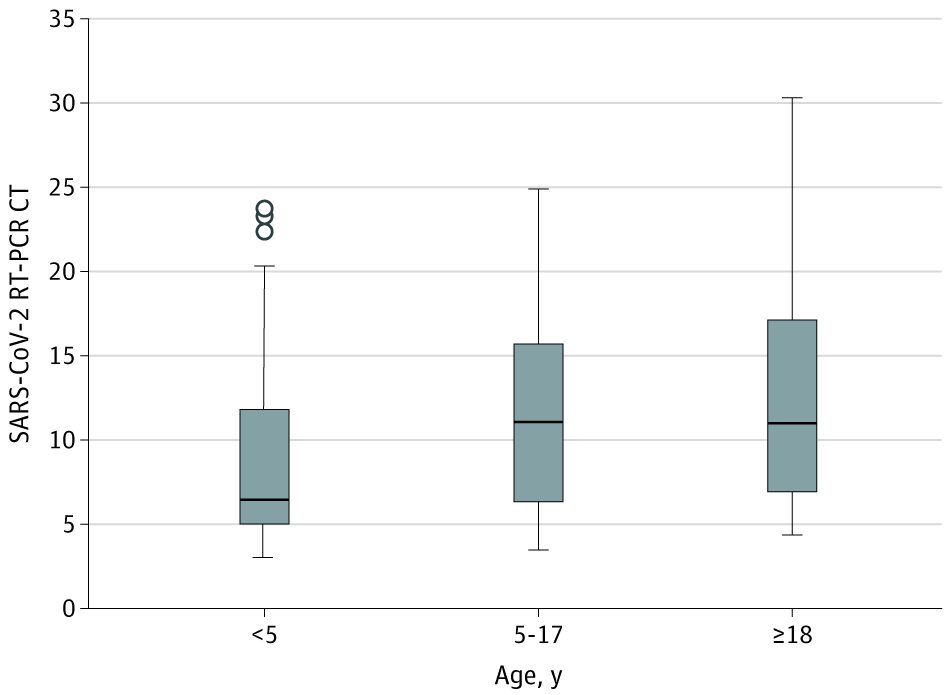COVID-19 Science Update released: August 11, 2020 Edition 38

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
Universities across the United States are wrestling with whether and how to reopen this fall in order to optimize learning while minimizing SARS-CoV-2 spread. Below, we summarize a model’s predicted impacts from various campus screening strategies, and related commentary from authors using assumptions for their institution.
PEER-REVIEWED
A. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United Statesexternal icon. Paltiel et al. JAMA Network Open (July 31, 2020).
Key findings:
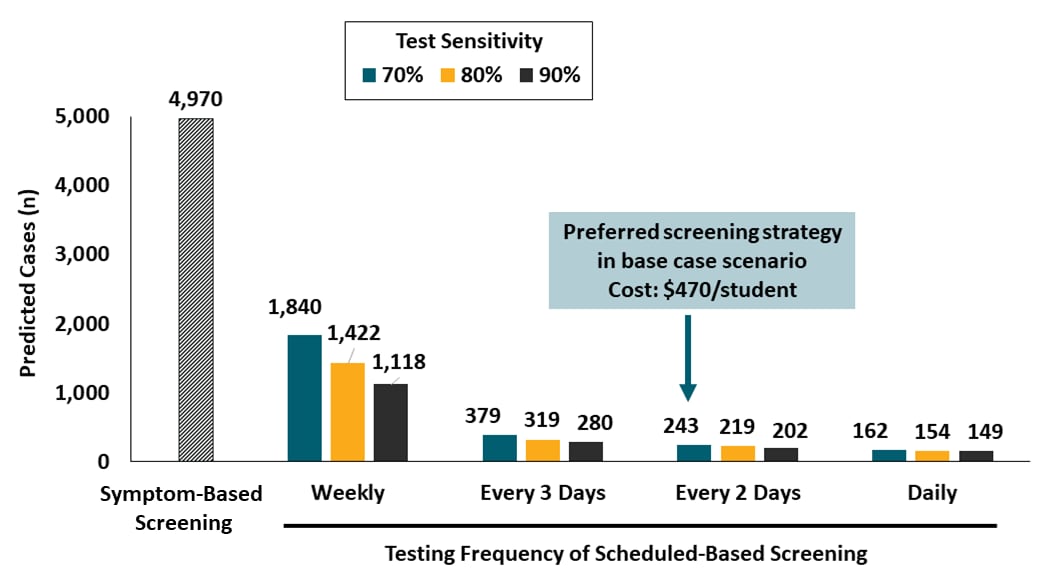
- Screening every 2 days with a rapid inexpensive test with sensitivity >70%, plus strict adherence to behavioral interventions could maintain a low number of COVID-19 cases within a pre-defined cost threshold (Figure).
- Screening strategies based on weekly testing or solely on symptoms would be comparably less effective.
- Cost to implement the preferred screening strategy ranged from $120-$910 per student.
Methods: Modeling study with a hypothetical college student cohort in three scenarios—base case, worst case, and best case defined by varying reproductive number (Rt), testing frequency, test characteristics, and number of weekly infections imported to the student population by university employees or community members. Model assumed an 80-day fall semester, 3-day incubation period, 14-day recovery time, and availability of an isolation dormitory. Inputs for the decision and cost-effectiveness analyses included symptom-based screening and varying test frequency, sensitivity, specificity, and cost. Limitations: Model results are based on assumptions; multiple parameters not included, e.g., clinical harms and costs incurred, effects of contact tracing, pre-existing immunity in the cohort, logistic challenges of testing, and faculty and staff screening.
