COVID-19 Science Update released: July 23, 2021 Edition 99

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update align with the CDC Science Agenda for COVID-19.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
A longitudinal study of convergence between Black and White COVID-19 mortality: A county fixed effects approach.external icon Lawton et al. The Lancet Regional Health Americas (July 13, 2021).
Key findings:
- COVID-19 deaths increased 61% for non-Hispanic Blacks and 90% for non-Hispanic Whites nationally between June 2020 and January 2021.
- The COVID-19 mortality rate ratio of Blacks to Whites decreased by ~25%.
- County level analysis revealed that the Black/White mortality ratio changed the least in higher population density areas and decreased the most in younger populations.
Methods: National Vital Statistics System (NVSS) COVID-19 mortality data were used for national (May 6, 2020–January 27, 2021) and county-level (June 24, 2020–January 27, 2021) analyses. Demographic information was obtained from the American Community Surveys (ACS), CDC Behavioral Risk Factor Surveillance System, and USAfacts.org. Limitations: Delays in county-level reporting could underestimate the number of deaths. Only counties with more than 10 deaths for Black and White individuals were included.
Implications: The Black/White COVID-19 mortality ratio has been declining and the driving force for the observed mortality convergence appears to be changes within counties rather than county level differences or regional dispersion of COVID-19.
PEER-REVIEWED
SARS-CoV-2 testing and sequencing for international arrivals reveals significant cross border transmission of high risk variants into the United Kingdomexternal icon. Williams et al. EClinical Medicine (July 14, 2021).
Key findings:
- 1.9% of international travelers (3,855/203,065) tested positive for SARS-CoV-2 after arriving in the UK.
- Prevalence of infection was 2.4% in unvaccinated travelers (2449/105,193), 1.6% in travelers with 1 vaccine dose (244/15,415) and 0.5% in travelers with 2 vaccine doses (31/5,844).
- While 49 variants were identified from 1,635 sequences, Alpha (B.1.1.7) accounted for 80.6%, Beta (B.1.351) for 4.2% and Delta (B.1.617.2) for 1.7% of infections.
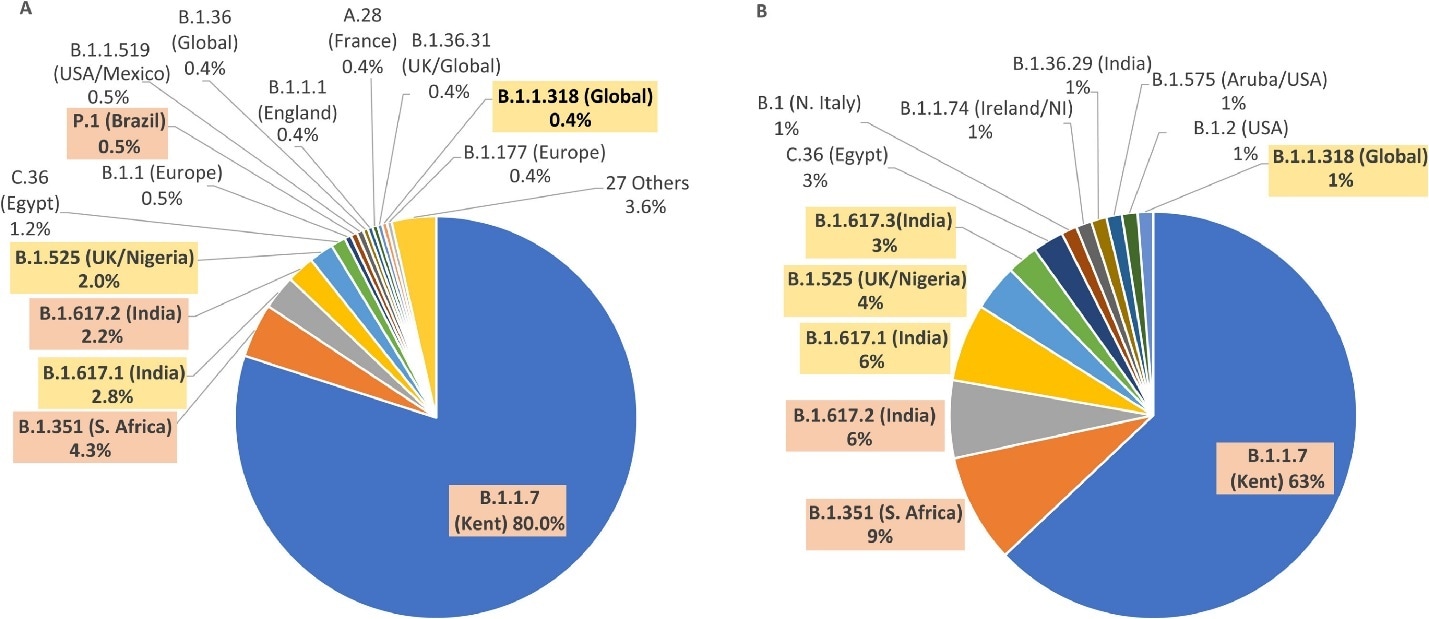
- Among 81 vaccinated travelers Alpha represented 63% of infections, compared to 80% of 759 unvaccinated travelers (Figure).
Methods: International travelers entering the United Kingdom between March 11, 2021 and April 14, 2021 had mandatory RT-PCR tests done 2- and 8-days post arrival. Specimens with Ct < 30 from Day 2 were chosen for sequencing (n = 1,913), which yielded 1,635 reads of sufficient quality for analysis. Limitations: Vaccination status was self-reported, missing for about 40% of travelers, and did not include vaccine type.
