COVID-19 Science Update released: July 14, 2020 Edition 30

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
Large population-based serological studies are being performed to estimate prevalence of prior SARS-CoV-2 infection, age- and sex-based seroprevalence, epidemiologic risks, case fatality and infection fatality rates.
PEER-REVIEWED
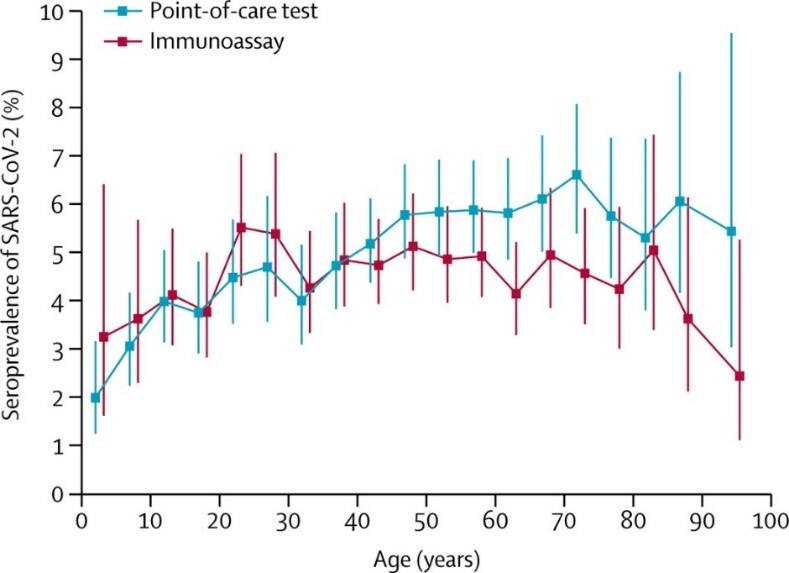
A. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological studyexternal icon. Pollan et al. Lancet (July 6, 2020).
Key findings:
- In a nationwide Spanish survey, seroprevalence of SARS-CoV-2 infection was 5.0% (95% CI 4.7–5.4) using a point-of-care (POC) test and 4.6% (95% CI 4.3–5.0) by immunoassay (Figure).
- No difference by sex.
- Lower seroprevalence in children age < 10 years (<3.1% by POC test).
- Higher prevalence around Madrid (>10%) and lower in coastal areas (<3%).
- An estimated 21.9%-35.8% of seropositive participants were asymptomatic.
- Only 19.5% (95% CI: 16.3–23.2) of symptomatic, seropositive participants reported a previous PCR test.
Methods: Nationwide population-based seroprevalence study, April 27-May 11, 2020. 61,075 participants had testing for antibody by POC and immunoassay tests. Limitations: Potential false-positive results.
Figure:
Note: Adapted from Pollan et al. Seroprevalence of SARS-CoV-2 by age by POC test and Immunoassay. Vertical lines represent 95% CIs. This article was published in Lancet, Vol 396, Pollan et al., Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study, Page 535-544, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource centre: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
B. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donorsexternal icon. Erikstrup et al. Clinical Infectious Diseases (June 25, 2020).
Key findings:
- Adjusted seroprevalence among Danish blood donors was approximately 1.9% (95% CI: 0.8-2.3).
- The ratio between estimated antibody-positive individuals and confirmed cases was 16 (CI: 7-20).
- Estimated infection fatality rate (IFR) was 89 per 100,000 (95% CI: 72-211).
Methods: Seroprevalence study of SARS-CoV-2 among 20,640 Danish blood donors aged 17–69 years April 6-May 3, 2020. Limitations: Relatively low sensitivity in serology test.
Implications for both studies (Pollan et al. & Erikstrup et al.): The prevalence of persons with evidence of prior infection exceeded the rate of reported COVID-19 diagnoses. Seroprevalence studies can better help understand true rates of infection and infection-related fatalities.
PEER-REVIEWED
Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients — Nashville, Tennesseeexternal icon. Stubblefield et al. Clinical Infectious Diseases (July 6, 2020).
Key findings:
- Among the 249 healthcare personnel (HCP), 35 (14.1%) reported a prior SARS-CoV-2 RT-PCR test.
- 19 (7.6%) had antibodies detected.
- 8/19 (42.1%) were asymptomatic.
- 7/19 (36.8%) had prior RT-PCR test but only 3 of these 7 had a positive result.
Methods: Cross-sectional seroprevalence study of SARS-CoV-2 infection among HCP from April 3-13, 2020. Limitations: Convenience sampling at a single center.
