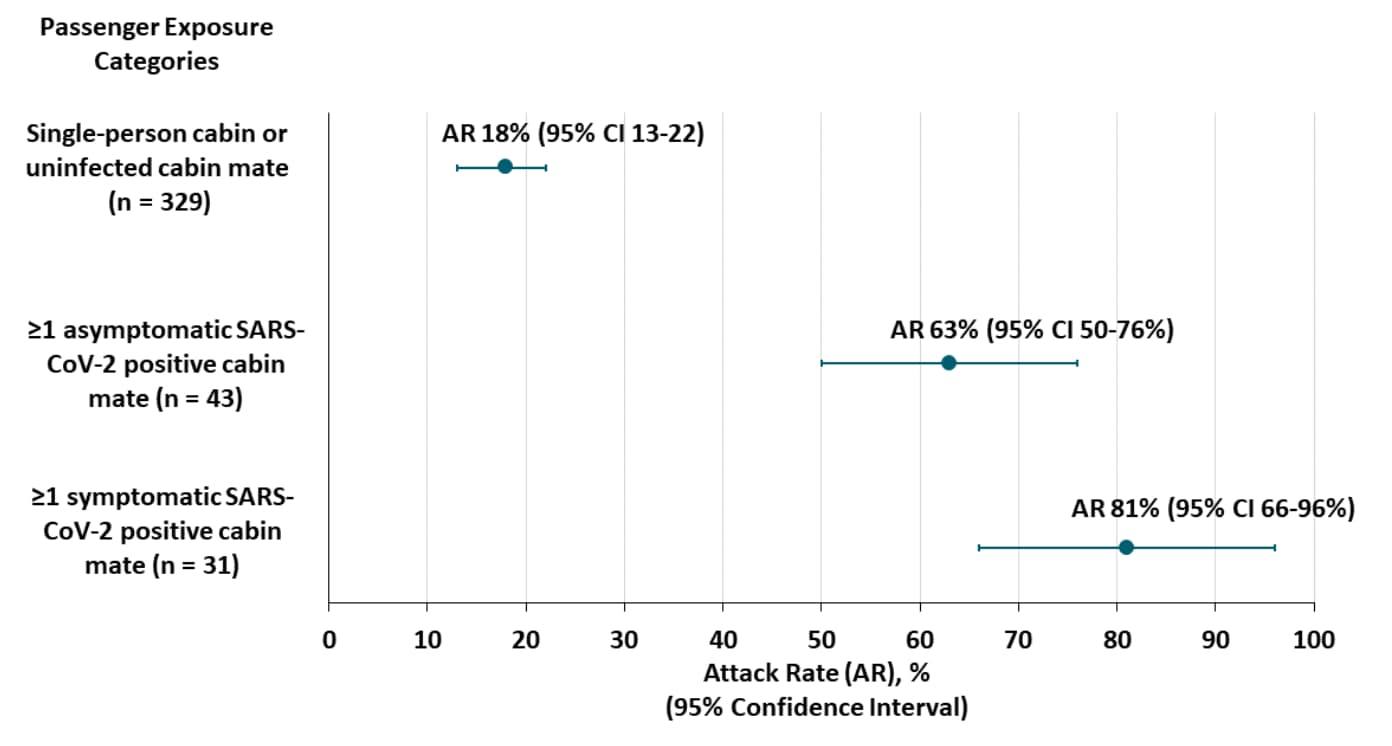
- Attack rates (AR) were higher for people who shared cabins with infected individuals (Figure).
- Among surveyed passengers, close contact with a case was associated with SARS-CoV-2 infection, adjusted odds ratio (aOR) 3.37 (95% CI 1.73-6.64).
Methods: Assessment of 437 American passengers and crew on the Diamond Princess, January 20 to February 17, 2020. Investigators collected RT-PCR results and cabin location for each passenger. A subset of passengers (n = 229) responded to a cross-sectional survey including an assessment of close contacts (defined as >15 minutes in a room with someone without a mask). Limitations: Survey response rate was 52%; very sick passengers were excluded; symptomatic persons may have been more likely to participate in the survey.
Implications: Both symptomatic and asymptomatic persons were likely sources of transmission. Data suggest prolonged exposure in small quarters increases transmission risk substantially.
Figure:
Note: Adapted from Plucinski et al. Attack rate (AR) on board the Diamond Princess stratified by exposure to infected passengers. Dots indicate AR; bars indicate 95% CI. Crew were excluded from AR calculations because their exposures differed from passengers. US Government work not subject to copyright.
Association between youth smoking, electronic cigarette use, and coronavirus disease 2019.external icon Gaiha et al. Journal of Adolescent Health (August 11, 2020).
