COVID-19 Science Update released: August 20, 2021 Edition 103

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update align with the CDC Science Agenda for COVID-19.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescentsexternal icon. Ali et al. NEJM (August 11, 2021).
Key findings:
- mRNA-1273 vaccine (100 micrograms per dose) had an acceptable safety profile in adolescents.
- Most common side effects within 7 days of 1st and 2nd dose, respectively, were injection site pain (93.1% and 92.4%), headache (44.6% and 70.2%), and fatigue (47.9% and 67.8%). No serious adverse events related to mRNA-1273 or placebo were noted.
- Most vaccinated persons seroconverted (98.8% of adolescents, 98.6% of young adults). Geometric mean titers (GMT) of neutralizing antibody were noninferior among adolescents compared with young adults (GMT ratio: 1.08, 95% CI 0.94-1.24).
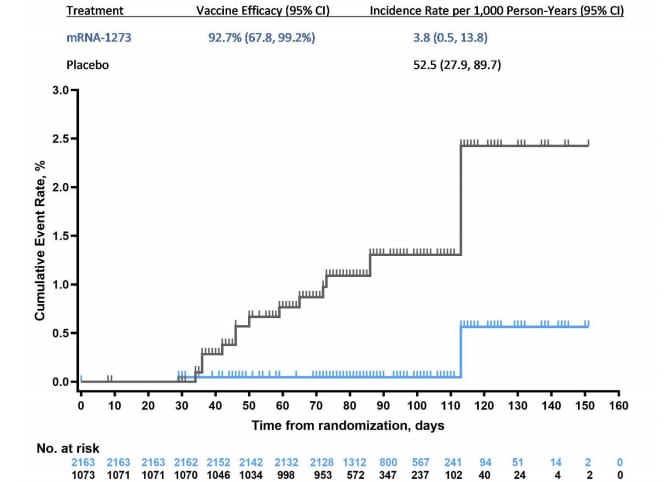
- Vaccine efficacy against symptomatic COVID-19 in adolescents was 93.3% (95% CI 47.9%-99.9%) in the per-protocol population and 92.7% (95% CI 67.8%-99.2%) in the intention-to-treat population. (Figure)
Methods: In this bridging study, data on immunogenicity as well as vaccine efficacy and adverse reactions among U.S. adolescents (aged 12–17 years) receiving 2 doses of mRNA-1273 (Moderna, n = 2,489) or saline placebo (n = 1,243) in December 2020–February 2021 as part of the manufacturer’s Teen COVE (coronavirus efficacy) phase 2-3 randomized trial were compared with previously reported phase 3 trial results from young adults (aged 18–25 years). Immunogenicity was assessed in a random sample of vaccinated adolescents (n = 340). Doses were administered 28 days apart to >98% of participants; median follow-up was 83 days (range 9–151 days). Limitations: Low incidence of COVID-19 in the trial population (4 cases in the placebo group only).
Implications: Adolescents vaccinated with 2 doses of mRNA-1273 had immunogenicity and vaccine efficacy comparable to young adults, with no serious adverse events noted.
Figure:
Note: Adapted from Ali et al. Cumulative incidence of symptomatic COVID-19 ≥14 days after the 1st injection in the modified intention-to-treat population. Four cases of COVID-19 occurred in the placebo group, and no cases of COVID-19 with an onset of ≥14 days after the 2nd injection were reported in the mRNA-1273 group. From the New England Journal of Medicine, Ali et al., Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. August 11, 2021, online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipientsexternal icon. Hall et al. NEJM (August 11, 2021).
Key findings:
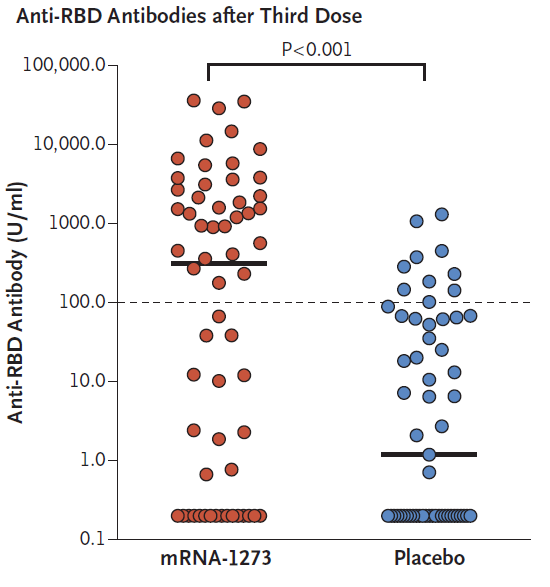
- Among solid organ transplant recipients who had received 2 doses of mRNA-1273 (Moderna) vaccine, a 3rd dose produced significantly higher immunogenicity than placebo.
- Antibody levels were high in 55% of the mRNA-1273 group compared with 18% of the placebo group (relative risk 3.1; 95% CI 1.7-5.8) (Figure)
- Median percent virus neutralization was 71% in the mRNA-1273 group and 13% in the placebo group (between-group difference 58 percentage points, 95% CI 11-76 percentage points).
- A 3rd dose had an acceptable safety profile.
- No severe adverse events or cases of acute rejection were reported.
Methods: Double-blind, randomized, controlled trial comparing a 3rd dose of mRNA-1273 (Moderna) vaccine (n = 60) versus placebo (n = 57) in solid organ transplant recipients who had received 2 doses of mRNA-1273 previously. Participants received either a 3rd vaccine dose or saline placebo. Anti-receptor-binding domain (anti-RDB) antibody levels (>100 U/mL) were measured after 1 month. Limitations: Cutoff selected for anti-RBD antibody level not necessarily predictive of resistance to infection.
Implications: Solid organ transplant recipients should be considered for an additional dose of mRNA COVID-19 vaccine after an initial 2-dose primary series. As of August 13, 2021, CDC recommends that people with moderate to severe immune compromise, including solid organ transplant recipients who are taking immunosuppressive therapy, should receive an additional dose of mRNA COVID-19 vaccine after their initial 2 doses.