Figure:
Note: Adapted from Paltiel et al. Base case scenario (Rt 2.5, test specificity 98%, 10 imported infections per week) during an 80-day semester, predicted number of infections vary by testing strategy. Model for symptom-based testing assumed a 90% sensitive test; scheduled screening models included estimates for tests with, 70%, 80% and 90% sensitivity.
Reopening colleges during the coronavirus disease 2019 (COVID-19) pandemic—One size does not fit allexternal icon. Bradley et al. JAMA Network Open (July 31, 2020).
Key findings:
- Both testing students before they arrive to limit initial infections and reducing the reproductive number (Rt) through multiple interventions could decrease the frequency of testing needed and substantially limit SARS-CoV-2 infections.
- By limiting initially infected students to 5 and Rt to 1.25, Vassar College might expect:
- 79 infections with testing every 4 weeks.
- 50 infections with testing every 2 weeks.
Methods: Applied an online version of the Paltiel et al.external icon model using assumptions for Vassar College: 2,500 students, 5 initially infected, Rt of 1.25, 1 new infection per week, and 80% sensitive of 80% and 99% specific PCR test. Limitations: Results depend on assumptions from the underlying model and for Vassar College, which include a very low initial infection rate and strictly limited interactions.
Implications for study (Paltiel et al.) and commentary (Bradley et al.): Frequent test-based screening may be necessary to reduce the risk of uncontrolled infections and allow the safe return of students to campus. Robust combinations of interventions that substantially reduce Rt may keep both SARS-CoV-2 infections and frequency of required testing as low as possible.
PEER-REVIEWED
Coronavirus disease 2019 (COVID-19) outbreak in a San Francisco homeless shelter.external icon Imbert et al. Clinical Infectious Disease (August 3, 2020).
Key findings:
- During the first 2 days of this outbreak investigation, only 8 of 22 high-risk shelter resident contacts could be identified through contract tracing, of whom 7 were additional cases.
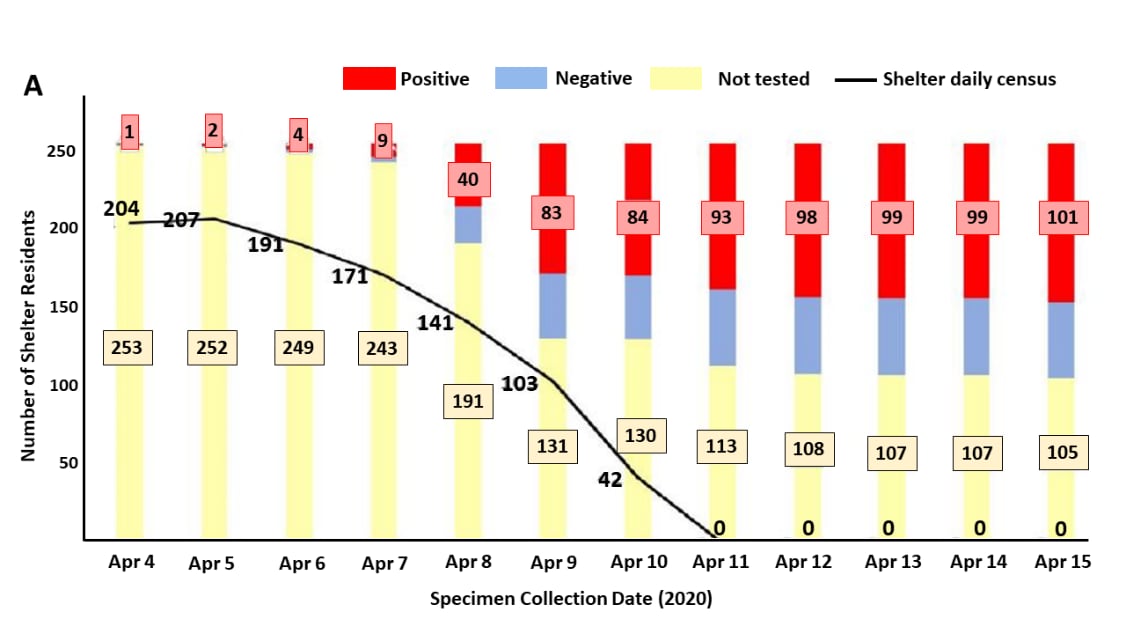
- During mass testing, 150 out of 255 (59%) residents were successfully tested; 101 (67%) tested positive (Figure).
- 52 (51%) were asymptomatic at time of testing, 21% were age > 60 years, and 27% had underlying medical conditions.
- Among 64 staff, 60 (94%) were tested, of whom 10 (17%) tested positive.
- After the shelter closed, 190 residents moved to single occupancy hotel rooms, including 100 of those who tested positive.
Methods: Describes a public health response to a COVID-19 outbreak in a 250-bed homeless shelter; two index cases tested positive for COVID-19 on April 4-5; contact tracing was initiated over the next two days, followed by mass testing until April 15, 2020. The shelter closed on April 11, and residents moved to hotels for isolation and quarantine. Limitations: Poor case interview completion rate; limited contact information provided; low acceptance of testing; underreporting of symptoms.
Implications: For outbreak response among people in crowded settings such as homeless shelters, mass testing can provide a faster and more informative snapshot of the outbreak than contact tracing.