Key findings:
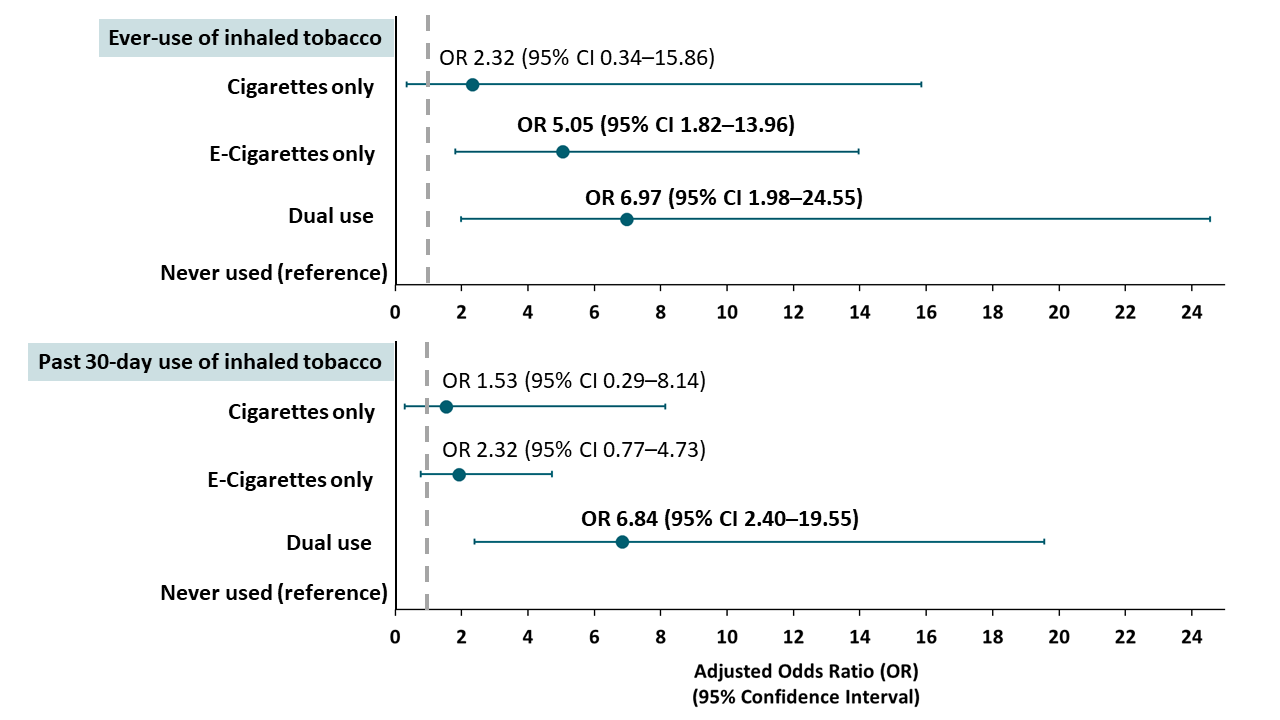
- Compared to adolescents and young adults who had never used inhaled tobacco, those who had ever used electronic cigarettes (e-cigarettes), with or without tobacco cigarette use, were more likely to report being diagnosed with COVID-19 (Figure).
- Reported diagnosis with COVID-19 did not differ between tobacco non-users and tobacco cigarette-only users (Figure).
- Tobacco users (all forms) were more likely to be tested for infection than were non-users.
- For tobacco cigarette use, aOR 3.94 (95% CI 1.43-10.86).
- For e-cigarette only, aOR 3.25 (95% CI 1.77-5.94).
- For e-cigarette use and tobacco cigarette use, aOR 3.58 (95% CI 1.96-6.54).
Methods: Online national-level cross-sectional survey in 4,351 U.S. adolescents and young adults, 13 to 24 years, May, 2020. Respondents reported history of testing and diagnosis of COVID-19, cigarette use and e-cigarette use. Limitations: Self-report data; obesity was the only measured comorbidity; testing more common in tobacco users.
Implications: Findings indicate that e-cigarette use appears to be a risk factor for adolescents and young adults. Targeted education is warranted.
Figure:
Note: Adapted from Gaiha et al. Associations between self-reported COVID-19 diagnosis and use of inhaled tobacco products. Dots represent point estimates; bars represent 95% CI. This article was published in Journal of Adolescent Health, Vol 67, Gaiha et al, Association between youth smoking, electronic cigarette use, and coronavirus disease 2019, Page 519-523, Copyright Society for Adolescent Health and Medicine 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
COVID-19 and the changes in sexual behavior of men who have sex with men: Results of an online surveyexternal icon. Shilo et al. Journal of Sexual Medicine (August 10, 2020).
Key findings:
- 90.0% of respondents reported casual sex before social distancing requirements.
- 39.5% reported casual sex before and during social distancing requirements.
- Casual sex was associated with single status, odds ratio (OR) 1.65 (95% CI 1.3-2.1) and higher levels of mental distress, OR 1.5 (95% CI 1.3-1.8).
- Frequency of phone or webcam use for sex increased during social distancing requirements, p < 0.001.
Methods: Anonymous cross-sectional survey about sexual practices and mental health in 2,562 Israeli men who have sex with men (MSM) >18 years, March to April, 2020 during social distancing requirements in Israel. Limitations: Convenience sample; conclusions limited to MSM.
Implications: MSM with a history of casual sex appear willing to decrease this activity during social distancing requirements, although mental health distress in this group might inhibit adherence to social distancing.
Obesity and mortality among patients diagnosed with COVID-19: Results from an integrated health care organizationexternal icon. Tartof et al. Annals of Internal Medicine (August 12, 2020).