Figure:
Note: Adapted from Hall et al. Immune response (anti-RBD antibodies) in solid organ transplant recipients who received mRNA-1273 vaccine or placebo. From the New England Journal of Medicine, Hall et al., Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. August 11, 2021, online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
PREPRINTS (NOT PEER-REVIEWED)
Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trialexternal icon. Gilbert et al. medRxiv (August 12, 2021). Published in Scienceexternal icon (November 23, 2021).
Key findings:
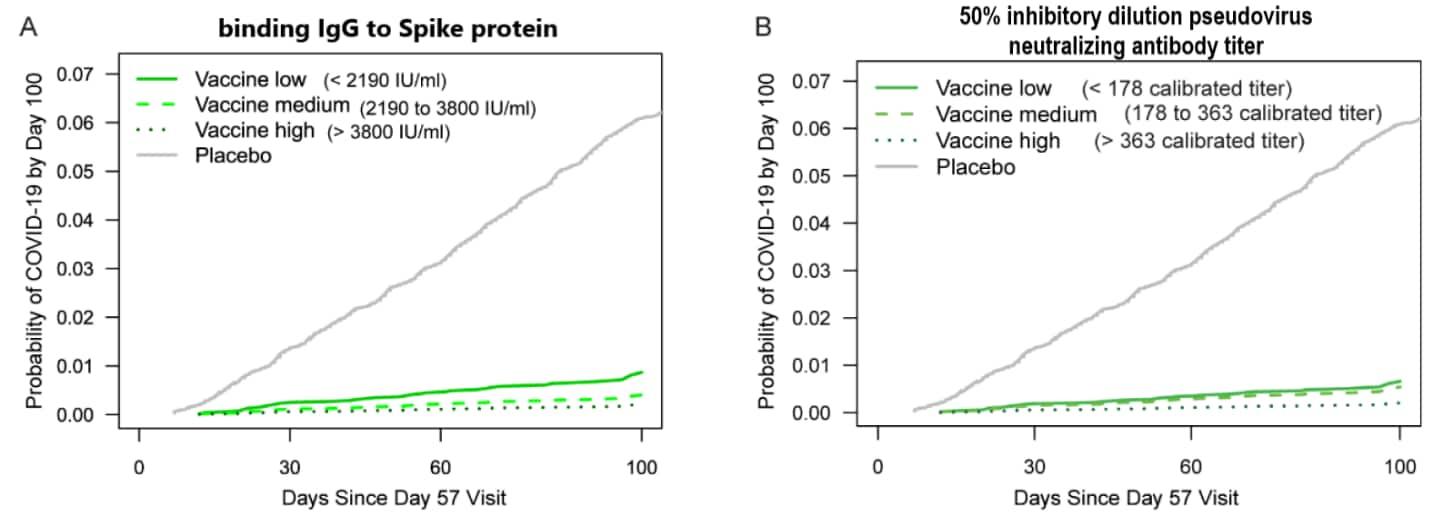
- Multiple antibody markers (IgG binding antibodies to SARS-CoV-2 spike protein and to receptor-binding domain, and 50% [ID50] and 80% [ID80] inhibitory dilution pseudovirus neutralizing antibody titers) were inversely correlated with COVID-19 risk, 4 months post-2nd mRNA-1273 (Moderna) vaccine dose. (Figure)
- At 4 months post 2nd dose, mRNA-1273 vaccine efficacy against symptomatic, laboratory-confirmed COVID-19 was 50.8% (95% CI -51.2%-83.0%), 90.7% (86.7%-93.6%), or 96.1% (94.0%-97.8%) when calibrated ID50 titers were undetectable, 100, or 1,000, respectively.
Methods: In the manufacturer’s COVE (coronavirus efficacy) phase 3 trial, adult participants were randomized to receive 2 doses of mRNA-1273 vaccine or placebo from July 27–October 23, 2020. In a case-cohort sample of vaccinated trial participants (n = 14,064), IgG binding antibodies to SARS-CoV-2 spike protein and receptor-binding domain, ID50 and ID80 were measured on day 1 (dose 1), day 29 (dose 2), and day 57 post-vaccination. Correlates of risk for acute COVID-19 (symptomatic infection with positive SARS-CoV-2 PCR test and no previous infection) and correlates of vaccine efficacy were determined up to 4 months post-vaccination. Limitations: Most cases were infected with Alpha (B.1.1.7) or a similar virus; correlates of protections against other variants cannot be inferred.
Implications: Reduced antibody levels might represent waning immunity to SARS-CoV-2 infection. This report adds to a small number of studies suggesting that antibody titer-based correlates corresponding with levels of protection against SARS-CoV-2 can be determined.
Figure:
Note: Adapted from Gilbert et al. Adjusted cumulative incidence of COVID-19 by placebo and low (solid green line), medium (dashed green line), and high (dotted green line) tertile of A) binding IgG antibody to spike protein titer and B) calibrated 50% inhibitory dilution pseudovirus neutralizing antibody titer, by time since day 57 visit. Incidence adjusted for at-risk status, membership in a community of color, and predicted risk score. ID50 neutralizing titers were calibrated to the WHO anti–SARS–CoV–2 immunoglobulin International Standard. Licensed under CC-BY-NC-ND 4.0.
PREPRINTS (NOT PEER-REVIEWED)
BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar.external icon Tang et al. medRxiv (August 11, 2021). Published in Nature Medicineexternal icon (November 2, 2021).
Key findings:
- Vaccine effectiveness (VE) against infection with Delta (B.1.617.2) ≥14 days following a 2nd dose was 53.5% (95% CI 43.9%-61.4%) for BNT162b2 and 84.8% (95% CI 75.9%-90.8%) for mRNA-1273 vaccines.