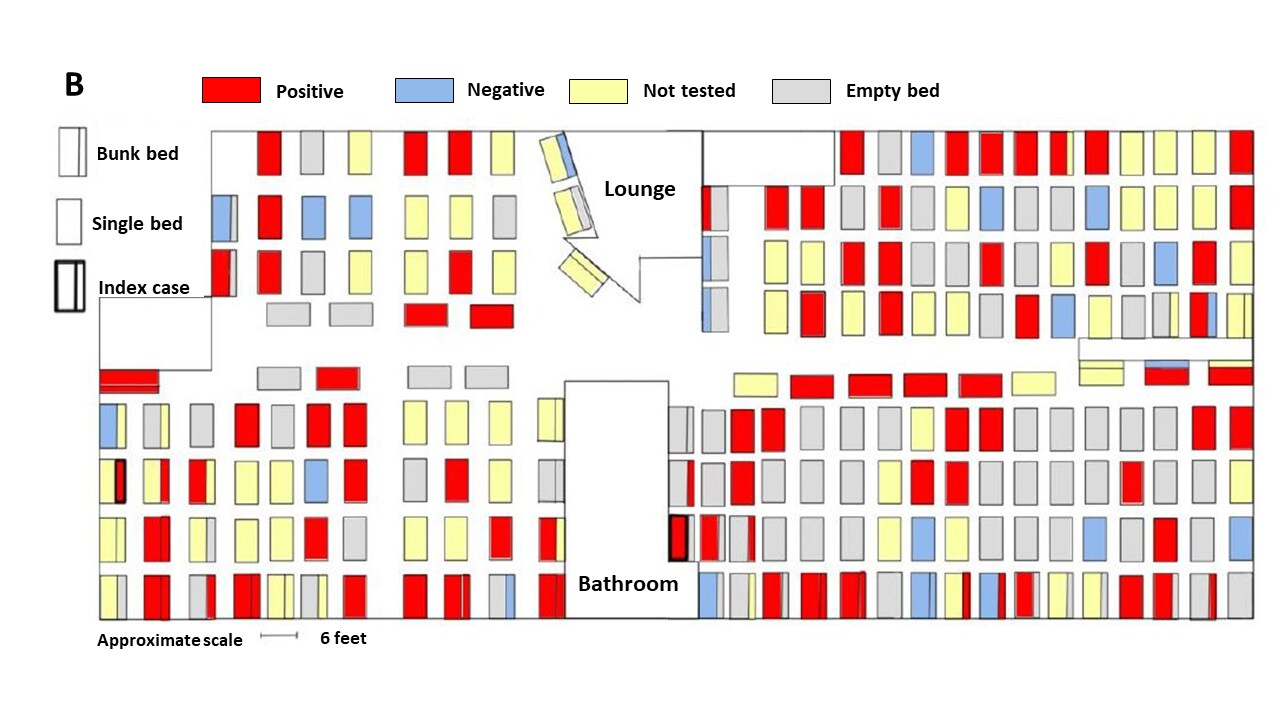
Figure:
Note: Adapted from Imbert et al. (A) Positive and negative results and proportion of those who were not tested among shelter residents during each day of the public health outbreak response. (B) Final SARS-CoV-2 testing outcomes on the second floor of the homeless shelter where 89 of 101 cases slept.
Transmission of SARS-CoV-2 in Australian educational settings: A prospective cohort study.external icon Macartney et al. Lancet Child & Adolescent Health (August 3, 2020).
Key findings:
- In 25 educational settings (15 schools, 10 early childhood education and care [ECEC]), 27 COVID-19 cases (12 children, 15 adults) exposed 1,448 other children and staff.
- 633/1,448 (43.7%) contacts were tested; 18 additional cases were identified.
- Of these, 5 (27.8%) were attributed to contacts in educational settings.
Methods: Cases associated with 25 educational settings, with symptom onset from January 13-May 1, 2020 in New South Wales, Australia, were identified through public health surveillance. Contacts were identified and tested with PCR, serology, or both. On March 23, distance learning was implemented for most students. Limitations: Staff and student enrollment data were not available for ECECs; relevance of these findings from a low prevalence setting to the U.S. may be limited.
Implications: In communities with low prevalence, risk of transmission in schools and early childhood settings appears low in the context of mitigation strategies.
Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 levels (SARS-CoV-2) in patients with mild to moderate coronavirus disease 2019 (COVID-19)external icon. Heald-Sargent et al. JAMA Pediatrics. (July 30, 2020).
Key findings:
- Children age < 5 years had lower median cycle threshold (Ct) values (6.5) than children aged 5-17 years (11.1; p = 0.02) and adults (11.0; p = 0.001), indicating that young children had a 10- to 100-fold greater amount of SARS-CoV-2 viral nucleic acid in the upper respiratory tract than older children and adults.
- Ct values were similar between children aged 5-17 years and adults (p= 0.34).
Methods: Cross-sectional study of 145 patients (46 children aged 1 month to 5 years, 51 children aged 5 to 17 years, and 48 adults aged 18 to 65 years) presenting for SARS-CoV-2 testing within 1 week of the onset of mild-to-moderate illness, Chicago, Illinois, March 23-April 27, 2020. NP swabs were collected and PCR Ct values were measured (NB: Ct values are inversely proportional to the amount of viral RNA present in a sample). Limitations: Relatively small sample, single-center, measured viral RNA and not infectious virus, did not assess person-to-person spread.