Implications: Cross-border introduction of variants is significant and traveler screening offers valuable information for understanding these introductions.
Figure:
Note: Adapted from Williams et al. SARS-CoV-2 strains sequenced from A) Unvaccinated and B) Vaccinated international travelers 2 days after arrival in UK. Variants of concern and variants of interest are highlighted. Licensed under CC-BY-NC-ND.
PEER-REVIEWED
Spike-antibody waning after second dose of BNT162b2 or ChAdOx1.external icon Shrotri et al. The Lancet (July 15, 2021).
Key findings:
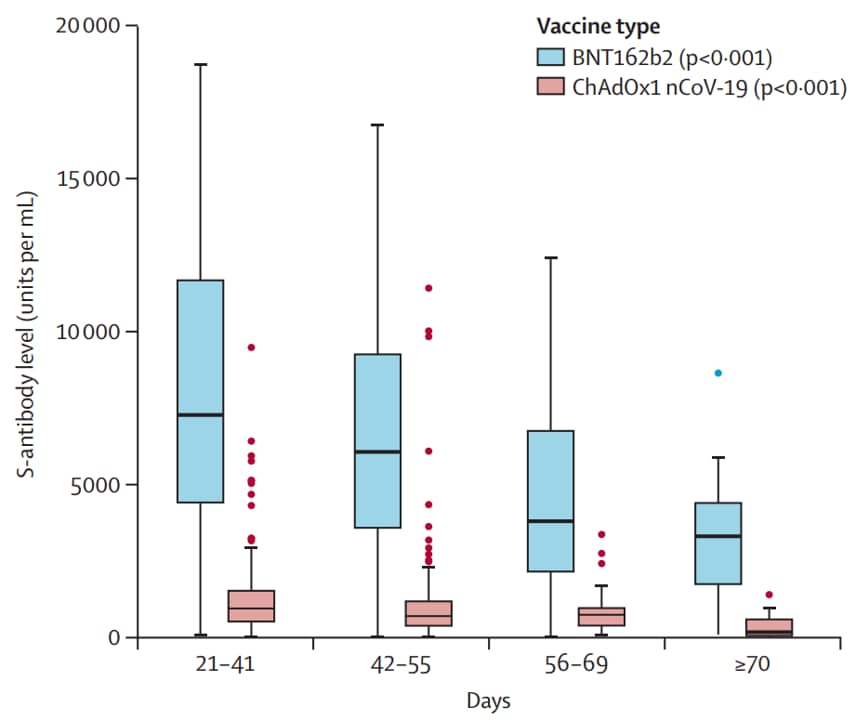
- Between 21-41 days and ≥70 days after 2nd dose, spike antibody (S-antibody) levels decreased in fully vaccinated adults by ~2-fold (BNT162b2) and ~5-fold (ChAdOx1) (Figure), regardless of sex, age, or clinical vulnerability.
- Average S-antibody levels were higher in individuals vaccinated with BNT162b2 than ChAdOx1.
- Women and individuals <65 years old had higher mean titers, regardless of vaccine received.
- At ≥70 days post-2nd dose, individuals classified as clinically vulnerable who received BNT162b2 had ~25-fold higher S-antibody levels than those vaccinated with ChAdOx1.
Methods: Spike antibody levels were measured in serum from fully vaccinated, previously uninfected adults (≥18 years old, n = 552) in England and Wales. Individuals were vaccinated with either BNT162b2 (Pfizer/BioNTech) or ChAdOx1 (Oxford/AstraZeneca), and a single sample was collected 14–154 days post-2nd dose. Limitations: Neutralization capability of the detected antibody levels is not reported.
Implications: The UK vaccination strategy of longer time interval between doses elicited antibody response, antibody levels for both BNT162b2 and ChAdOx1 vaccines, however, declined over time and there was a significant difference between vaccine types.
Figure:
Note: Box plots of antibody levels (units/mL) against SARS-CoV-2 spike glycoprotein (S-antibody) at defined timepoints after 2nd vaccine dose in individuals with no previous infection. Median interval between 1st and 2nd doses was 77 days. P-values derived from non-parametric tests for trends. Reprinted from The Lancet, online July 15, 2021, Shrotri et al., Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Copyright 2021, with permission from Elsevier.
Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis.external icon John et al. JAMA Internal Medicine (July 13, 2021).
Key findings:
- Among veterans with cirrhosis, a single dose of mRNA vaccine reduced SARS-CoV-2 infections by 64.8%, and hospitalizations or death by 100%, after 28 days. Two doses reduced infection by 78.6%.