Key findings:
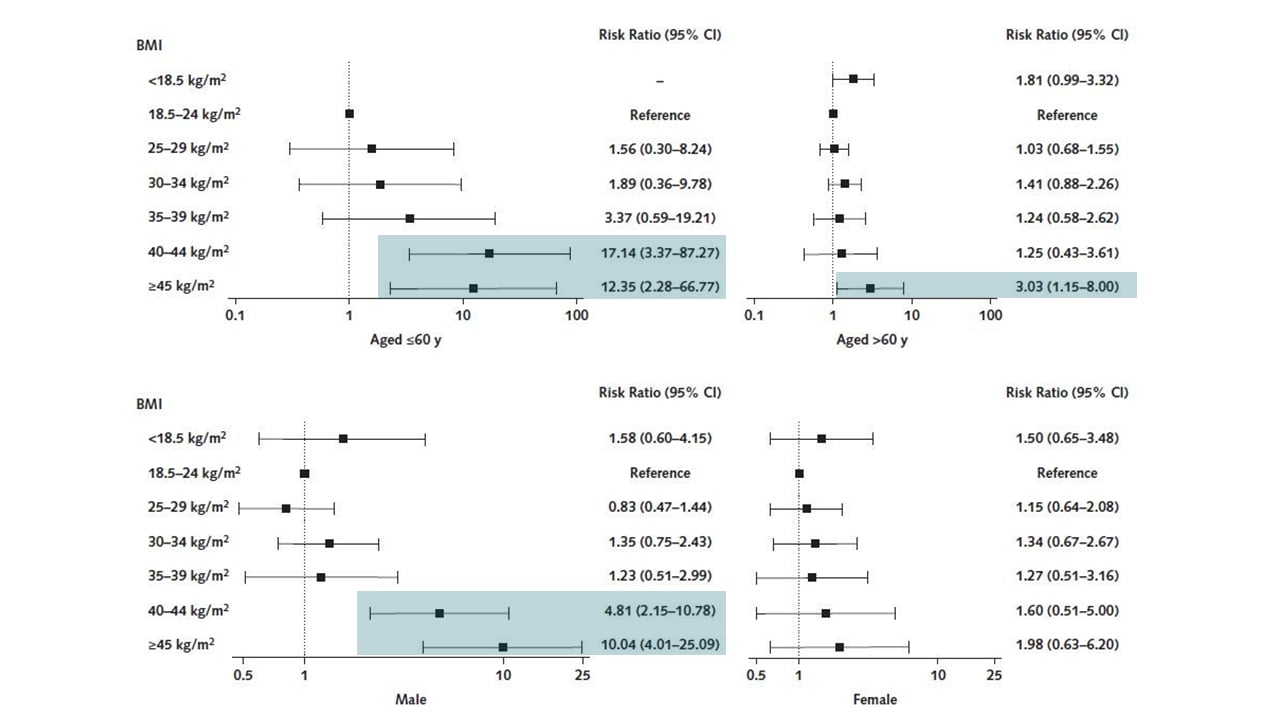
- Body mass index (BMI) ≥45 kg/m2, compared with BMI 18.5 to 24.0, was associated with greater risk for death: adjusted risk ratio (RR) 4.18 (95% CI 2.12-26).
- High BMI was more closely associated with death in persons aged ≤60 years, compared with patients aged >60 years and in men, compared with women (Figure).
Methods: Retrospective cohort of 6,916 Kaiser Permanente Southern California members diagnosed with COVID-19, February 13 to May 2, 2020. Primary outcome was death up to 21 days after the diagnostic code or lab-confirmed positive test in the patient record. Data were adjusted for race/ethnicity, comorbidities, and social determinants of health. Limitations: Mortality after 21 days was not recorded; BMI measurements were up to four years prior to enrollment.
Implications: Severe obesity is associated with substantially increased risk of death in men and patients <60 years. Vigilance with care and targeted prevention efforts to reduce infection rates may mitigate COVID-associated mortality among severely obese people.
Figure:
Note: Adapted from Tartof et al. Interaction of age and BMI on death (top row) and sex and BMI on death (bottom row). Black squares represent adjusted RR; bars represent 95% CI; shading indicates RR with 95% CI not including 1.0. Available via American College of Physicians Public Health Emergency Collection through PubMed Central.
Transfusion of convalescent plasma (CP) collected from donors who have recently recovered from COVID-19 is a potential therapy for COVID-19 patients. Studies of neutralizing antibody (NAb) titers in donor and recipient plasma, timing of CP transfusion, and signals of therapeutic efficacy in COVID-19 patients may provide data regarding use of CP for treatment of COVID-19.
PEER-REVIEWED
A. SARS-CoV-2 neutralizing antibody titers in convalescent plasma and recipients in New Mexico: An open treatment study in COVID-19 patientsexternal icon. Bradfute et al. Journal of Infectious Diseases. (August 11, 2020).
Key findings:
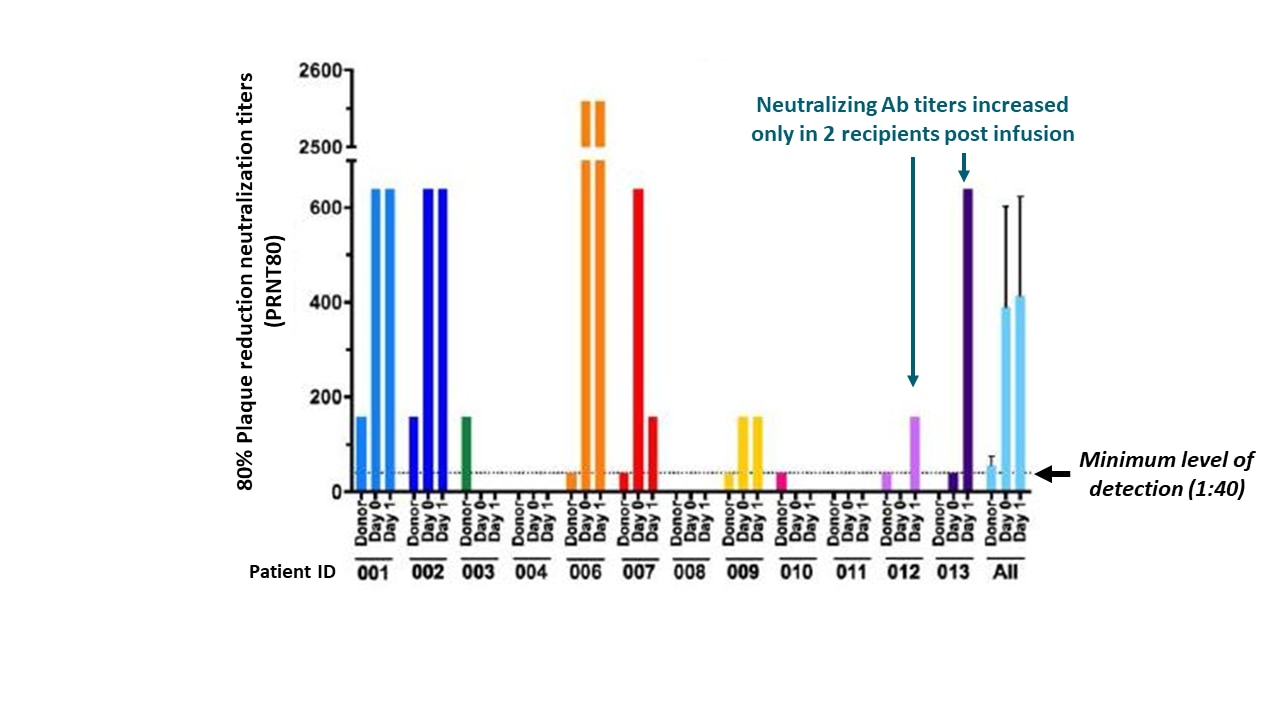
- Neutralizing antibody (NAb) titers in donor convalescent plasma (CP) units were low (<1:40 to 1:160) and had no effect on recipient NAb titer one day after transfusion (Figure 1).