- Against severe, critical, or fatal disease due to Delta, VE was 89.7% (95% CI 61.0%-98.1%) for BNT162b2 and 100.0% (95% CI 41.2%-100.0%) for mRNA-1273 vaccines.
Methods: Matched, test-negative, case-control study of general population vaccinated from December 21, 2020–July 21, 2021 in Qatar, a highly vaccinated population (>87%) where community transmission of Delta was first detected in March 2021. Median time between vaccine doses was 21 days for BNT162b2 (Pfizer-BioNTech) and 28 days for mRNA-1273 (Moderna) vaccine. Limitations: Unique population demographics; only 9% aged >50 years, and 89% are expatriates from >150 countries.
Implications: BNT162b2 and mRNA-1273 both appeared to be more effective at preventing severe or fatal COVID-19 than at preventing infection with the Delta variant in a real-world setting.
Progress of the Delta variant and erosion of vaccine effectiveness, a warning from Utah.external icon Keegan et al. medRxiv (August 10, 2021).
Key findings:
- 54% of eligible Utah residents were fully vaccinated by late June 2021, including 52.9% with BNT162b2 (Pfizer/BioNTech), 38.1% with mRNA-1273 (Moderna), and 9.1% with Ad26.COV2.S (Johnson & Johnson/Janssen) vaccine.
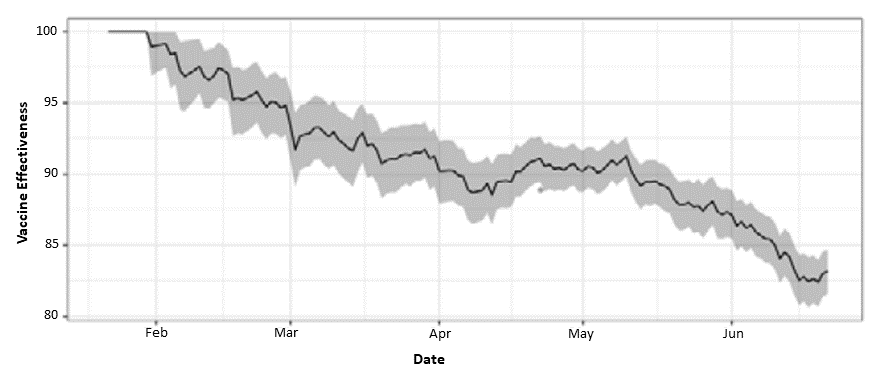
- Vaccination effectiveness (VE) decreased from January–June 2021, as Delta (B.1.617.2) outcompeted other SARS-CoV-2 variants (Figure).
- As of June 20, 2021, 70% of all SARS-CoV-2 viruses sequenced in Utah were Delta.
- Proportion of breakthrough cases increased faster than expected, reaching 10.5% of cases by the end of June, beyond the 6.4% that would have been expected with a 90% effective vaccine.
- Estimated VE against Delta infection was 82% (95% CI 78%-85%).
Methods: Combined daily vaccine effectiveness was estimated using a field evaluation approach based on reported COVID-19 and vaccination status of cases obtained from the Utah Department of Health (UDOH). Limitations: Only 10–15% of daily cases were sequenced; vaccination status might be overestimated in persons with breakthrough infections who may self-report their vaccination status to UDOH.
Implications: Protection against SARS-CoV-2 infection remained high, but appeared to decrease over time following initial vaccinations, in association with the increase in the Delta variant. In addition to increasing vaccination coverage, non-pharmaceutical interventions such as masking, physical distancing, and avoiding crowds and poorly ventilated indoor spaces may be needed to reduce transmission.
Figure:
Note: Adapted from Keegan et al. 14-day moving average of VE against SARS-CoV-2 infection in Utah over time (95% CI). Licensed under CC BY 4.0.
PEER-REVIEWED
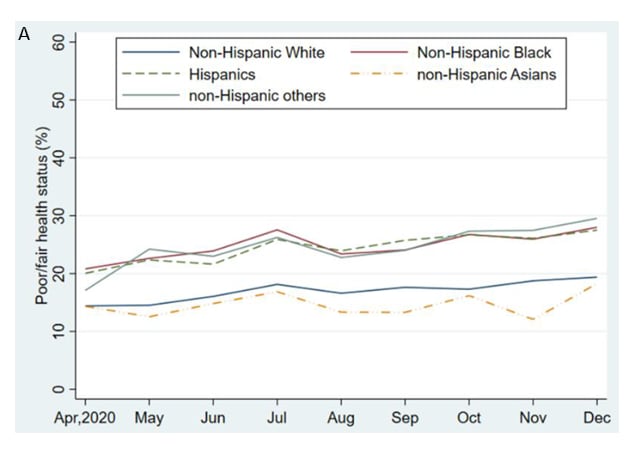
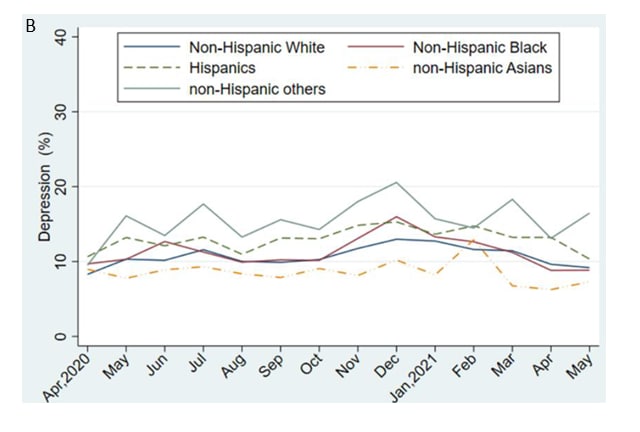
Monthly trends in self-reported health status and depression by race/ethnicity and socioeconomic status during the COVID-19 pandemic, United States, April 2020 – May 2021.external icon Lee et al. Annals of Epidemiology (August 4, 2021).