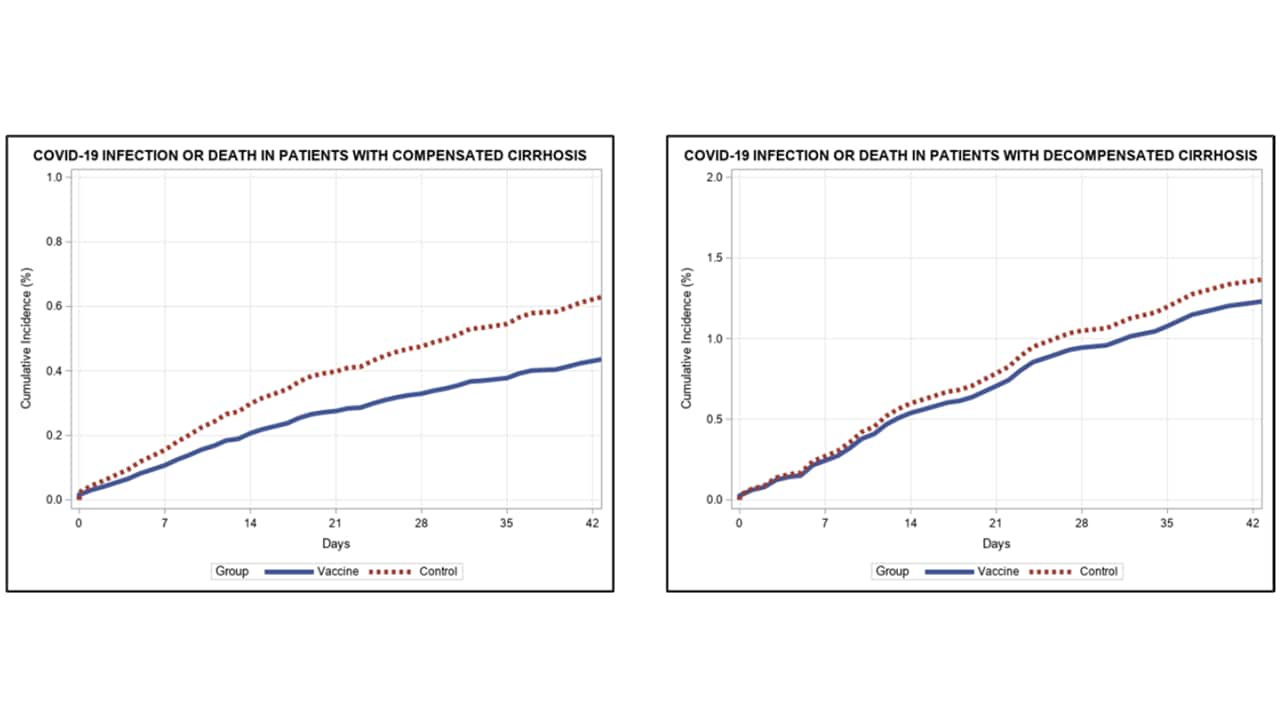
- The adjusted association between vaccination and infection was significant for patients with compensated cirrhosis (adjusted hazard ratio [aHR], 0.69; 95% CI 0.54-0.89) but not for patients with decompensated cirrhosis (aHR, 0.90; 95% CI 0.62-1.30) (Figure).
Methods: Retrospective cohort study among US veterans (18 years or older) diagnosed with cirrhosis. Vaccine recipients (n = 20,037) were matched by propensity score based on age group, sex, race, alcohol use, electronic Child-Turcotte-Pugh score, and cirrhosis comorbidity score to unvaccinated controls (n = 20,037). Primary outcomes were assessed 28 days following 1st dose and secondary outcomes 7 days after 2nd dose. Limitations: Study only tested mRNA-based vaccines; participants were predominantly male (97.3%) and white (60.6%).
Implications: Patients with cirrhosis benefit from mRNA vaccines, although vaccine efficacy related to infection is lower than in RCTs for both mRNA-1273external icon and BNT162b2external icon. Vaccinated cirrhosis patients, especially decompensated ones, should continue practicing nonpharmaceutical interventions following vaccination.
Figure:
NOTE: Adapted from John et al. Adjusted time from receipt of 1st dose of mRNA vaccine and COVID-19 infection or death in vaccine and control group patients with compensated cirrhosis (left) and decompensated (right). Reproduced with permission from JAMA Internal Medicine, 2021. Published online July 13, 2021. https://doi.org/10.1001/jamainternmed.2021.4325. Copyright© 2021 American Medical Association. All rights reserved.
Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccinationexternal icon. Barouch et al. NEJM (July 14, 2021).
Key findings:
- Vaccination with a single dose of Ad26.COV2.S continued to produce humoral and cellular immunity for at least 239 days.
- Median CD8+ T-cell responses was 0.0545%, 0.0554%, and 0.0734% on days 57, 85, and 239, respectively.
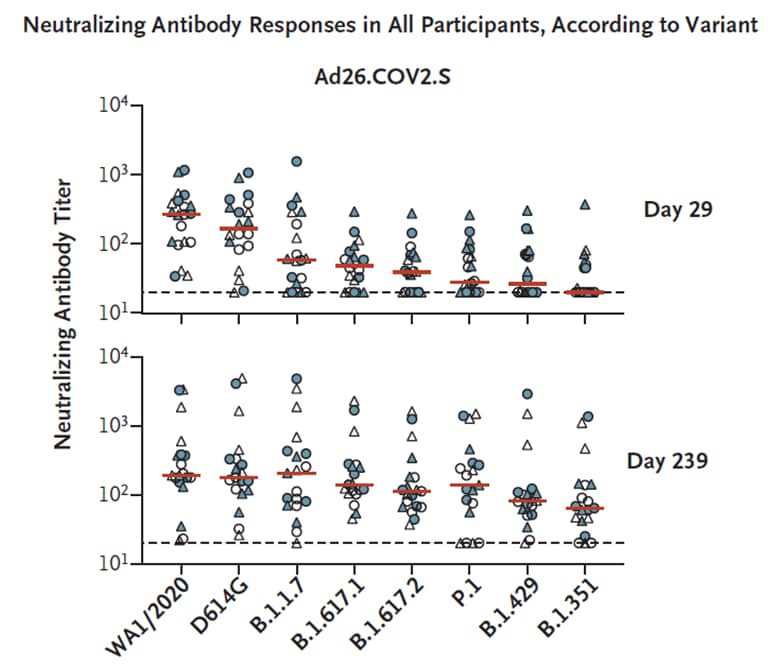
- Increased neutralization over time of SARS-CoV-2 variants, including B.1.617.2 (Delta), B.1.351 (Beta) and P.1 (Gamma) suggests maturation of B-cell responses (Figure).