Implications: Despite milder symptoms of COVID-19 in children age < 5 years, these data suggest such children could be more infectious than adults and older children. More information on the correlation of respiratory tract viral load and infectiousness is needed as communities work to re-open day cares and schools.
Figure:
Note: Adapted from Heald-Sargent et al. Distribution of SARS-CoV-2 PCR Ct values from NP swabs collected from patients with COVID-19. Midlines indicate the medians, boxes indicate IQRs, whiskers indicate the upper and lower adjacent values (within 1.5-fold the IQR), and isolated data points indicate outliers.
PREPRINTS (NOT PEER-REVIEWED)
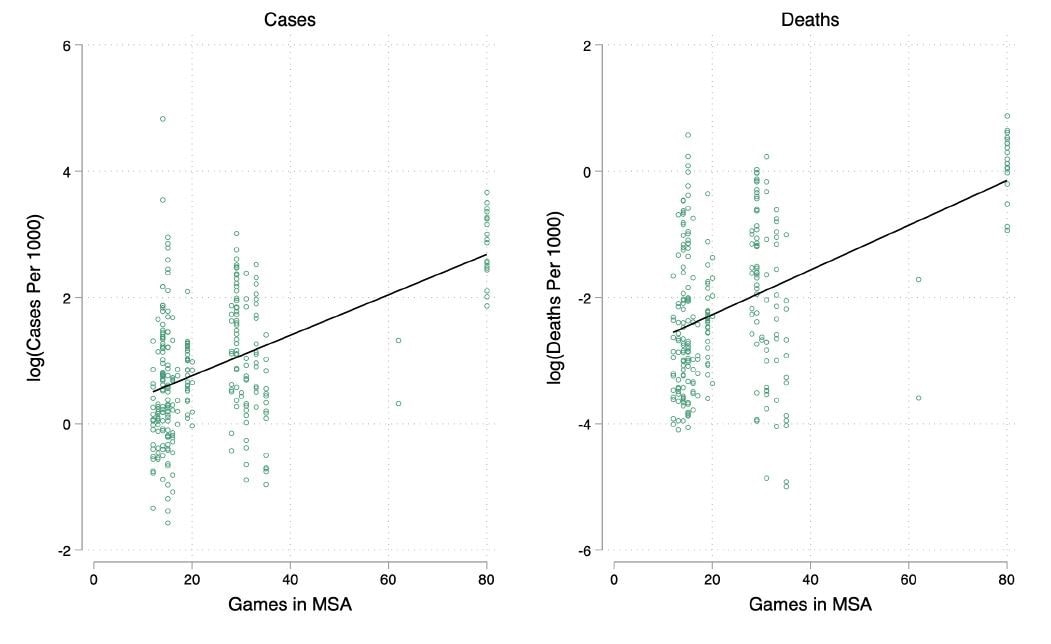
Effects of large gatherings on the COVID-19 epidemic: Evidence from professional and college sportsexternal icon. Wing et al. SSRN (July 21, 2020).
Key findings:
- Mass gatherings at large professional sporting events held January 1-March 12, 2020 may have resulted in an estimated 17,000 cases and 1,160 deaths in metropolitan statistical areas (MSAs) where they occurred (Figure).
- Each additional event played was associated with a 2.9-4.0% increase in cases per 1,000 residents per county within the MSA.
- Effects on counties directly hosting events were greater than on nearby counties in the same MSA.
Methods: Assessment of COVID-19 cases and mortality (up to May 17, 2020) across areas that hosted NHL, NBA, and NCAA games using modeling based on observed data. Analyses controlled for population density, risk factors for COVID-19 (i.e. age, gender, race), and number of professional sports teams per area. Limitations: Does not present complete statistics or assess behavioral mitigation strategies or testing (likely minimal during the period of assessment); no attendance numbers per game; no travel patterns to and from geographical areas.
Implications: Despite modest statistical correlations that limit interpretation of the modeled effect sizes, the results validate decisions to halt sporting events and caution against fans returning to college or professional games in the absence of other mitigation strategies.
Figure:
Note: From Wing et al. Each point in the graph represents one of 296 counties located in an MSA where at least one county has one NHL or NBA team. The x-axis is the combined number of NHL and NBA games played between January 1-March 12, 2020. The y-axis is the log cumulative number of confirmed cases of COVID-19 per 1000 people in the county as of May 17, 2020. The solid line is the least squares regression for cases (β = 0.035, p < 0.05, R2 = 0.56 for the model) and deaths (β = 0.051, p < 0.05, R2 = 0.59 for the model) per 1,000 population, respectively.
PEER-REVIEWED
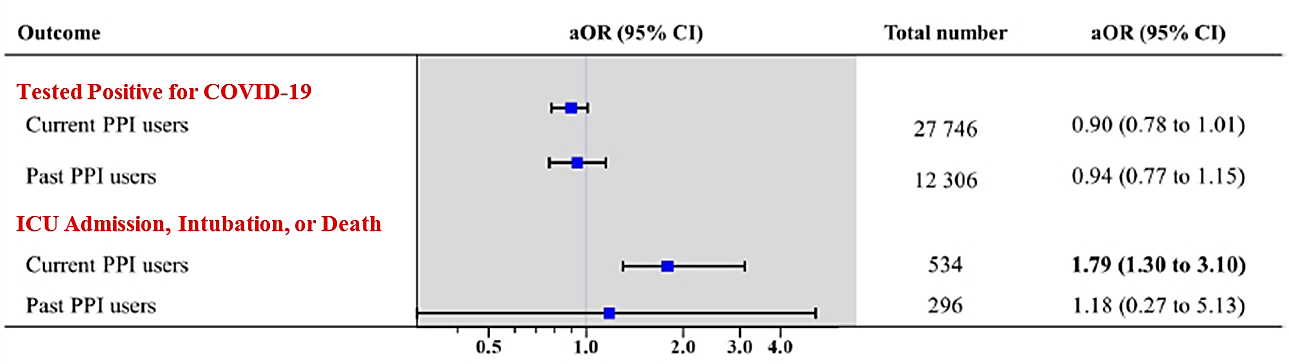
Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: A nationwide cohort study with propensity score matchingexternal icon. Lee et al. Gut (July 30, 2020).