Key findings:
- From April–December 2020, weighted prevalence of self-reported fair or poor health among U.S. adults increased, with differences by race/ethnicity and by socioeconomic status (SES).
- Non-Hispanic Black (aOR = 1.07; 95% CI 1.01-1.14), Hispanic (aOR = 1.19; 95% CI 1.12-1.26), and non-Hispanic Asian (aOR = 1.10; 95% CI 1.00-1.22) adults had higher odds of fair or poor health than non-Hispanic White adults (Figure A).
- Adults with less than a high school education, annual income <$25,000, and renters had higher odds of fair or poor health than adults with a master’s degree or higher, annual income ≥$200,000, and homeowners.
- From April 2020–May 2021, weighted prevalence of serious depression among U.S. adults increased until December and then declined (Figure B).
- Non-Hispanic other and Hispanic adults had higher odds of serious depression than Non-Hispanic Black, non-Hispanic White, and non-Hispanic Asian adults.
- Adults with less education, lower annual income, and who were renters had higher odds of serious depression than their counterparts with higher SES.
Methods: The U.S. Census Bureau’s nationally representative Household Pulse Survey assessed trends in health status (April 2020–December 2020) and serious depression (April 2020–May 2021) among adults aged ≥18 years (n = 1,144,405) using an online questionnaire. Limitations: Depression was self-reported; prevalence could be underestimated due to limited participation in this internet-based survey by persons with lower SES.
Implications: Differences in prevalence of self-reported health status and serious depression by race/ethnicity and socioeconomic factors, including housing status, highlight the need to address health disparities related to the COVID-19 pandemic.
Figures:
Note: Adapted from Lee et al. Monthly trends among U.S. adults. A) Prevalence of self-reported fair/poor health by race/ethnicity, April–December 2020. Respondents who reported fair or poor when asked “Would you say your health in general is excellent, very good, good, fair, or poor?” were defined as having fair/poor health. B) Prevalence of serious depression by race/ethnicity, April 2020–May 2021. Respondents who responded, “nearly every day” when asked “Over the last 7 days, how often have you been bothered by feeling down, depressed, or hopeless?” were defined as having serious depression. Reprinted from Annals of Epidemiology, online August 4, 2021, Monthly trends in self-reported health status and depression by race/ethnicity and socioeconomic status during the COVID-19 pandemic, United States, April 2020 – May 2021. Lee et al. Copyright 2021, with permission from Elsevier.
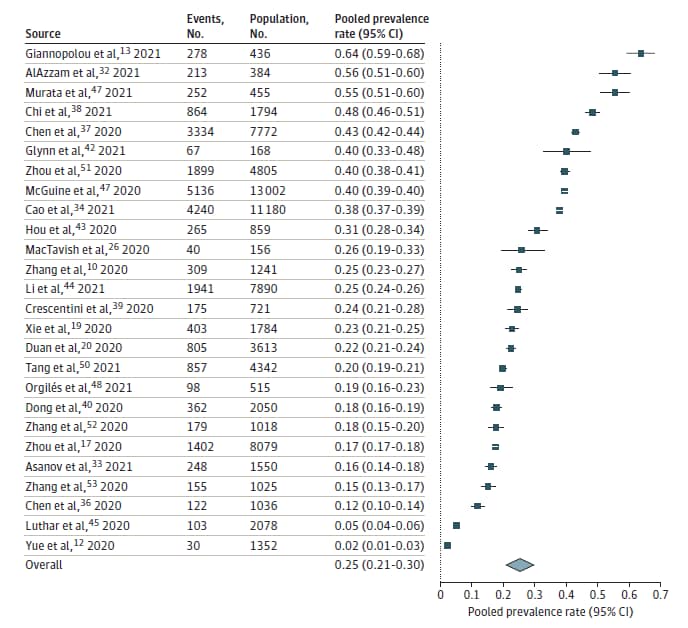
Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19—a meta-analysisexternal icon. Racine et al. JAMA Pediatrics (August 9, 2021).
Key findings:
- In 29 studies including 80,879 youth, pooled prevalence of clinically elevated depressive symptoms was 25.2% (95% CI 21.2%-29.7%), and pooled prevalence of clinically elevated anxiety symptoms was 20.5% (95% CI 17.2%-24.4%). (Figure)
- Estimates ranged widely across studies (2%–64% for depression and 2%–50% for anxiety).
- Prevalence of depression and anxiety symptoms increased with each month of the pandemic, with increasing child age, and with higher proportion of females participating in the study.
Methods: Random-effect meta-analyses were conducted of studies published in English from January 1, 2020–February 16, 2021 containing prevalence rates of clinically elevated depression or anxiety in youth (aged ≤18 years) during the COVID-19 pandemic. Quality of studies was assessed using a 5-item questionnaire. Limitations: Most studies were cross-sectional, limiting examination of associations over time.
Implications: Globally, the high prevalence of children and adolescents experiencing moderate or severe symptoms of depression (1 in 4 youth) or anxiety (1 in 5 youth) during the COVID-19 pandemic warrants attention to resource allocation for essential mental health services.
Figure:
Note: Adapted from Racine et al. Forest plot of the pooled prevalence of clinically elevated depressive symptoms in youth during the COVID-19 pandemic. Squares represent prevalence rates, with lines indicating 95% CIs. The diamond represents the overall effect size. Reproduced with permission from JAMA Pediatrics, 2021. Published online August 9, 2021. https://doi.org/10.1001/jamapediatrics.2021.2482. Copyright© 2021 American Medical Association. All rights reserved.