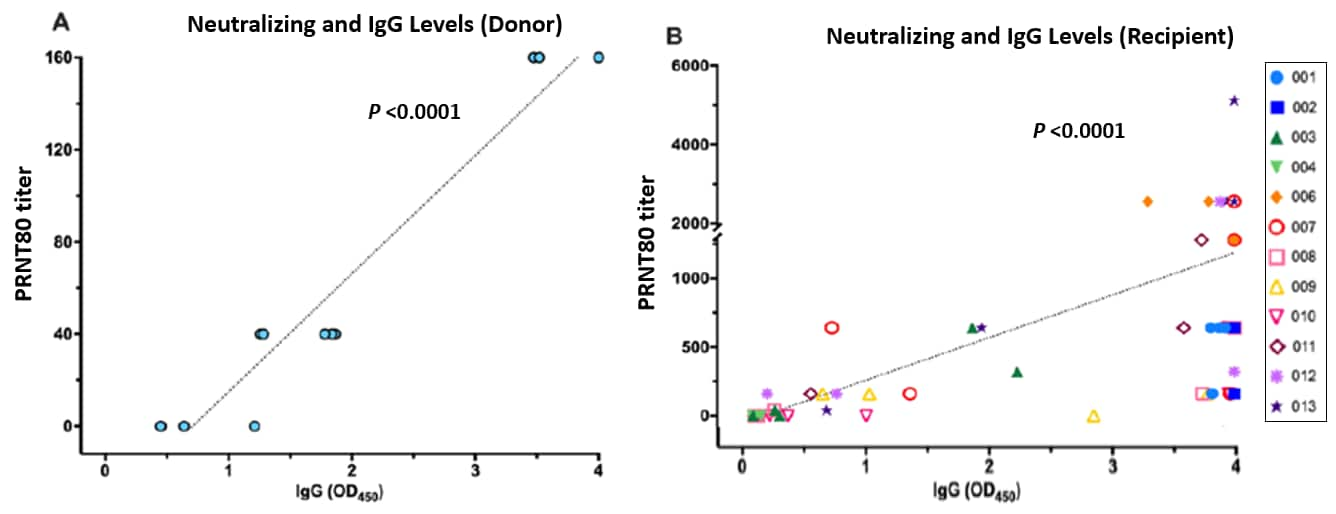
- NAb and the total amount of IgG antibody (IgG titers) in donor plasma were correlated, Spearman correlation (ρ) = 0.938; positive correlation was also observed in CP recipients (r) = 0.781, both p <0.0001 (Figure 2).
Methods: Single-arm interventional trial measuring NAb and total IgG titers before and after CP transfusion over a 14-day period among 12 hospitalized COVID-19 patients, median age 52 years, New Mexico, May 11 to 29, 2020. Limitations: Single arm, non-randomized controlled trial, small cohort, short follow up time.
Figure 1
Note: Adapted from Bradfute et al. SARS-CoV-2 NAb in CP units (Donor), and pre-infusion (day 0) and post-infusion (day 1) in each recipient and all patients. The minimal level of detection was 1:40 for NAb shown as dashed line. Bars in all patients indicate the standard error of measurement. Plaque reduction neutralizing titers (PRNT80) detect neutralizing antibodies that prevent ability of virus to infect a cell monolayer. Adapted and reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiaa505/5890960external icon. OUP and the IDSA are not responsible or in any way liable for the accuracy of the adaptation. Licensee is solely responsible for the adaptation in this publication/reprint.
Figure 2
Note: Adapted from Bradfute et al. Relationship between NAb and IgG levels in donor units (A) and in recipients (B). Colored shapes in B represent individual patients. Plaque reduction neutralizing titers (PRNT80) detect neutralizing antibodies that prevent ability of virus to infect a cell monolayer. Adapted and reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiaa505/5890960external icon. OUP and the IDSA are not responsible or in any way liable for the accuracy of the adaptation. Licensee is solely responsible for the adaptation in this publication/reprint.
B. Treatment of COVID-19 patients with convalescent plasma reveals a signal of significantly decreased mortalityexternal icon. Salazar et al. American Journal of Pathology. (August 10, 2020).