Key findings:
- Current use of PPIs, especially within 1 month of testing for SARS-CoV-2, was associated with more serious clinical outcomes (i.e., ICU admission, intubation, or death).
Methods: Cohort study (N = 132,316) of persons age > 18 years tested for SARS-CoV-2, January 1-May 1, 2020, using the Korean national health insurance claims database. Examined differences in SARS-CoV-2 positivity and clinical outcomes associated with PPI use (current user n = 14,163; former user n = 6,242; never-PPI user n = 111,911). Current users took PPIs 1–30 days before the testing date; past users took PPIs 31–365 days before the testing date; never users had never received PPIs within 1 year before the testing date. Propensity score matching was used to calculate predicted probabilities for COVID-19 positivity and clinical outcomes. Limitations: Observational cohort study, PPI use categories were defined based only on past 1 year.
Implications: Clinicians may wish to carefully consider initiating use of PPIs in patients during the current COVID-19 pandemic as well as consider alternatives to PPIs for patients diagnosed with COVID-19 who are already using them. Additional studies may be needed to further explain these findings.
Figure:
Note: Adapted from Lee et al. COVID-19 positive tests and clinical outcomes by PPI use. Adjusted for age, sex, urban vs rural residence, underlying disease, Charlson comorbidity index as well as current use systemic steroids, metformin, and aspirin. aOR = fully adjusted odds ratio. Number in bold indicate significant differences (p < 0.05).
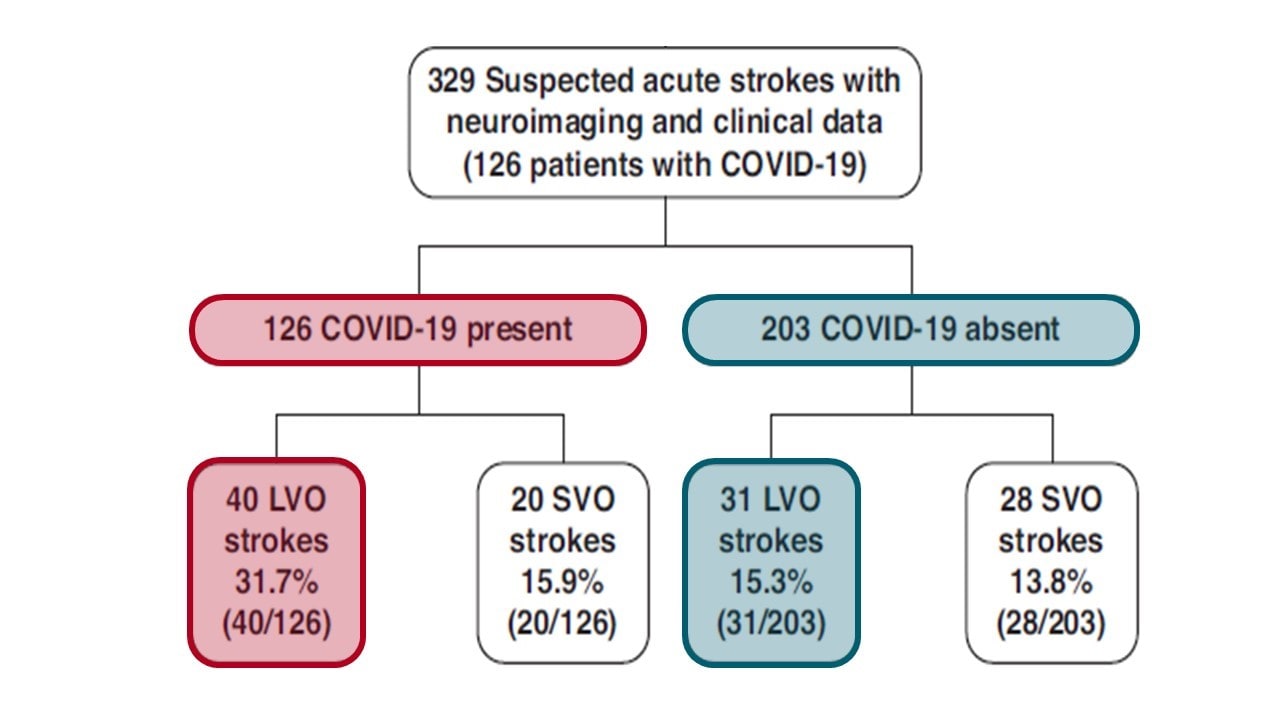
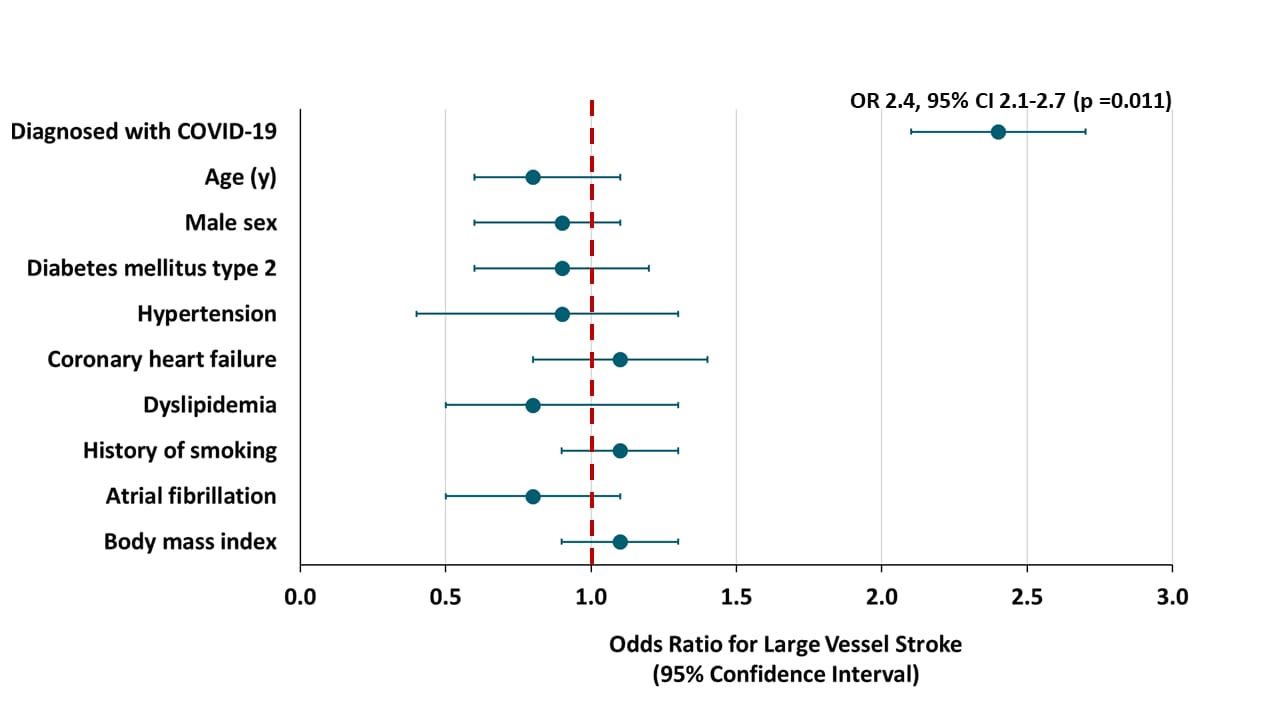
Association of coronavirus disease (COVID-19) with large vessel occlusion strokes: A case-control studyexternal icon. Kihira et al. American Journal of Roentgenology (July 29, 2020]).
Key findings:
- Large vessel occlusion strokes were more common in persons diagnosed with COVID-19 (31.7% vs. 15.3%, p = 0.001) (Figure 1).
- No association found with small vessel occlusion.