Methods: Durability of humoral and cellular immune responses in experimental group receiving Ad26.COV2.S (Johnson & Johnson/Janssen) vaccination (n = 20) and placebo (n = 5) studied over 8 months. Median neutralizing antibody titers were evaluated by ELISA for the WA1/2020, D614G, B.1.1.7 (Alpha), B.1.617.1 (Kappa), B.1.617.2 (Delta), P.1, B.1.429 (Epsilon), and B.1.351 (Beta) variants. Interferon-γ CD8+ and CD4+ T-cell responses were measured on days 29, 57, 71 or 85, and 239. Limitations: Small sample size.
Implications: The Ad26.COV2.S vaccine induces prolonged immune response against WA1/2020. Neutralization effects expanded against other variants over time, likely through maturation of B-cell responses.
Figure:
NOTE: Adapted from Barouch et al. Neutralizing antibody responses in all participants, according to variant. Humoral and cellular immune responses after Ad26.COV2.S vaccination. Pseudovirus neutralizing antibody titers (y-axis) against parental strain (WA1/2020), D614G, B.1.1.7 (Alpha), B.1.617.1 (Kappa), B.1.617.2 (Delta), P.1 (Gamma), B.1.429 (Epsilon), and B.1.351 (Beta) on days 29 and 239. Red bars denote medians; horizontal dashed line indicates lower limit of quantitation. From NEJM, Barouch et al., Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination, published online July 14, 2021. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israelexternal icon. Brosh-Nissimov et al. Clinical Microbiology and Infection (July 6, 2021).
Key findings:
- 96% of 152 fully vaccinated hospitalized patients with SARS-CoV-2 infections had at least 1 co-morbidity, including diabetes (73, 48%), hypertension (108, 71%), chronic renal failure (48, 32%), congestive heart failure (41, 27%), chronic lung disease (37, 24%), and cancer (36, 24%).
- Immunosuppression was present in 40% (60) of patients, including 11% (16) with a solid organ transplant and 7% (10) on anti-CD20 therapy.
- 38 patients had poor outcomes including 34 (22%) who died.
- Among 45 patients with available genotyping, 89% (40) were infected with B.1.1.7 (Alpha), 7% (3) with wild-type, and 4% (2) with B.1.351 (Beta) SARS-CoV-2 variants.
Methods: Multicenter retrospective cohort of 152 hospitalized patients fully vaccinated with BNT162b2 (Pfizer/BioNTech), conducted between January 18 and April 20, 2021. Breakthrough infections were identified as symptom onset, SARS-CoV-2 positive PCR test, or admission 8 days after a second dose of BNT162b2 vaccine. Patients’ outcomes were either poor: requiring ventilation or in-hospital death; or favorable: not requiring ventilation or being discharged. Limitations: Study not designed to explore risk factors for vaccine failures; small cohort; patients were predominantly male and median age 71 years.
Implications: Hospitalized patients with breakthrough infections had a high burden of comorbidities and immunosuppression.
PEER-REVIEWED
Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant womenexternal icon. Goldshtein et al. JAMA (July 12, 2021).
Key findings:
- Among vaccinated pregnant women, effectiveness of BNT162b2 >28 days after 1st dose was 78% (adjusted hazard ratio of infection 0.22; 95% CI 0.11-0.43).
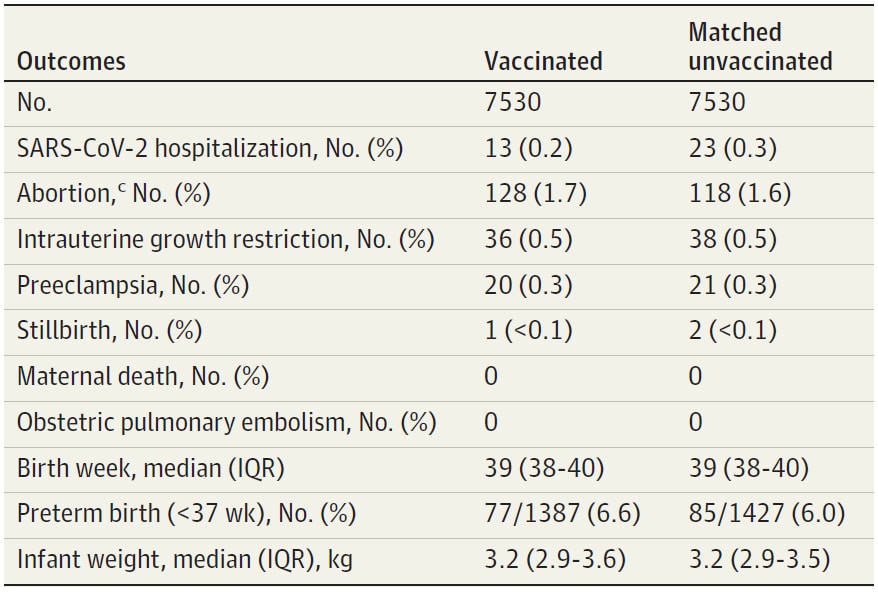
- There were no notable differences in pregnancy-related outcomes between vaccinated and unvaccinated women in exploratory analysis (Table).
Methods: Retrospective cohort study using an Israeli health care organization pregnancy registry. Pregnant women receiving 1st dose of BNT162b2 (Pfizer/BioNTech) between December 9, 2020 and February 28, 2021 (n = 7,530) were matched by age and gestational age with unvaccinated pregnant women (n = 7,530). Limitations: Observational study underpowered to compare adverse events; no data on strain of SARS-CoV-2 infections.
