Methods
Recommendations for Prevention and Control of Infections in NICU Patients: CLABSI
This guideline is based on a targeted, systematic review of the best available evidence on the prevention and control of infections in NICUs.
D.1. Development of Key Questions
In order to inform the development of the Central Line-associated Blood Stream Infections (CLABSI) Key Questions, electronic searches were conducted to retrieve existing relevant guidelines and to identify gaps and areas where new evidence may have been published. The strategies used for the guideline searches and results can be found in the Appendix. (Appendix Section A) The results of this initial review informed the development of a preliminary list of Key Questions. Key Questions were finalized after vetting them with HICPAC.
D.2. Literature Search
A list of search terms was developed to identify the literature most relevant to the Key Questions. The terms were incorporated into search strategies, and these searches were performed in MEDLINE, EMBASE, CINAHL, and the Cochrane Library. Subject matter experts supplemented the literature search results by recommending relevant references published after the final search in May 2021.
D.3. Study Selection
Titles and abstracts from references were screened by dual review (A.E., C.H., J.H., M.I., A.D.O., K.T.R., S.S., or E.C.S.). Full-text articles were retrieved if they were:
- 1Relevant to one or more Key Question(s);
- 2Primary research, systematic reviews, or meta-analyses; and
- Written in English.
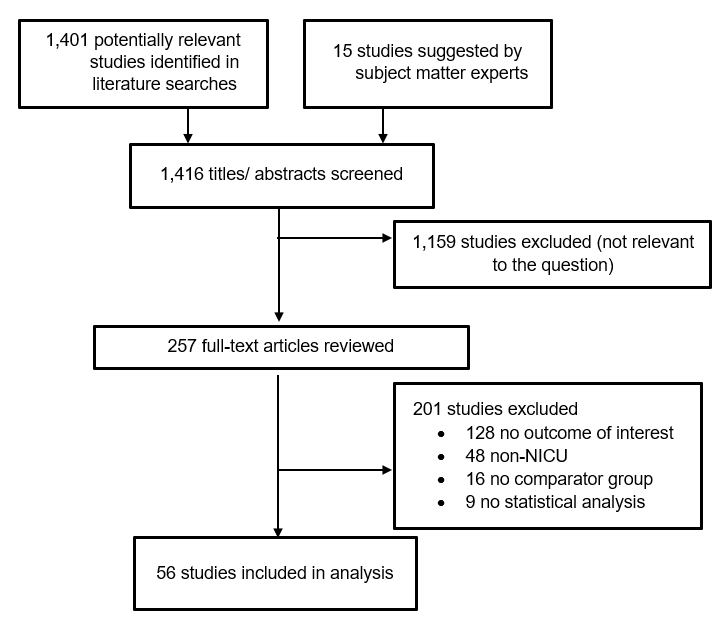
The Appendix presents the full list of exclusion criteria. (Appendix Section B) The full texts of selected articles were then screened by two independent reviewers, and disagreements were resolved by discussion (K.B., A.E., L.F., J.H., W.C.H., M.I., A.M., A.D.O., K.T.R., S.S., N.S., or E.C.S.). After the full-text screening was complete, a bibliography of the articles selected for inclusion was vetted with subject matter experts. Additional studies suggested by the subject matter experts were screened for inclusion as described above. The results of the study selection process are depicted in Figure 1.
Figure 1. Results of the Study Selection Process
D.4. Data Extraction and Synthesis
Methodologic data and results of clinically relevant outcomes from the studies meeting inclusion criteria were extracted into standardized evidence tables. Data and analyses were extracted as presented in the studies. For the purposes of this review, statistical significance was defined as p ≤ 0.05.
D.5. Grading of the Evidence
The risk of bias associated with each study was assessed using scales developed by the University of Pennsylvania Center for Evidence-based Practice, and scores were recorded in the evidence tables. (Appendix Section D) The Appendix includes the questions used to assess the quality of each study design. The quality of the evidence base was then assessed using methods from the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group, which considers randomized controlled trials (RCTs) the gold standard. (Appendix Section D) The GRADE approach[65], [66] was applied to provide explicit links between the available evidence and the resulting recommendations.
D.6. Formulating Recommendations
The criteria used to formulate the strength of each recommendation are described in the HICPAC Update to the Recommendation Categorization Scheme[67], and in the Appendix. (Appendix Section E.) The recommendation justification tables for each recommendation outline the factors weighed when determining the recommendation’s strength.
D.7. Reviewing and Finalizing the Guideline
Recommendations, including narrative evidence reviews, recommendation justification tables, GRADE tables, and evidence tables, were presented to HICPAC for review and input at public meetings in November 2017; May and November 2018; and May, August, and November 2019. Following further revisions, the Guideline was announced in the Federal Register and published on Regulations.gov for a public comment period of 60 days. After this period, the public comments received were reviewed at a HICPAC meeting. The Recommendations were revised accordingly and finalized.
D.8. Updating the Guideline
Future revisions to this Guideline will be guided by new research and technological advancements for preventing and managing infections and infectious disease outbreaks in the NICU setting.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. Apr 2011;64(4):383-94. doi:10.1016/j.jclinepi.2010.04.026
- Schunemann HJ, Oxman AD, Brozek J, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. ACP J Club. Dec 16 2008;149(6):2.
- The Healthcare Infection Control Practices Advisory Committee (HICPAC). Update to the CDC and the HICPAC Recommendation Categorization Scheme for Infection Control and Prevention Guideline Recommendations Updated October 8, 2019. Accessed August 14, 2020. https://www.cdc.gov/hicpac/workgroup/recommendation-scheme-update.html