- The association with large vessel stroke remained significant after controlling for White race and Hispanic ethnicity (2.4 odds ratio, p = 0.011); other stroke risk factors were not associated with COVID-19 status (Figure 2).
Methods: Retrospective case control study in a New York City hospital system March 15-April 30, 2020 of 329 persons evaluated for suspected acute strokes (126 with COVID-19; 203 without COVID-19) clinically and with neuroimaging. Stroke-related variables were compared in the COVID-19 group versus controls. Limitations: Small sample; causality not evaluated.
Implications: Among persons with COVID-19, a lower threshold for stroke evaluation for large vessel occlusion is warranted. Patients presenting with large vessel occlusion stroke may warrant evaluation for COVID-19 depending on community prevalence of infection. Studies with larger patient populations would be helpful.
Figure 1
Note: Adapted from Kihira et al. Study flowchart. CTA = computed tomography angiography, LVO = large vessel occlusion, SVO =small vessel occlusion.
Figure 2
Note: Adapted from Kihira et al. Association of stroke risk factors with large vessel stroke, data controlled for race and ethnicity.
Sufficient levels of neutralizing antibodies (NAbs) protect from infection across a variety of viral pathogens. Anti-SARS-CoV-2 NAbs are found in convalescent plasma obtained from persons who have recovered from natural infection and have been elicited with some experimental vaccines. Characterizing NAbs from COVID-19 patients may provide relevant data for approaches to prevention and treatment of COVID-19.
PEER-REVIEWED
Potent neutralizing antibodies directed to multiple epitopes on SARS-CoV-2 spikeexternal icon. Liu et al. Nature (July 22, 2020).
Key findings:
- Identification of 61 different SARS-CoV-2 neutralizing antibodies (NAb) (Figure 1).
- Nine were highly potent (able to neutralize the virus in vitro at concentrations of 9 ng/mL or less) and bound the top area of the spike protein: four to the receptor binding domain, three to the N-terminal domain, and two to novel nearby epitopes (Figure 2).