PEER-REVIEWED
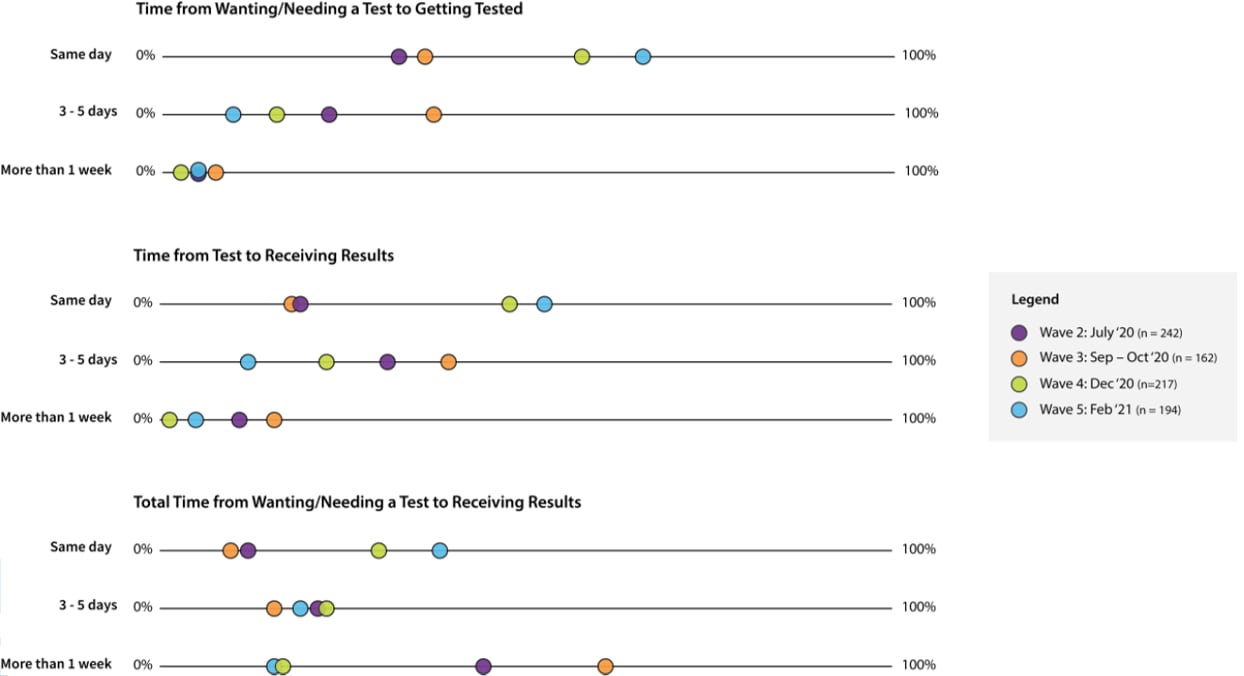
Improvements in SARS-CoV-2 testing cascade in the U.S.: data from serial cross-sectional assessmentsexternal icon. Clipman et al. Clinical Infectious Diseases (August 10, 2021).
Key findings:
- In February 2021, 11% of survey participants reported wanting a SARS-CoV-2 test in the prior 2 weeks.
- 63% of participants who wanted a SARS-CoV-2 test reported receiving one.
- Among participants not tested, the most commonly reported barriers were not knowing where to go (27%), not having time (20%), and not having a doctor’s order for a test (16%).
- In 3 states with baseline data from July 2020, access to testing improved significantly by February 2021, with increases in the number of participants reporting receiving a test if they wanted one (from 52% to 62%), same day test availability (from 28% to 56%), and same day test results (from 17% to 45%) (Figure).
- Not knowing where to go (32% to 23%) remained the most common barrier to testing.
Methods: Adults (aged ≥18 years) in 10 U.S. states were asked about SARS-CoV-2 testing experiences using serial cross-sectional online surveys sent at 4 different times: July 2020 (wave 2, n = 3,009), September–October 2020 (wave 3, n = 3,074), December 2020 (wave 4, n = 7,905), and February 2021 (wave 5, n = 8,029). People in Florida, Illinois, and Maryland were surveyed during all 4 waves, and people in California, Massachusetts, Nebraska, North Dakota, South Dakota, Texas, and Wisconsin were surveyed only during waves 4 and 5. Limitations: Access to testing might be overestimated among volunteers for online surveys.
Implications: In several U.S. states, access to SARS-CoV-2 testing and turnaround times for results improved over the past year. Ongoing efforts are needed to further reduce barriers to testing.
Figure:
Note: Adapted from Clipman et al. Tunaround times from wanting a test to receiving a test (top), from receiving a test to receiving results (middle), and combined (bottom) for survey participants in Florida, Illinois, and Maryland in July 2020, September–October 2020, December 2020, and February 2021. Licensed under CC-BY-NC-ND.
Vaccines
- SARS-CoV-2 vaccine effectiveness in immunosuppressed kidney transplant recipients.external icon Chemaitelly et al. medRxiv (Preprint; August 9, 2021). Among 782 kidney transplant recipients in Qatar, vaccine effectiveness against severe, critical, or fatal COVID-19 increased slowly with time from 2nd dose, reaching 72.3% (95% CI 0-90.9%) at ≥14 days, 85.0% (95% CI 35.7%-96.5%) at ≥42 days, and 83.8% (95% CI 31.3%-96.2%) at ≥56 days. Estimated cumulative incidence of infection through July 21, 2021, was 1.2% (95% CI 0.5%-2.6%) at ≥56 days in vaccinated individuals, compared with 4.7% (95% CI 3.0%-7.1%) for unvaccinated individuals.
Note: Adapted from Chemaitelly et al. Cumulative incidence of documented SARS-CoV-2 infection in vaccinated and unvaccinated kidney transplant recipients in Qatar ≥56 days after 2nd vaccine dose. Used by permission of authors.