Implications: Vaccination with BNT162b2, consistent with a US-based preliminary analysis on the safety of mRNA vaccinesexternal icon, appears to be effective and safe in pregnant woman, a demographic currently without published Phase 3 data.
Table:
Note: Adapted from Goldshtein et al. Exploratory outcomes among the matched study population. Median follow up time was 37 days (IQR 21–54 days) for both groups; matched by age, gestational age, residential area, population subgroup, number of prior children and seasonal influenza vaccination in the prior year; (c) abortion category encompassed either spontaneous or induced abortion. Reproduced with permission from JAMA, 2021. Published online July 12, 2021. https://doi.org/10.1001/jama.2021.11035. Copyright© 2021 American Medical Association. All rights reserved.
Detection, Burden, and Impact
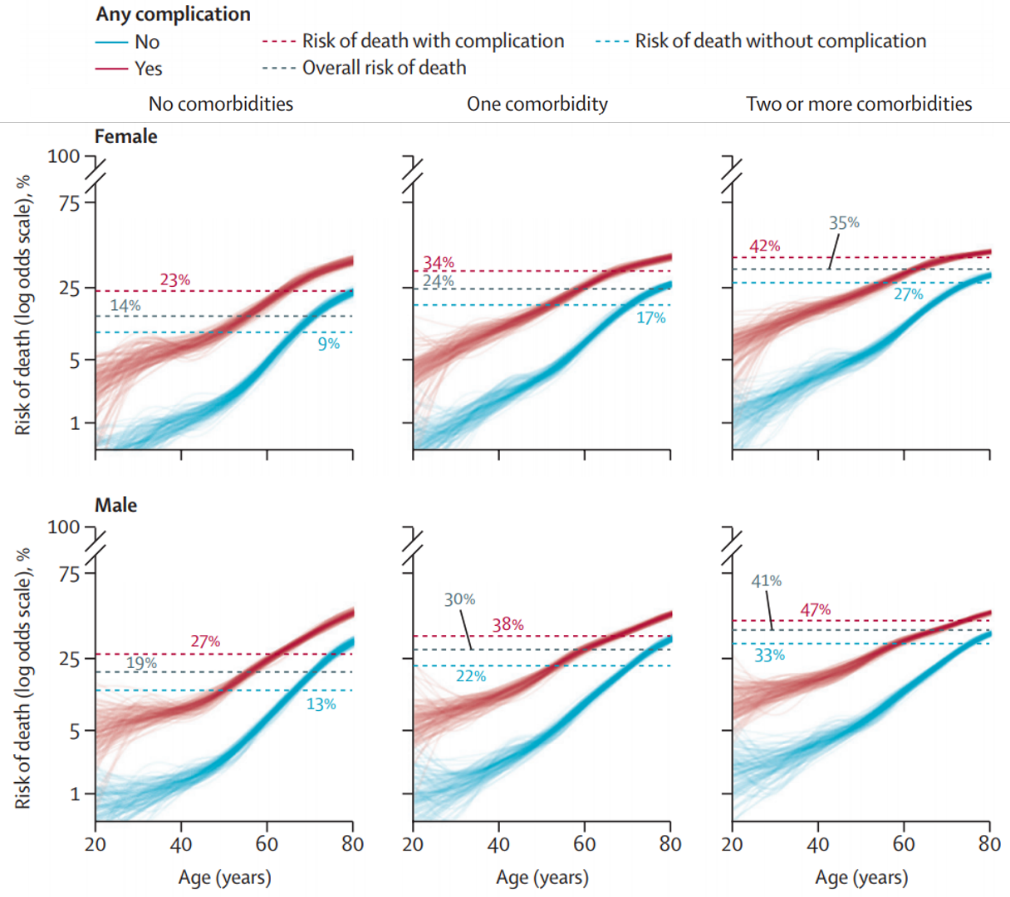
- Drake et al. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort studyexternal icon. The Lancet (July 17, 2021). Nearly 50% (n = 73,197) of adults with COVID-19 in 302 UK health care facilities developed at least 1 in-hospital complication. Presence of complications correlated with a higher risk of death. Complications were more likely in older patients and were more prevalent in males than in females.
Note: Adapted from Drake et al. Risk of death by sex, age, comorbidities, and development of complications while hospitalized for COVID-19 treatment. Licensed under CC-BY.