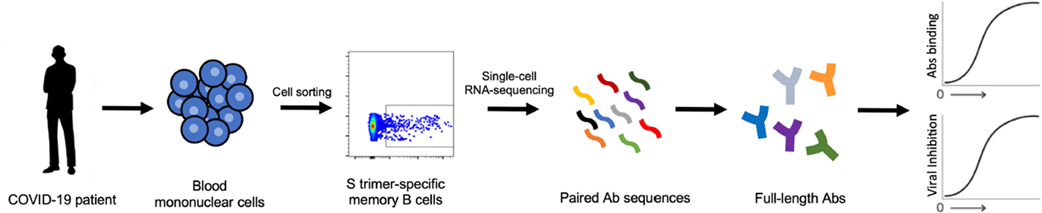
Methods: Plasma from 40 COVID-19 patients was screened for NAbs against SARS-CoV-2: 5 patients with the highest titers were selected for analysis and monoclonal antibody isolation. 252 unique antibodies that bound the SARS-CoV-2 spike protein were examined for neutralizing activity.
Figure 1
Note: Adapted from Liu et al. Flow diagram illustrating how the NAb that bind specifically to the SARS-CoV-2 spike (S) trimer were isolated from memory B cells in the blood of COVID-19 infected patients.
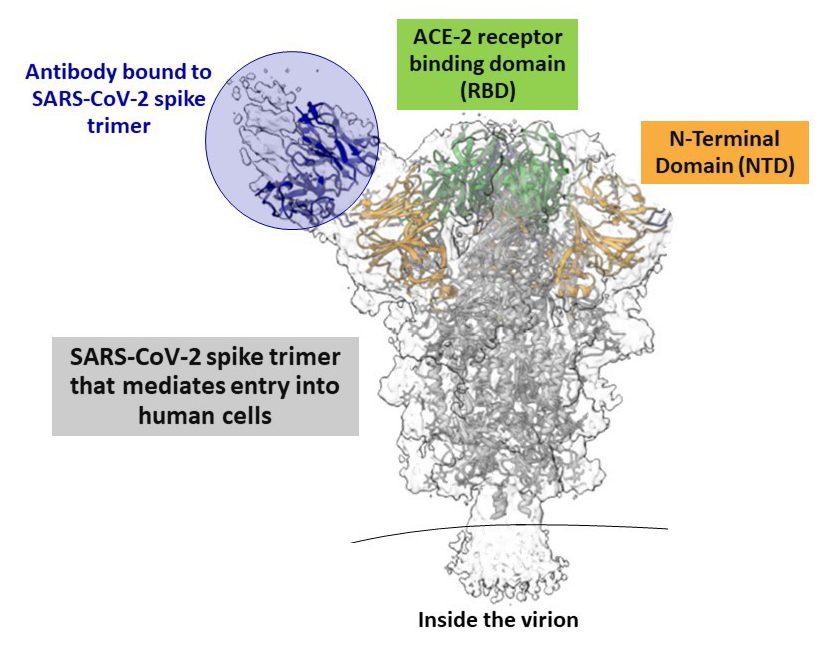
Figure 2
Note: Adapted from Liu et al. Cryo-electron microscopy reconstruction of antibody fragment of a neutralizing antibody bound to the N-terminal domain of the SARS-CoV-2 spike protein. NAbs were found to bind to the top of the spike on the receptor binding domain as well.
Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerabilityexternal icon. Brouwer et al. Science (Aug 7, 2020).
Key findings:
- 19 SARS-CoV-2 neutralizing antibodies (NAbs) against the SARS-CoV-2 spike protein were identified.
- 2 highly potent NAbs (able neutralize the virus at concentrations of 9 ng/ml or less) bound to the receptor binding domain of the spike protein.
Methods: Isolated and examined 323 unique antibodies produced by 3 patients diagnosed with COVID-19 approximately 4 weeks after symptom onset. 84 antibodies showing high-affinity binding to the SARS-CoV-2 spike protein were further examined for neutralization activity.
Implications for Liu et al. and Brouwer et al: Both studies demonstrated that naturally occurring, genetically diverse NAbs bind multiple sites on the SARS-CoV-2 spike protein, towards the top of the spike. Vaccines that mimic these sites could potentially elicit NAbs that block SARS-CoV-2 infection. Along with guiding vaccine design, several of these antibodies are promising candidates for clinical development to treat or prevent SARS CoV-2 infection and COVID-19.
PEER-REVIEWED
A vaccine to prevent SARS-CoV-2 is urgently needed and multiple candidates are currently being tested. At present, it is unknown if any of the vaccines being tested will prove efficacious and if so, the necessary number of doses for primary vaccination and whether revaccination will be needed. A vaccine that would provide high-level, long-lasting immunity with a single dose would be optimal.
Single-shot Ad26 vaccine protects against SARS-CoV2 in rhesus macaquesexternal icon. Mercado et al. Nature (July 24, 2020).
Key findings:
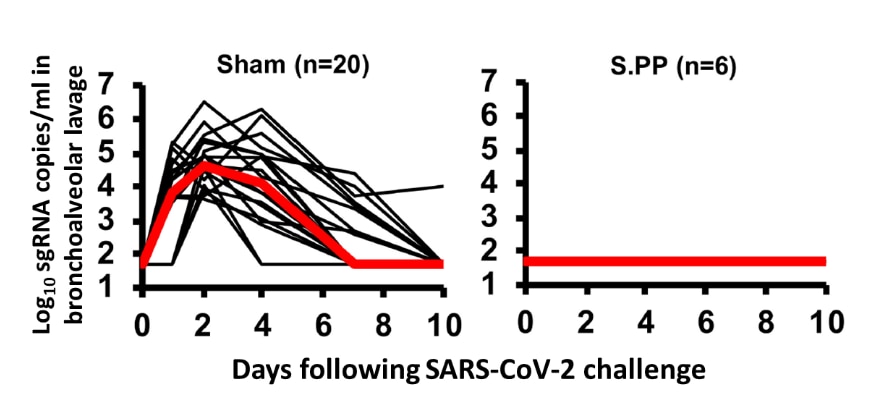
- Compared with control sham injections, vaccine candidates based on adenovirus serotype 26 (Ad26) vectors provided partial protection, although protection was optimal with the Ad26-S.PP vaccine.