- Immunogenicity of BNT162b2 vaccine against the Alpha and Delta variants in immunocompromised patients.external icon Hadjadj et al. medRxiv (Preprint; August 9, 2021). Published in Annals of the Rheumatic Diseases as Immunogenicity of BNT162b2 vaccine against the Alpha and Delta variants in immunocompromised patients with systemic inflammatory diseasesexternal icon (January 12, 2022). In a prospective study of patients with systemic inflammatory disease (n = 64) receiving immunosuppressive or immunomodulatory medications (rituximab, methotrexate, disease-modifying antirheumatic drugs, or other), administration of 2 doses of BNT162b2 (Pfizer/BioNTech) vaccine generated a neutralizing response against both Alpha and Delta in 100% of controls (n = 21), but not all cases. Rituximab and methotrexate might impact immunogenicity of BNT162b2 by impairing B cell and T cell responses, respectively.
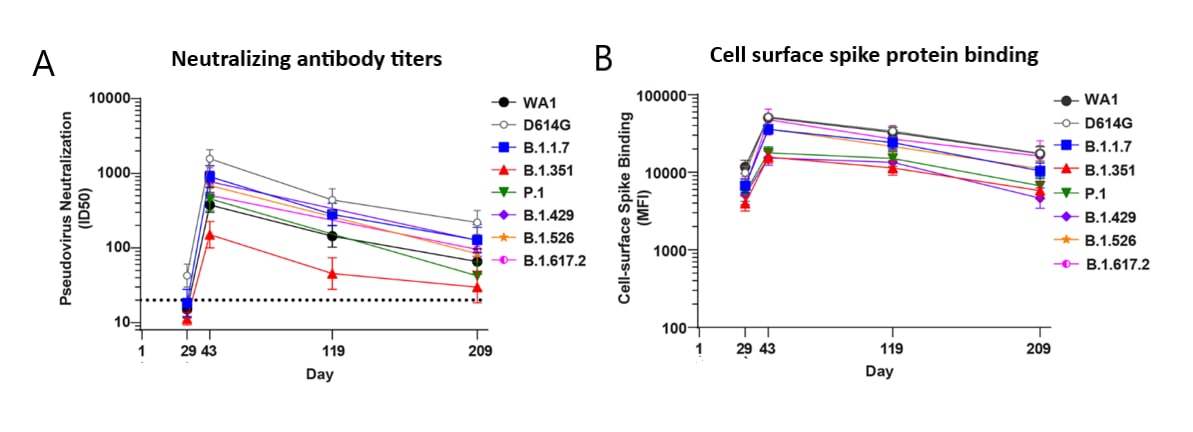
- Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants.external icon Pegu et al. Science (August 12, 2021). Vaccine-induced antibody responses to 6 SARS-CoV-2 variants Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), Epsilon (B.1.429), and Iota (B.1.526) were assessed in 24 subjects who received 2 doses of mRNA-1273 (Moderna) vaccine. Neutralizing antibody titers decreased over time yet persisted at low levels in ≥85% of persons at 6 months post-vaccination against Delta and other variants (except Beta). High levels of binding antibodies against each variant, including Delta, were maintained in all subjects over the 7-month study period.
Note: Adapted from Pegu et al. A) Neutralizing antibody titers and B) binding to cell-surface expressed full length spike protein for 6 SARS-CoV-2 variants (WA1 (wild-type) and D614G (predominant circulating virus as of summer 2020), B.1.1.7, B.1.351, P.1, B.1.429, B.1.526, and B.1.617.2, sampled at 4 timepoints. Pseudovirus neutralization expressed as 50% inhibitory dilution (ID50). Cell surface spike protein binding expressed as median fluorescence intensity (MFI). Dotted line, limit of detection (>20). Pegu et al. (August 12, 2021). Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science. Reprinted with permission from AAAS.
Variants
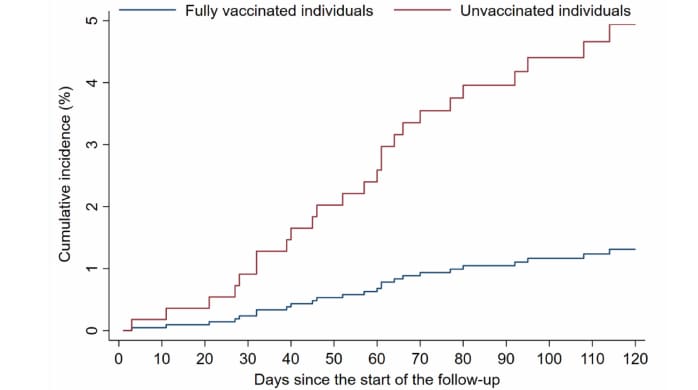
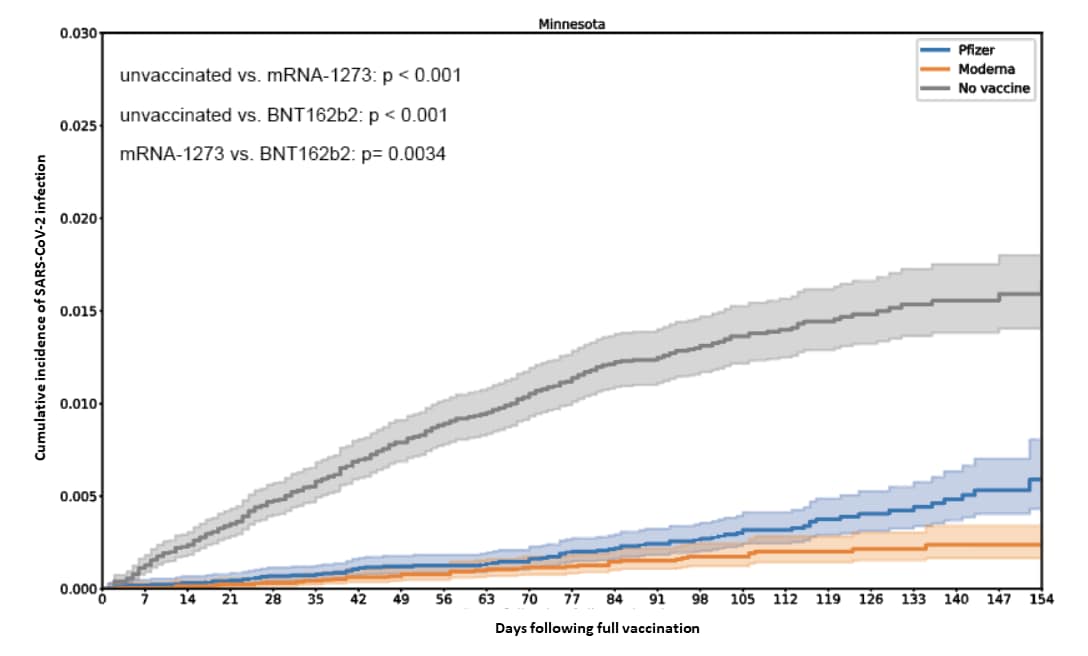
- Comparison of two highly effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence.external icon Puranik et al. medRxiv (Preprint; August 9, 2021). In matched cohorts of vaccinated and unvaccinated individuals from Minnesota (n = 25,869/group), vaccine effectiveness (VE) against SARS-CoV-2 infection appeared to decrease after Delta (B.1.617.2) became the dominant variant in July 2021, to 76% (95% CI 58%-87%) with mRNA-1273 (Moderna) and 42% (95% CI 13%-62%) with BNT162b2 (Pfizer/BioNTech). Persons vaccinated with mRNA-1273 had lower odds of breakthrough infection compared to BNT162b2 (incidence rate ratio = 0.50, 95% CI 0.39-0.64), but VE remained high (≥85%) against severe COVID-19 requiring hospitalization or intensive care unit admission. No deaths occurred in either vaccinated cohort.