Implications: Asymptomatic and minimally symptomatic HCP may be a source for SARS-CoV-2 transmission, emphasizing that enhanced surveillance of SARS-CoV-2 infection in HCP is an important prevention strategy.
Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: Cross sectional studyexternal icon. Liu et al. BMJ (June 10, 2020).
Key findings:
- No frontline healthcare workers (HCWs) who used recommended PPE and were exposed in high-risk settings contracted SARS-CoV-2 infection.
- All tested negative for SARS-CoV-2 by PCR and IgM or IgG antibodies.
Methods: Cross-sectional study of 420 HCWs attending severe or critical COVID-19 patients, Wuhan, China for 6–8 weeks, January 24-April 7, 2020 to assess whether PPE (the combination of protective suits, N95 respirators combined with surgical masks, gloves, goggles, face shields, and gowns) protected HCWs from SARS-CoV-2 infection. RT-PCR testing conducted during 2-week quarantine; antibody testing done at end of quarantine. Limitations: Potential false-negative test results; findings not generalizable to community settings.
Implications: Protection for HCWs from SARS-CoV-2 infection is possible with appropriate PPE.
PREPRINTS (NOT PEER-REVIEWED)
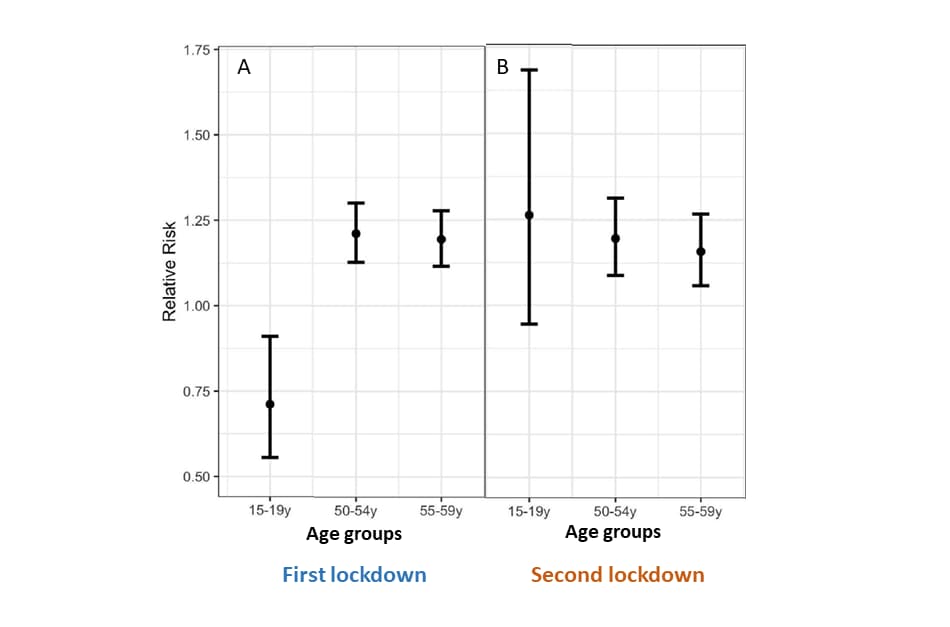
Lockdown measures and relative changes in the age-specific incidence of SARS-CoV-2 in Spainexternal icon. Salazar et al. medRxiv (July 2, 2020). Publishedexternal icon in Epidemiology & Infection (October 21, 2020).
Key findings:
- In the first lockdown, the highest proportion ratio (PR) of COVID-19 cases was in people aged 50-59 years (50-54 years: 1.21; 55-59 years: 1.19) (Figure A).
- In the second lockdown period, the highest PR was in people 15-19 years: 1.26 (Figure B).
Methods: Age-specific PRs in 5-year increments were estimated for two lockdown periods, the first, between March 25 and April 3, 2020 (non-essential workers were allowed to go to work) and the second, between April 8 and 17, 2020 (non-essential workers were not allowed to go to work) compared with pre-lockdown period (March 1–10, 2020). Limitations: No data on interventions.
Implications: Relative incidence of SARS-CoV-2 infection in different age groups exists and may be determined by age-based employment patterns; differential impact of various measures such as physical distancing and workforce-related policies may have implications for epidemic control.
Figure:
Note: Adopted from Salazar et al. Estimates of PR and 95% CIs of COVID-19 cases by age groups: (A) during first lockdown period (March 25-April 3, 2020) vs pre-lockdown period (March 1-10), and (B) second lockdown period (April 8-17) vs. pre-lockdown period, for each 15-19y (Left dot), 50-54y (Middle dot), and 55-59y (Right dot). Licensed under CC-BY-NC-ND 4.0.