Key findings:
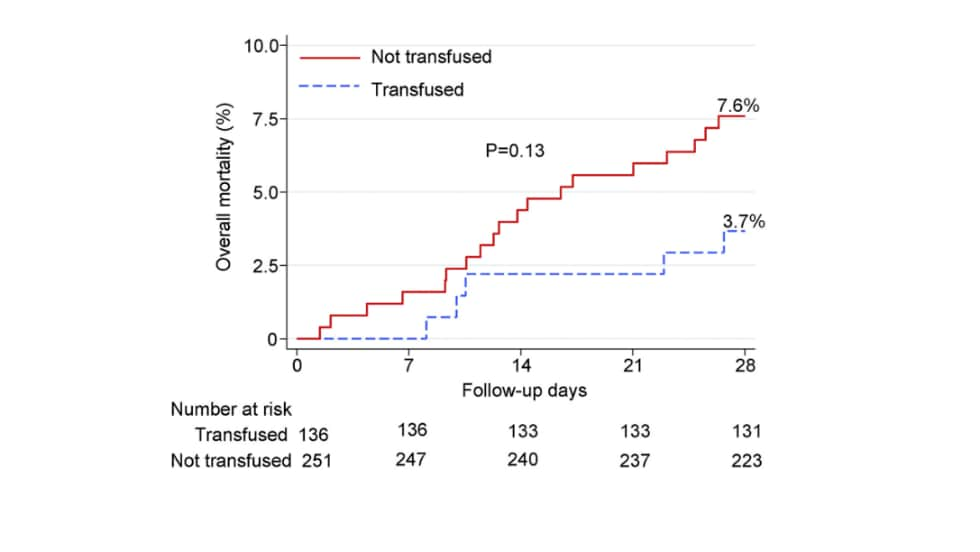
- Mortality at 28 days was reduced among all patients transfused with convalescent plasma (CP) versus non-transfused patients, although not significantly, (Figure 1).
- Non-transfused patients, compared with patients transfused within 72 hours of admission and with high-titer CP had higher overall mortality, relative risk (RR) 7.53 (95% CI 1.12-50.46).
- Non-transfused patients did not differ from patients transfused >72 hours after admission, RR 0.81 (95% CI 0.25-2.69).
- Mortality at 28 days was reduced among patients transfused ≤72 hours of admission, compared to patients transfused >72 hours after admission, p = 0.01, (Figure 2A).
- Mortality at 28 days was reduced among CP recipients who were transfused within 72 hours of hospital admission with high IgG titer (≥1:1,350) plasma (Figure 2B).
Methods: Prospective cohort of 316 COVID-19 patients with severe or life-threatening COVID-19 receiving CP transfusion, Houston, March 28 to July 6, 2020. 136 patients were propensity-matched to 251 controls who had not received CP, and mortality was measured at 28 days. CP titers for IgG measured by ELISA; high titer defined as ≥1:1,350 because this level indicates an 80% probability of viral neutralization titer ≥1:160 (recommended viral neutralization cutoff for therapeutic purposes). Limitations: Small cohort; interim analysis.
Figure 1
Figure 2
Note: Adapted from Salazar et al. Mortality in CP-transfused patients and propensity-matched controls not transfused. Day 0 indicates date of transfusion for patients. Licensed under CC-BY-NC-ND 4.0
Note: Adapted from Salazar et al. A: Mortality in patients transfused with CP ≤72 hours of admission vs. >72 hours. B: Mortality in those with transfusion >72 hours and with CP with IgG titer <1,350 vs. those with transfusion <72 hours and IgG titer >1,350. Day 0 indicates date of transfusion for patients. Licensed under CC-BY-NC-ND 4.0
PREPRINTS (NOT PEER-REVIEWED)
C. Effect of convalescent plasma on mortality among 2 hospitalized patients with COVID-19: Initial three-month experienceexternal icon. Joyner et al. medRxiv. (August 12, 2020). Published in NEJM as Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19 (January 13, 2021)external icon.
Key findings:
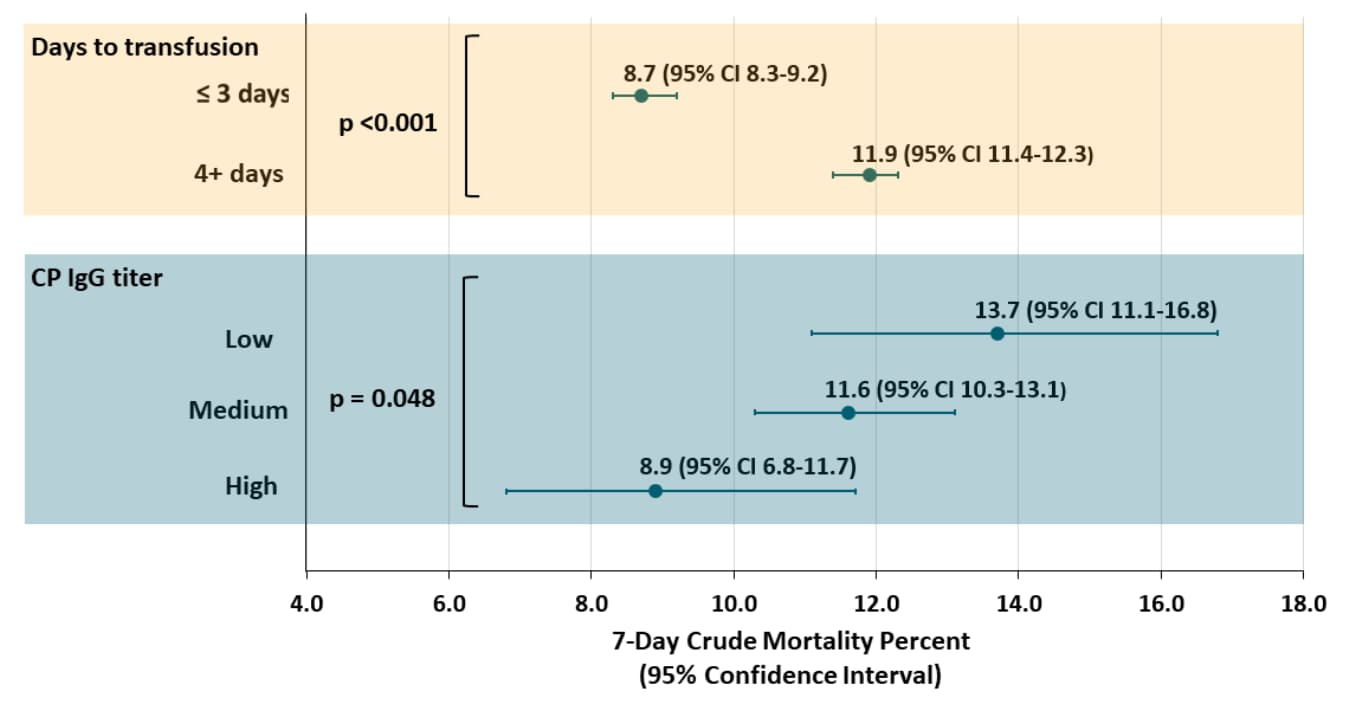
- 7-day mortality was lower in patients transfused ≤3 days of COVID-19 diagnosis, compared with patients transfused four or more days after diagnosis, p <0.001 (Figure 1).