Note: Adapted from Puranik et aI. Cumulative incidence of SARS-CoV-2 infection in unvaccinated, mRNA-1273-vaccinated and BNT162b2-vaccinated people in Minnesota. Licensed under CC-BY-ND 4.0.
- Serial intervals in SARS-CoV-2 B.1.617.2 variant cases.external icon Pung et al. The Lancet (August 10, 2021). Drivers of rapid growth of COVID-19 cases in Singapore were examined using serial intervals (i.e., onset-to-onset delay, a proxy for the generation interval). Serial intervals were determined in household transmission pairs infected with Delta (B.1.617.2) from April 27–May 22, 2021 (n = 32) and compared to those of household pairs infected prior to the partial lockdown in Singapore during April 2020 (n = 63). Mean, median, and mode of the serial interval distributions were determined and found not to be statistically different between groups, supporting the hypothesis that recent rapid growth in SARS-CoV-2 infections is driven by an increase in the average number of secondary cases generated by each case infected with the Delta variant.
Natural History, Reinfection, and Health Impact
- More than 50 long-term effects of COVID-19: a systematic review and meta-analysis.external icon Lopez-Leon et al. Scientific Reports (August 9, 2021). In a meta-analysis of 19 studies of COVID-19 patients aged 17–87 years (n = 47,910) followed for up to 110 days, 80% (95% CI 65%-92%) developed at least 1 of 55 long-term effects. Most common symptoms were fatigue (58%, 95% CI 42%-73%), headache (44%, 95% CI 13%-78%), attention disorder (27%, 95% CI 19%-36%), hair loss (25%, 95% CI 17%-34%), and dyspnea (24%, 95% CI 14%-36%).
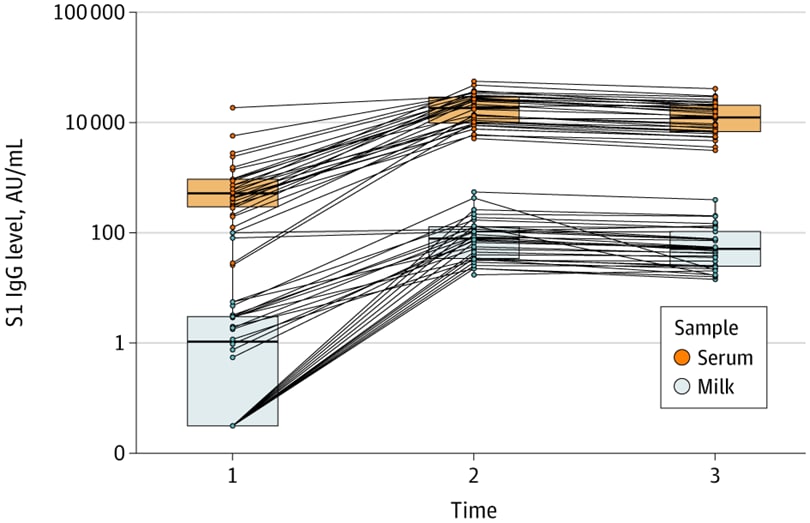
- Quantification of specific antibodies against SARS-CoV-2 in breast milk of lactating women vaccinated with an mRNA vaccine.external icon Esteve-Palau et al. JAMA Network Open (August 11, 2021). In a prospective cohort study (n = 33) in Spain, breast milk from women (mean age 37 years, mean postpartum time 17.5 months) vaccinated with BNT162b2 (Pfizer-BioNTech) contained immunoglobin (Ig) G antibodies against the spike protein (S1 subunit). After the 2nd dose, breast milk IgG(S1) levels increased and were positively associated with serum IgG(S1). CDC recommends COVID-19 vaccination for people who are pregnant or breastfeeding.
Note: Adapted from Esteve-Palau et al. SARS-CoV-2 antibody levels (S1 IgG) in breast milk and serum of vaccinated women at Time 1 (2 weeks after 1st dose), Time 2 (2 weeks after 2nd dose), and Time 3 (4 weeks after 2nd dose). Shaded boxes indicate interquartile range; horizontal lines are medians. AU, arbitrary units. Licensed under CC BY.