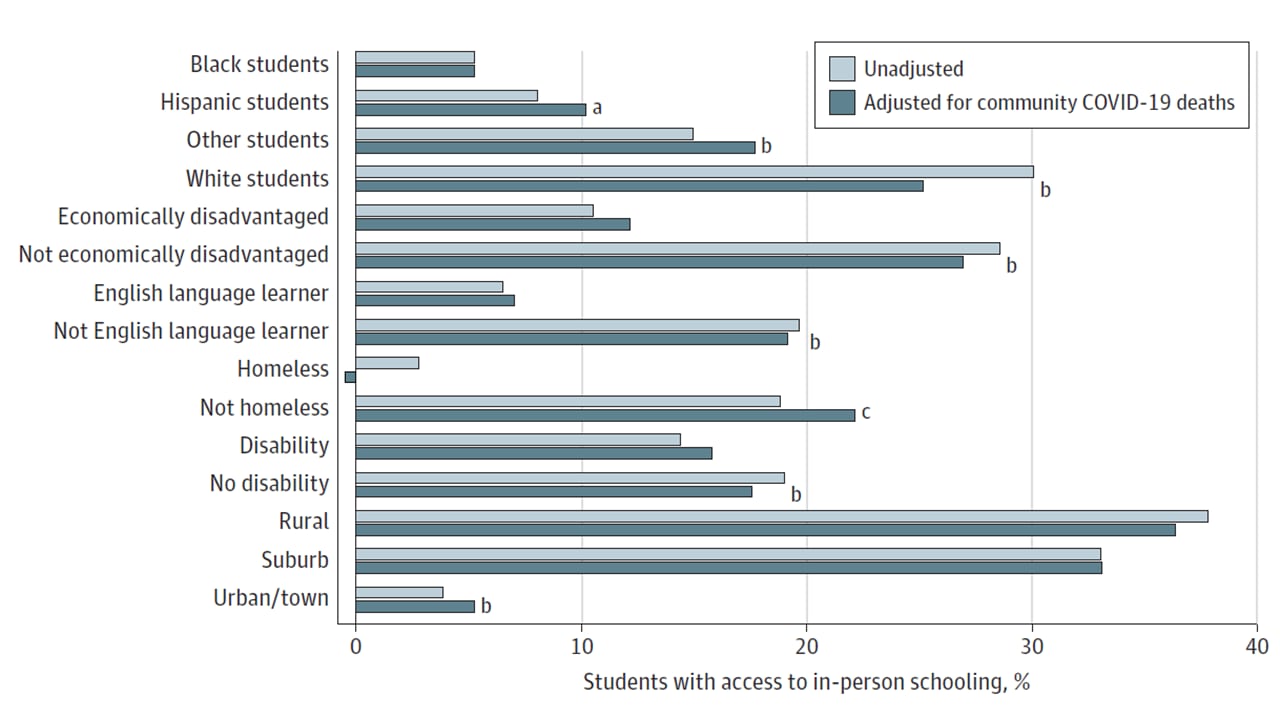
- Fox et al. Sociodemographic characteristics and inequities associated with access to in-person and remote elementary schooling during the COVID-19 pandemic in New York State.external icon JAMA Network Open (July 15, 2021). In October 2020, 38.5% of elementary schools remained open for in-person instruction in New York State (1.14 million students). Access to in-person instruction based on race/ethnicity was 30.1% for White students, 8.1% for Hispanics, and 5.3% for Blacks. Disadvantaged students were less likely to have in-person instruction. Local COVID-19 burden did not explain school reopening decisions.
Note: Adapted from Fox et al. Proportion of students with access to in-person learning by sociodemographic factor in Fall 2020. Adjusted values control for different mortality rates by county. a: p <0.10, b: p <0.01, c: p <0.05. Licensed under CC-BY.
- Frediani et al. Multidisciplinary assessment of the Abbott BinaxNOW SARS-CoV-2 point-of-care antigen test in the context of emerging viral variants and self-administration.external icon Scientific Reports (July 16, 2021). BinaxNOW accurately detected B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma) and B.1.617.2 (Delta). Concordance with RT-PCR decreased with increasing Ct values. BinaxNOW sensitivity/specificity for healthcare staff (n = 297, 77 positive) was 74%/99% compared to 57%/100% for parent or self-collected anterior nasal swabs (n = 44, 16 positive).
Note: Adapted from Frediani et al. BinaxNOW agreement with gold standard PCR by Ct values and self-collected samples. (A) Collection of diagnostic samples was completed by either trained healthcare workers (staff) or individual participants. % Agreement was calculated as the percentage of concordant test results per group. (B) Pictures of BinaxNOW results for positive and negative findings. Licensed under CC BY 4.0.
- Radtke et al. Long-term symptoms after SARS-CoV-2 infection in children and adolescents.external icon JAMA (July 15, 2021). In a school-based longitudinal cohort in Zurich, Switzerland 4% (4/109) of seropositive and 2% (28/1246) of seronegative children in October and November 2020 reported at least 1 symptom lasting more than 12 weeks. Good or excellent health was reported in similar proportions in both groups, and there were no hospitalizations among seropositive children. No severe infections were captured among children in this study.
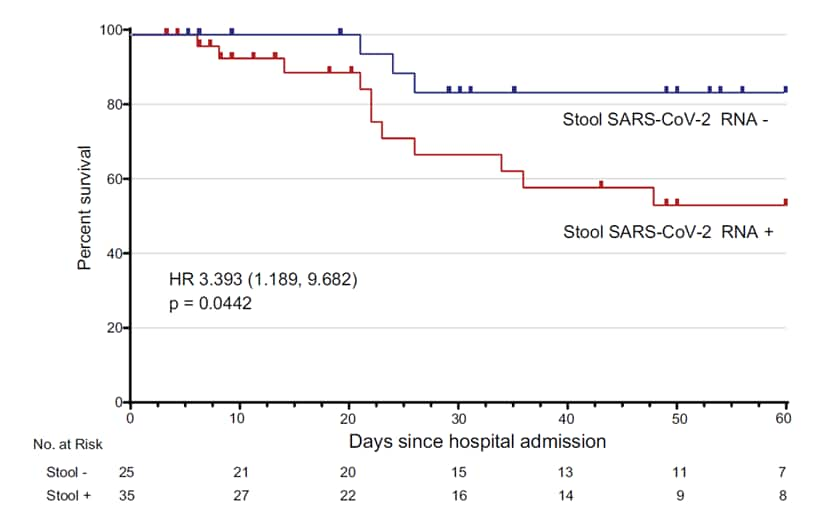
- Das Adhikari et al. Fecal SARS-CoV-2 RNA is associated with decreased COVID-19 survival.external icon Clinical Infectious Diseases (July 10, 2021). Hospitalized patients with detectable SARS-CoV-2 RNA in their stool (35/60) had higher mortality compared with those without detectable RNA but did not differ clinically or demographically. Stool viral loads did not correlate with plasma or nasal-pharyngeal swab RNA levels.