- 7-day mortality was lower in patients who received high-IgG plasma compared with medium- and low-IgG plasma, p = 0.048 (Figure 1).
- Similar findings were observed for 30-day mortality for transfusion timing, p <0.0001, and IgG titer level, p = 0.021 (Figure 2).
Methods: Cohort of 35,322 U.S. adults who were hospitalized with, or at risk of, severe or life-threatening acute COVID-19 respiratory syndrome with transfusion of ≥1 unit of CP, between April 4 and July 4, 2020. Seven- and 30-day mortality were measured. CP IgG titers were measured and defined as high: >18.45 signal to cutoff ratio [S/Co]), medium: 4.62 to 18.45 S/Co and low: <4.62 S/Co). Limitations: Not randomized; antibody levels in the CP units collected from donors were unknown at the time of transfusion; conclusions apply to patients with severe illness.
Figure 1
Note: Adapted from Joyner et al. 7-day mortality in CP-transfused patients stratified by timing of transfusion and by IgG titer of CP. Dots indicate point estimates; bars indicate 95% CI. Used by permission from the author.
Figure 2
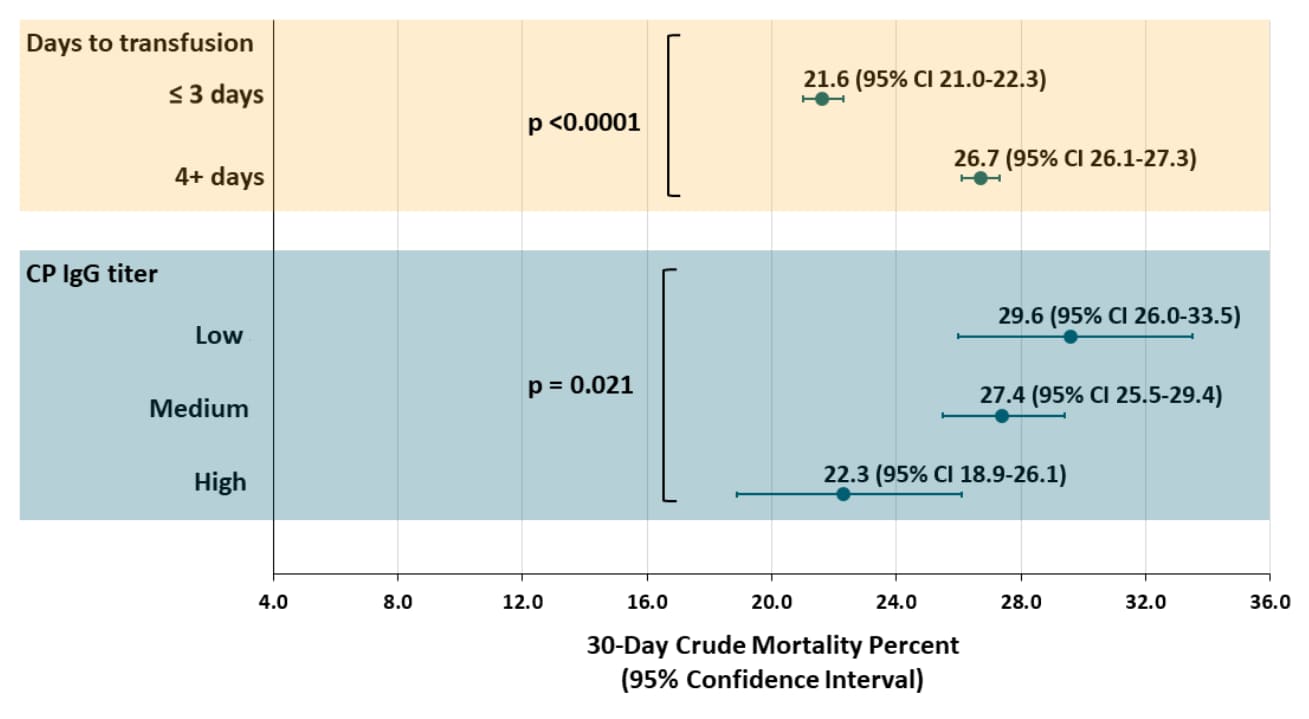
Note: Adapted from Joyner et al. 30-day mortality in CP-transfused patients stratified by timing of transfusion and by IgG titer of CP. Dots indicate point estimates; bars indicate 95% CI. Used by permission from the author.