Testing
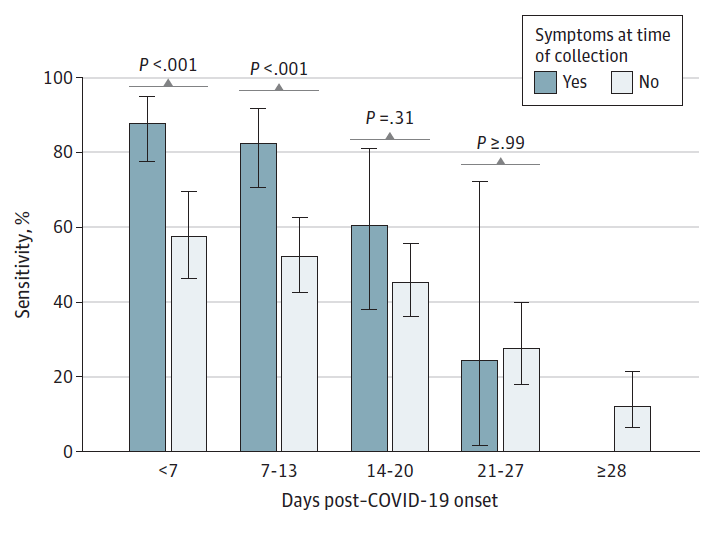
- Change in saliva RT-PCR sensitivity over the course of SARS-CoV-2 infection.external icon Congrave-Wilson et al. JAMA (August 13, 2021). In a longitudinal study of paired nasopharyngeal and saliva specimens collected every 3–7 days, saliva sensitivity for SARS-CoV-2 detection was highest in specimens collected during the 1st week of infection (71.2%, 95% CI 62.6%-78.8%) and decreased each subsequent week. Saliva testing might be useful for detecting SARS-CoV-2 in the first 2 weeks after symptom onset, but sensitivity for screening asymptomatic people was <60% at all time points.
Note: Adapted from Congrave-Wilson et al. Saliva sensitivity for SARS-CoV-2 detection among symptomatic vs. asymptomatic participants at time of specimen collection. Error bars indicate 95% CIs. Reproduced with permission from JAMA, 2021. Published online August 13, 2021. https://doi.org/10.1001/jama.2021.13967. Copyright© 2021 American Medical Association. All rights reserved.
Health Equity
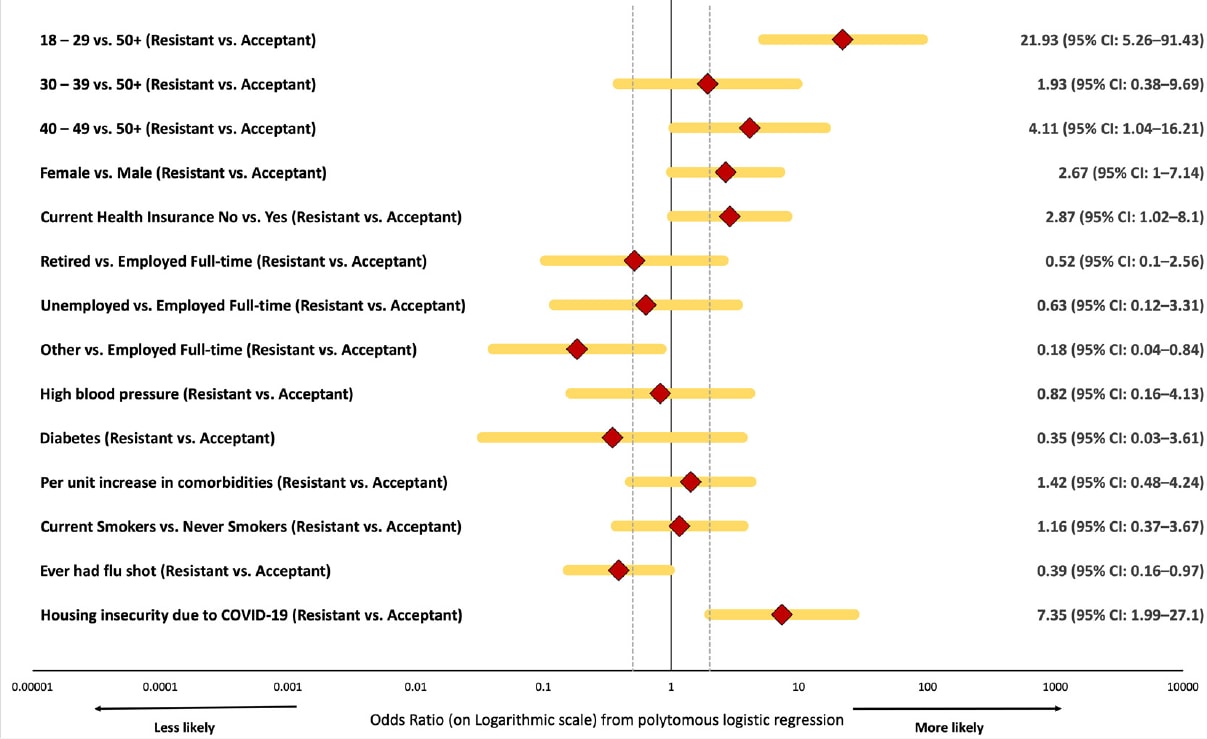
- Correlates of COVID-19 vaccine hesitancy among a community sample of African Americans living in the southern United States.external icon Moore et al. Vaccines (August 8, 2021). Among 257 African American adults residing in the Central Savannah River Area in December 2020–April 2021, about 1/3 reported resistance (n = 42) or hesitancy (n = 40) to receiving free COVID-19 vaccination. Vaccine resistance was associated with age <30 years or housing insecurity due to COVID-19.
Note: Adapted from Moore et al. Characteristics associated with vaccine resistance versus acceptance included age <30 years (OR: 21.93, 95% CI 5.26–91.43) and housing insecurity (OR: 7.35, 95% CI 1.99-27.10). Licensed under CC BY 4.0.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.