PEER-REVIEWED
Thyroid function analysis in 50 patients with COVID-19: a retrospective studyexternal icon. Chen et al. Thyroid (June 29, 2020).
Key findings:
- Levels of thyroid stimulating hormone (TSH) and serum total triiodothyronine (TT3) were lower among COVID-19 patients with no history of thyroid disease than in two control groups (healthy persons and non-COVID-19 pneumonia patients) (Figure 1).
- Lower levels of TSH and TT3 were observed in COVID-19 patients with more severe disease (Figure 2).
- After recovery from infection, TSH and TT3 levels in COVID-19 patients normalized.
Methods: Retrospective analysis comparing thyroid function test results in 50 COVID-19 patients without thyroid disease with healthy participants of similar age and sex (n = 54) and non-COVID-19 pneumonia patients with similar disease severity (n = 50). Limitations: Other thyroid and pituitary hormones were not assessed; most patients were receiving glucocorticoids, which affect pituitary-endocrine axis feedback loops; patients with mild COVID-19 disease not included.
Implications: SARS-CoV-2 may affect TSH-secreting cells directly, or indirectly such as through activation of proinflammatory cytokines or hormonal changes due to treatments. Changes in thyroid hormone levels may be important manifestations of COVID-19.
Figure 1
Note: Adapted from Chen et al. Comparison of median (1st quartile, 3rd quartile) serum TT3 and TSH levels between non-COVID-19 pneumonia patients, healthy controls and COVID-19 patients. *p <0.01 compared with healthy controls; #p <0.05; ##p <0.01 compared with non-COVID-19 pneumonia patients. Data in Figure 1 used by permission from Thyroid and Mary Ann Liebert, Inc. Publishers.
Note: Adapted from Chen et al. Comparison of median (1st quartile, 3rd quartile) serum TT3 and TSH levels among healthy controls and COVID-19 patients according to clinical classification of disease severity (moderate, severe, critical). p <0.001 for overall differences between the groups on TT3 and TSH levels. Data in Figure 2 used by permission of Thyroid and Mary Ann Liebert, Inc. Publishers.
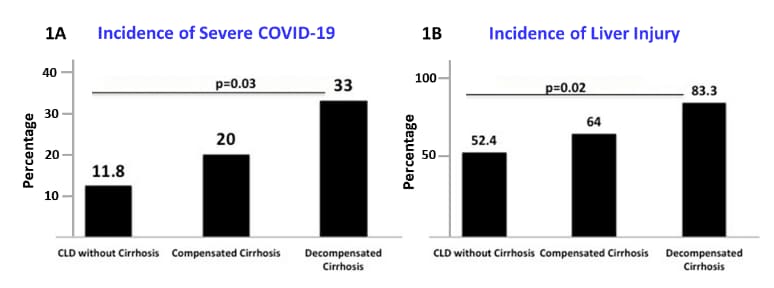
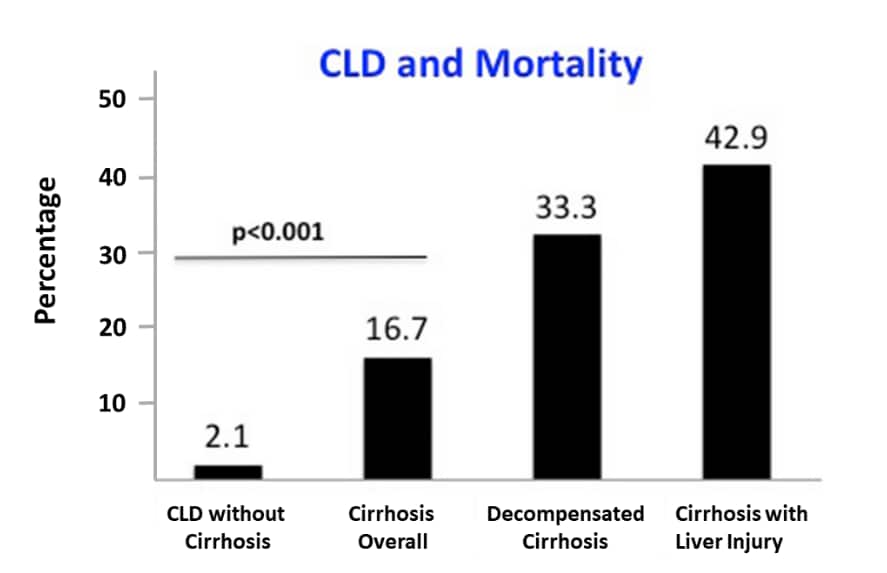
Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study)external icon. Sarin et al. Hepatology International (June 27, 2020).