Implications for 3 studies (Bradfute et al., Salazar et al., & Joyner et al.): Early initiation of transfusion of CP with sufficient levels of NAbs may promote better clinical outcomes of critically ill COVID-19 patients. CP therapy merits as a treatment for hospitalized COVID-19 patients; however, randomized controlled trials may still be needed.
PREPRINTS (NOT PEER-REVIEWED)
First-in-human trial of a SARS CoV 2 recombinant spike protein nanoparticle vaccineexternal icon. Keech et al. medRxiv. (August 6, 2020). Published in NEJM as Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccineexternal icon (September 2, 2020).
Key findings:
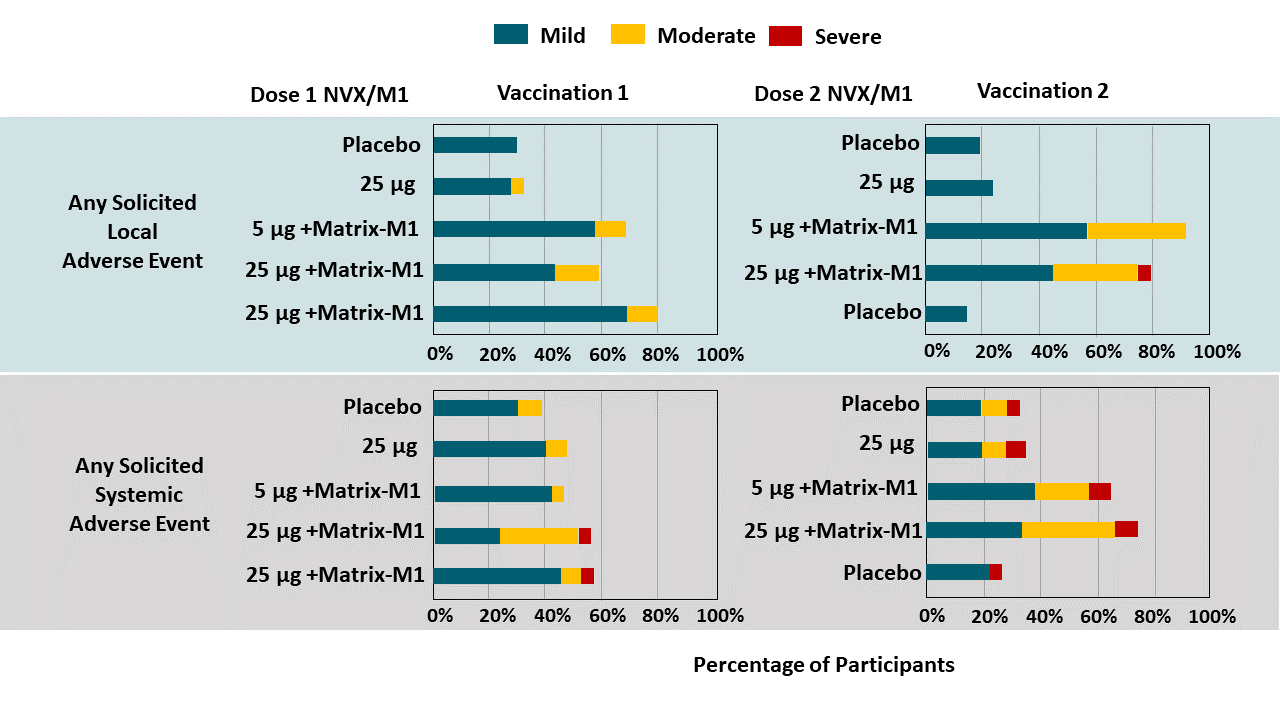
- Adverse reactions were typically mild or moderate and of short duration (mean ≤2 days), (Figure 1).
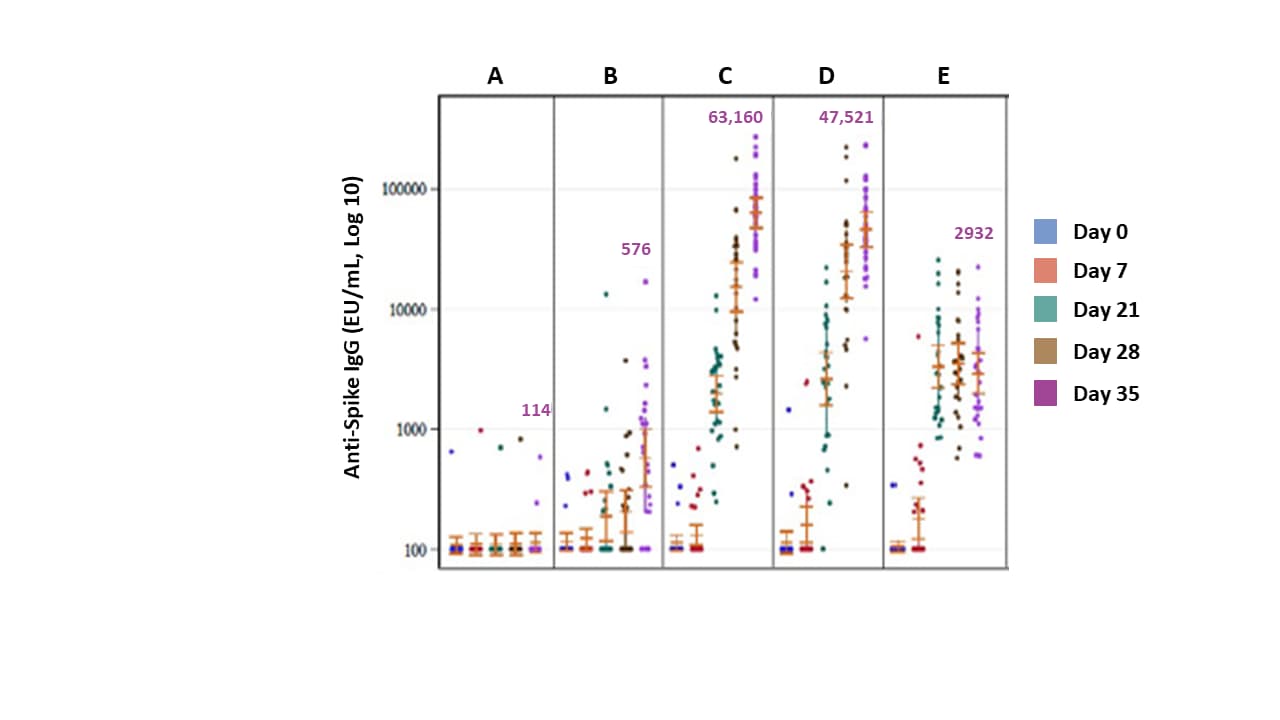
- Geometric mean titers (GMT) of anti-spike IgG and neutralizing antibody were highest in vaccine recipients also receiving adjuvant (Figure 2).