- Ad26-S.PP-vaccinated macaques (n = 6) had no detectable virus in bronchoalveolar lavage after challenge with SARS-CoV-2 (Figure).
- Median neutralizing antibody (NAb) titers in the Ad26-S.PP-vaccinated macaques were 4-times higher than median NAb titers in previously reported cohorts of 9 macaques and 27 humans following recovery from SARS-CoV-2 infection (P < 0.0001) .
Methods: 32 adult rhesus macaques were immunized with a single dose of one of seven Ad26 vectors expressing the SARS-CoV-2 spike protein; 20 received a sham injection (control). Antibody responses were assessed using neutralization assays. At week 6, all animals were challenged with SARS-CoV-2 by the intranasal and intratracheal routes. Viral loads in bronchoalveolar lavage and nasal swabs were assessed by PCR. Limitations: Did not evaluate the durability of NAb responses; study was not designed to assess safety, vaccine-associated enhanced respiratory disease, or antibody-dependent enhancement of infection.
Implications: A single immunization with Ad26 vector-based vaccines provided complete or near-complete protection against SARS-CoV-2 challenge in rhesus macaques. A single dose SARS-CoV-2 vaccine would have important logistic and practical advantages compared with multiple-dose vaccines for mass vaccination campaigns and pandemic control. The Ad26-S.PP vaccine from this study is currently being evaluated in clinical trials.
Figure:
Note: Adapted from Mercado et al. Viral load in bronchoalveolar lavage in (left) sham controls and (right) in vaccinated (Ad26-S.PP vaccine) animals at baseline and following SARS-CoV-2 challenge. Days following challenge is on the x-axis. Red lines reflect median values.
Decision Tools for Policy Makers
- Duque et al. Timing social distancing to avert unmanageable COVID-19 hospital surgesexternal icon. Proceedings of National Academy of Sciences of the USA. Presents an optimization model for triggering short-term shelter-in-place orders based on 7-day rolling averages of hospital admissions in Austin, Texas.
- Panovska-Griffiths et al. Determining the optimal strategy for reopening schools, the impact of test and trace interventions, and the risk of occurrence of a second COVID-19 epidemic wave in the UK: a modelling studyexternal icon. The Lancet Child and Adolescent Health. Modeling suggests that reopening schools and society full-time or in alternating schedules should be accompanied by large-scale testing and contact tracing to prevent a second COVID-19 wave in the UK.
COVID-19 Related Health Disparities
- Weill et al. Social distancing responses to COVID-19 emergency declarations strongly differentiated by income.external icon Proceedings of National Academy of Sciences of United States of America. People in wealthier areas went from travelling the most before the pandemic to travelling least, whereas the opposite pattern occurred in the poorest areas.
- Kandil et al. African Americans struggle with the current COVID-19external icon. Annals of Surgery. Hospitalized African Americans with COVID-19 were over-represented in hospital admissions (77% African American; 23% Caucasians) and of younger age (63 years old) than Caucasians (76 years old).
- Evans, M. COVID’s color line — Infectious disease, inequity, and racial justiceexternal icon. Perspective on adverse outcomes for racial/ethnic minorities during pandemics past and present with recommendations for today.
- Mello et al. Respecting disability rights — Toward improved crisis standards of careexternal icon. NEJM. Offers a set of principles to reduce bias in health care triage decisions that affect persons with disabilities.
Pandemic Effects on Mental Health
- Kelly, M. The pandemic’s psychological toll: An emergency physician’s suicideexternal icon. Annals of Emergency Medicine. Suicide by an emergency physician at the height of the COVID-19 pandemic introduces efforts to reduce stigma and provide psychiatric and psychotherapeutic support for physicians.
- Tng et al. Psychological sequelae within different populations during the COVID-19 pandemic: a rapid review of extant evidenceexternal icon. Singapore Medical Journal. Reviews the impact of COVID-19 on the psychological well-being of the general adult population, healthcare workers, and persons with preexisting physical and psychiatric illnesses.
Diagnostic and Clinical Issues
- Zampino et al. Liver injury in remdesivir-treated COVID-19 patientsexternal icon. Hepatology International. Remdesivir caused hepatocellular injury in 5 COVID-19 patients without prior liver disease.
- Pranata et al. Body mass index and outcome in patients with COVID-19: A dose-response meta-analysis.external icon Diabetes & Metabolism. The risk for poor outcomes increased 5% for every 5 kg/mg2 increase in body mass index and became steeper from 30-35 kg/mg2 upwards.
- Petrucca et al. Validation of small-size-pooling approach targeted to hospital surveillance of SARS-CoV-2 infection.external icon Infection Control and Hospital Epidemiology. In a low-incidence setting, a 5-pool strategy to detect SAR-CoV-2 proved to be highly sensitive and specific while reducing the cost of laboratory resources.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.