Note: Adapted from Das Adhikari et al. Kaplan-Meier survival curve of patients with and without detectable fecal SARS-CoV-2 RNA. Reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab623/6318824external icon.
Transmission of SARS-CoV-2
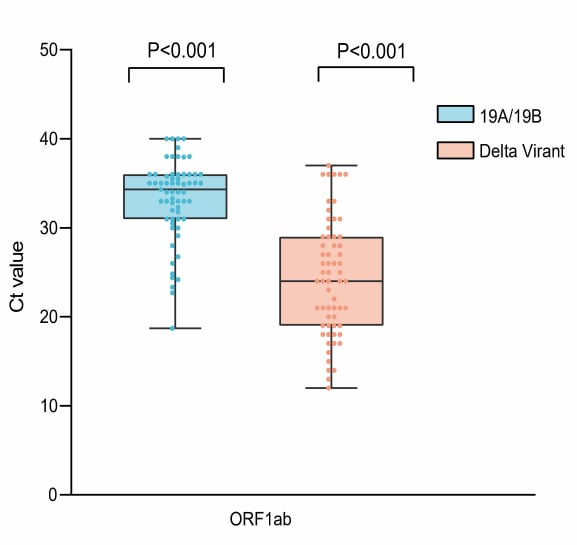
- Li et al. Viral infection and transmission in a large well-traced outbreak caused by the Delta SARS-CoV-2 variant.external icon medRxiv (Preprint; July 12, 2021). Published in Nature Communicationsexternal icon (January 24, 2022). What is believed to be the 1st locally acquired transmission of Delta on mainland China was identified in Guangdong May 21, 2021. The average viral load (Ct values) of 167 infections traced back to the index case were approximately 1,000-fold higher than the 19A/19B strains of the initial 2020 epidemic. The time interval from the exposure to PCR test positive was 4 days compared to 6 days during the 2020 epidemic.
Note: Adapted from Li et al. Ct values of Delta variant infections (n = 62) and previous 19A/19B strains infections (n = 63) in quarantined subjects. Dots represent Ct value distribution tested by RT-PCR in ORF1ab gene (left) and N gene (right). Box plots indicate the median and interquartile range (IQR); whiskers represent the maximum and minimum values. Licensed under CC-BY-NC-ND 4.0.
Natural History of SARS-CoV-2 Infection
- Redjoul et al. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients.external icon The Lancet (July 13, 2021). Among 88 allogenic hematopoietic stem cell transplant (HSCT) patients receiving 2 doses of BNT162b2, titers against SARS-CoV-2 reached neutralization levels > 4160 AU/mL in those more than 12 months beyond HSCT (n = 52) and having absolute lymphocyte counts in peripheral blood above 1G/L. Immunosuppressive treatments within 3 months of vaccination together with lymphocyte count below 1G/L remained independently correlated with low IgG (S-RBD) titers in multivariable analysis.
- Prendecki et al. Comparison of humoral and cellular responses in kidney transplant recipients receiving BNT162b2 and ChAdOx1 SARS-CoV-2 vaccines.external icon medRxiv (Preprint; July 14, 2021). Among infection-naïve kidney transplant recipients, 65.6% (269/410) of those fully vaccinated with BNT162b2 seroconverted compared to 43.6% (156/358) of those fully vaccinated with ChAdOx1 (p <0.0001). Only 26.4% (28/106) of patients had detectable T-cell responses with no significant difference by vaccine.
- Clarke et al. Comparison of immunogenicity between BNT162b2 and ChAdOx1 SARS-CoV-2 vaccines in a large haemodialysis population.external icon medRxiv (Preprint; July 14, 2021). Of 1,021 fully vaccinated COVID-19 naïve hemodialysis patients, those receiving BNT162b (n = 523) had higher anti-spike protein concentrations compared to those receiving ChAdOx1 (n = 498) (462 vs. 79 BAU/ml, p<0.0001). Immunosuppression was associated with a failure for vaccinees to cause seroconversion (p <0.0001). Only 38.2% of patients had detectable T-cell responses with no significant difference by vaccine.
Prevention, Mitigation, and Intervention Strategies
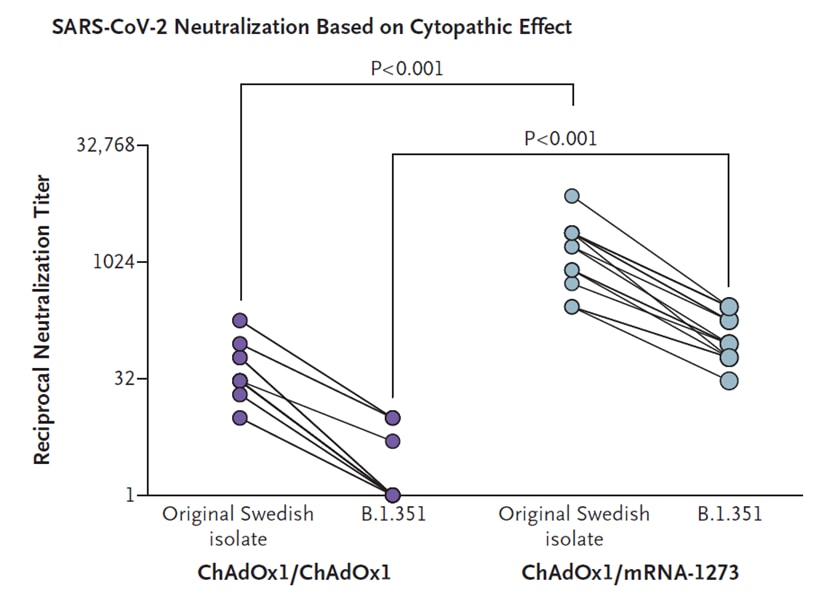
- Normark et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination.external icon NEJM (July 14, 2021). A dose of mRNA-1273 following, by 9 to 12 weeks, a primary dose of ChAdOx1 efficiently stimulated SARS-CoV-2 specific B-cell memory. Those receiving heterologous doses mounted significantly higher reciprocal neutralizing titers and had higher titers against B.1.351 than those receiving homologous doses (p <0.001).