Key findings:
- Among 228 COVID-19 patients with pre-existing chronic liver disease (CLD), severe COVID-19 and acute liver injury were seen among those without cirrhosis (n = 185), compensated cirrhosis (n = 25) and decompensated cirrhosis (n = 18) (Figure 1a and 1b).
- Mortality increased significantly among persons with cirrhosis and persons with decompensated cirrhosis compared with persons with CLD without cirrhosis (Figure 2).
Methods: Analysis of 228 COVID-19 patients with CLD (185 without cirrhosis; 43 with cirrhosis). January-April 2020. Predictors of severity of liver injury was analyzed. Severe disease includes severe pneumonia, acute respiratory distress syndrome, acute kidney, heart or circulatory failure, altered sensorium. Limitations: Small sample of cirrhotics; criteria for cirrhosis with liver injury not defined.
Implications: COVID-19 patients with pre-existing liver complications are at higher risk for severe complications and death.
Figure 1
Note: Adapted from Sarin et al. Severe COVID-19 (1A) and acute liver injury incidence rates (1B) stratified by spectrum of CLD groups. (1A) Severe disease due to COVID-19 increased progressively among those without cirrhosis, compensated cirrhosis and decompensated cirrhosis (p = 0.03). (1B) Liver injury due to COVID-19 increased progressively among groups (p = 0.02). Available via Nature Public Health Emergency Collection through PubMed Central.
Figure 2
Note: Adapted from Sarin et al. Mortality stratified by CLD group. Mortality increased among those with cirrhosis, decompensated cirrhosis, and onset of liver injury. Available via Nature Public Health Emergency Collection through PubMed Central.
High incidence of barotrauma in patients with COVID-19 infection on invasive mechanical ventilationexternal icon. McGuinness et al. Radiology (July 2, 2020).
Key findings:
- Proportion with of patients with barotrauma (lung injury as a result of invasive mechanical ventilation (IMV)) among COVID-19 vs. non-COVID-19 patients, 15% vs 0.5%, respectively.
- In a historical cohort of patients with acute respiratory distress syndrome (ARDS), the proportion with barotrauma was 10%.
Methods: A retrospective analysis of barotrauma between March 1 and April 6, 2020 among COVID-19 patients receiving IMV compared with patients without COVID-9 receiving IMV. Historical comparisons with ARDS patients February 2016-February 2020. Limitations: Potential bias from the selection of control cohort and confounding effects from pandemic health care situation.
Implications: Healthcare providers need to be aware of the increased risk of barotrauma during management of patients with COVID-19 receiving IMV.
PEER-REVIEWED
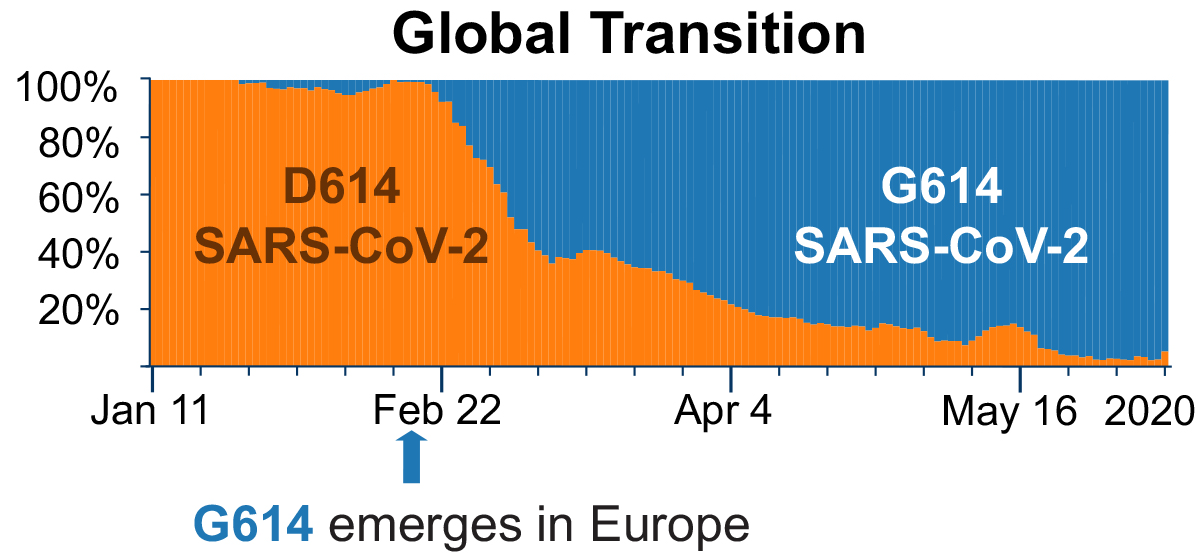
Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virusexternal icon. Korber et al. Cell (June 26, 2020).