- GMT increases were seen at 5 and 25 μg doses (Figure 2).
- The vaccine induced CD4+ T-cell responses.
Methods: Randomized, blinded, placebo-controlled, phase 1 trial of NVX-CoV2373 (recombinant nanoparticle vaccine composed of SARS-CoV-2 spike glycoproteins) in 131 healthy adults, Melbourne, Australia, May, 2020. Six participants received open label injections and were monitored for 48 hours. Remaining participants (n = 100, 25 per group in 4 groups) received 2 doses of 5 μg or 25 μg of vaccine with adjuvant; 2 doses of 25 μg of vaccine without adjuvant; 1 dose of 25 μg of vaccine with adjuvant and 1 dose of placebo; or 2 doses of placebo (saline). All participants received two intramuscular injections, 21 days apart. Primary outcomes at 35 days were reactogenicity, safety, IgG anti-spike protein response. Limitations: Phase 1 trial only.
Implications: NVX-CoV2373 with Matrix-M1 adjuvant appears well tolerated and induces immunological responses, supporting transition to a Phase 3 efficacy trial.
Figure 1
Note: Adapted from Keech et al. Solicited local and systemic adverse events through 7 days following each vaccination. There were no Grade 4 (life-threatening) events. Matrix-M1- adjuvant (50 μg for all conditions). Used by permission from the author.
Figure 2
Note: Adapted from Keech et al. SARS-CoV-2 Anti-Spike IgG and neutralizing antibody responses. A: placebo (administered Day 0 and 21); B: 25 μg vaccine without adjuvant (Day 0 and 21); C: 5 μg vaccine with 50 μg adjuvant (Day 0 and 21); D: 25 μg vaccine with adjuvant (Day 0 and 21); E: 25 μg vaccine at Day 0, placebo at Day 21. Purple numbers represent GMT of anti-spike IgG on Day 35. Used by permission from the author.
Prediction and Clinical Support Tools
- Burdick et al. Prediction of respiratory decompensation in COVID-19 patients using machine learning: The READY trial.external icon Computers in Biology and Medicine. Shows a machine learning algorithm predicts ventilation needs among COVID-19 patients more accurately than an existing scoring system and might assist care teams effectively triage patients and allocate resources.
- Jehi et al. Development and validation of a model for individualized prediction of hospitalization risk in 4,536 patients with COVID-19.external icon PLOS ONE. Presents an online risk calculator for predicting hospitalization associated with COVID-19.
Ethical Considerations and Opportunities
- Chastain et al. Racial disproportionality in COVID clinical trials.external icon NEJM. Discusses reasons for underrepresentation of racial and ethnic minorities in U.S. COVID-19 prevention and treatment trials along with recommendations for addressing deficiencies.
- Klar et al. The ethics of COVID-19 tracking apps – challenges and voluntarinessexternal icon. Research Ethics. Discusses design of tracing apps with an emphasis on voluntariness to minimize threats to privacy and equity while maintaining effectiveness.
Clinical Outcomes
- Radia et al. Multi-system Inflammatory Syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation.external icon Paediatric Respiratory Reviews. Concludes MIS-C, although rare, requires critical care for 68% of patients.
- Restivo et al. Myasthenia Gravis associated with SARS-CoV-2 infection.external icon Annals of Internal Medicine. Describes a case series of three COVID-19 patients without prior autoimmune or neurologic disorders who were diagnosed with myasthenia gravis.
- Jeronimo et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): A randomised, double-blind, Phase IIb, placebo-controlled trial.external icon Clinical Infectious Diseases. A short course of methylprednisone in hospitalized patients with COVID-19 did not reduce mortality in the overall population.
Other Topics
- Shultz et al. Cascading risks of COVID-19 resurgence during an active 2020 Atlantic hurricane season.external icon JAMA. Discusses management of social distancing and other COVID-19 mitigation interventions in the context of evacuation requirements for hurricanes.
- Orlowski et al. Four months into the COVID-19 pandemic, Sweden’s prized herd immunity is nowhere in sight.external icon Journal of the Royal Society of Medicine. Discusses the outcomes of the Swedish approach aimed at facilitating herd immunity in the context of sociopolitical conditions in Sweden.
- Jeremias et al. Prevalence of SARS-CoV-2 among Health Care Workers in a Tertiary Care Hospital.external icon JAMA Internal Medicine. Demonstrates seroprevalence among HCW is comparable to the general population when HCW use appropriate PPE.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.