Note: Adapted from Normark et al. Serum neutralization of the original SARS-CoV-2 isolate from Sweden (SARS-CoV-2/01/human/ 2020/SWE) and the B.1.351 variant at the 7-to-10-day time point, with neutralization evaluated as the lowest reciprocal serum dilution at which the cytopathic effect of SARS-CoV-2 on Vero E6 cells was reduced by 50% or more (50% cytopathic effect). ChAdOx1 nCoV-19 boost (n = 18); mRNA-1273 boost (n = 16). rom NEJM, Normark et al., Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination, published online July 14, 2021. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
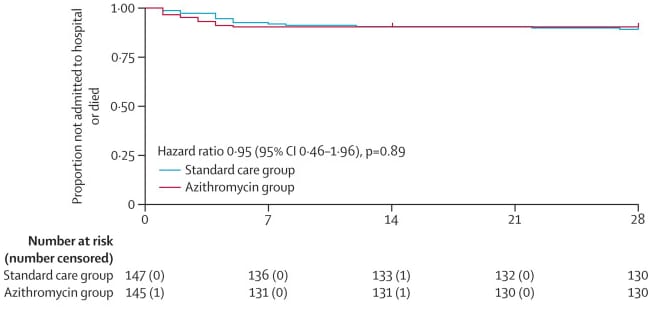
- Hinks et al. Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): an open-label, randomised trial.external icon The Lancet Respiratory Medicine (July 9, 2021). Of 292 outpatients diagnosed with COVID-19 between June 3, 2020 and January 29, 2021 in the UK, those receiving 500 mg azithromycin (n = 147) were no less likely to be admitted to hospital or die than those receiving symptomatic relief with rest and paracetamol.
Note: Adapted from Hinks et al. Kaplan-Meier plot of time to hospital admission or death in the intention-to-treat population. Azithromycin group compared to the standard care group (symptomatic relief). Licensed under CC-BY.
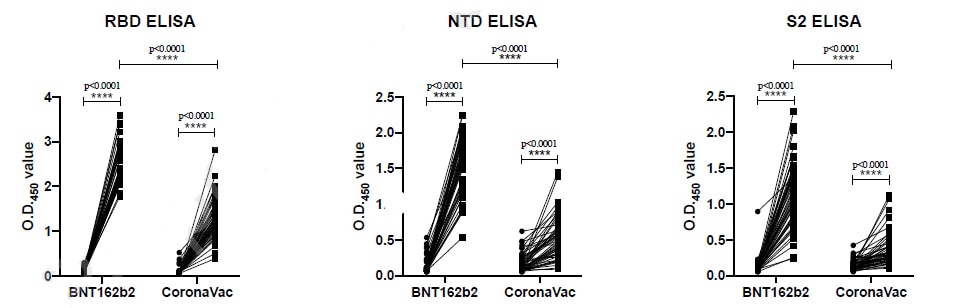
- Mok et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong: an observational cohort study.external icon SSRN (Preprint; July 12, 2021). Published in Respirology as Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kongexternal icon (November 24, 2021). One month after 2nd dose of BNT162b2, participants (n = 49) had significantly higher antibody levels to SARS-CoV-2 in tests of plaque reduction neutralization, surrogate virus neutralization, spike receptor binding (RBD), spike N terminal domain binding (NTD), and spike S2 domain binding than did those receiving 2 doses of CoronaVac (n = 49). Both vaccines elicited comparable T cell (CD4 and CD8) responses to spike protein.
Note: Adapted from Mok et al. Antibody responses of individuals before and after BNT162b2 or CoronaVac vaccination. The levels of RBD-specific (D), NTD-specific (E), and S2-specific (F) IgG antibodies, as tested by ELISAs, in plasma collected pre-vaccination and 1 month after 2nd dose. Permission request in progress.
- Regev-Yochay et al. Decreased infectivity following BNT162b2 vaccination: A prospective cohort study in Israel.external icon The Lancet Regional Health – Europe (July 7, 2021). Among 3,578 health care workers that reported exposure to someone with COVID-19 between December 19, 2020 and March 14, 2021, 295 (8.2%) had a positive RT-PCR test. Compared to unvaccinated cases, fully vaccinated cases had a higher mean Ct value (27.3±1.2 vs. 22.2±1.0, p<0.001). Vaccine effectiveness to prevent infectious cases (presumptive based on Ct value <30) was 70% (95% CI 43-84%).
Social, Behavioral, and Communication Science
- Petersen et al. Transparent communication about negative features of COVID-19 vaccines decreases acceptance but increases trust.external icon Proceedings of the National Academy of Sciences of the United States of America (July 20, 2021). Transparent neutral communication about a fictional COVID-19 vaccine (compared to vague communication) increased vaccine support (p <0.001) and transparent communication of any variety (positive, neutral, or negative) was associated with trust in healthcare authorities (p <0.05), regardless of reported vaccine efficacy or acceptance.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.