Key findings:
- The D614G spike mutation has become the dominant form of SARS-CoV-2 globally (Figure).
- The new variant was associated with higher viral loads.
- No significant association with disease severity as measured by hospitalization outcomes.
Methods: An analysis of SARS-CoV-2 spike protein mutations in 28,576 sequences from the global genomic database, January to May 2020. Limitations: Systematic sampling biases across regions.
Implications: The higher viral loads seen with the new variant may indicate increased infectiousness (i.e., risk of transmission). Tracking genetic variations is important to ensure the development of effective vaccines or antibody therapeutics for SARS CoV-2.
Figure:
Note: Adapted from Korber et al. Percentage distribution of 2 genetic variants of sampled SARS-CoV-2 sequences January to May 2020. The original D614 variant is shaded in orange and the new G614 variant is shaded in blue. The G614 variant was found in 10% of sequences before March 1, in 67% of sequences from March 1–31, and in 78% of those from April 1–30. Licensed under CC-BY-NC-ND 4.0.
- Morawska et al. It is time to address airborne transmission of COVID-19external icon. Clinical Infectious Diseases. Letter highlighting the need to recognize the potential of airborne spread of COVID-19.
- Brelie et al. Parosmia as an early symptom of acute SARS-CoV-2 infectionexternal icon. Deutsches Arzteblatt International. Parosmia in a 30-year-old otherwise asymptomatic woman, preceding complete loss of smell and taste and could be a new early symptom of SARS-CoV-2 infection.
- Li et al. The role of children in transmission of SARS-CoV-2: A rapid reviewexternal icon. Journal of Global Health. Narrative concludes that there is limited evidence on pediatric cases acting as a source of infection and highlights importance of obtaining robust data on transmission dynamics in children This rapid review will be updated external iconas new data becomes available.
- Ramnath et al. The challenge of COVID-19 has accelerated the use of new data-sharing technologiesexternal icon. Respirology. Describes how technology is supporting medicine and science during COVID‐19 pandemic: 1) Tech‐based tools expedite appraisal of medical information; 2) Fast dissemination of information; 3) Virtual conferences on demand; 4) Telemedicine and medical ‘virtual learning’; 5) Novel data analytics stratifies risk of disease in real time.
- Cheng et al. How to safely reopen colleges and universities during COVID-19: Experiences from Taiwanexternal icon. Annals of Internal Medicine. Describes Taiwan’s successful experience with reopening colleges and universities, however, it also notes that Taiwan’s case numbers are lower than other countries and it is unknown if the strategies would be sufficient for other settings.
- McMullen et al. Impact of SARS-CoV-2 on hospital acquired infection rates in the United States: Predictions and early resultsexternal icon. American Journal of Infection Control. Reviews the impact of waived reporting requirements for healthcare-associated infections through June 2020 by describing observed increases in central line-associated blood stream infections, catheter-associated urinary tract infections, surgical site infections, and Clostridioides difficile infections in hospitals in New York City, NY and St. Louis, MO.
- Leary et al. We got this and we don’t: Pediatricians going to battle for the “Big Children” of COVID-19external icon. Academic Pediatrics. A pediatric hospitalist’s experience with caring for adult COVID-19 patients describes the strengths and weaknesses brought into play by pediatricians caring for COVID-19 adult patients.
- Velioglu et al. Care of asymptomatic SARS‐CoV‐2 positive kidney transplant recipientsexternal icon. Transplant International. Case report of a kidney transplant recipient with asymptomatic COVID-19 that discusses treatment and follow-up strategies.
- Casas et al. Classification of the cutaneous manifestations of COVID ‐19: a rapid prospective nationwide consensus study in Spain with 375 casesexternal icon. Characterization of 5 cutaneous clinical patterns associated with COVID-19.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.
![Figure 1 Comparison of median [1rst quarter, 3rd quarter] serum TT3 and TSH levels between non-COVID-19 pneumonia patients, healthy controls and COVID-19 patients.](/library/covid19/images/0714_Figure_3a.png?_=62387)
![Figure 2 Comparison of median [1rst quarter, 3rd quarter] serum TT3 and TSH levels among healthy controls and COVID-19 patients according to clinical classification of disease severity (moderate, severe, critical).](/library/covid19/images/0714_Figure_3b.png?_=62385)