Volume 28, Number 12—December 2022
Research
Hedgehogs as Amplifying Hosts of Severe Fever with Thrombocytopenia Syndrome Virus, China
Abstract
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a tickborne bandavirus mainly transmitted by Haemaphysalis longicornis ticks in East Asia, mostly in rural areas. As of April 2022, the amplifying host involved in the natural transmission of SFTSV remained unidentified. Our epidemiologic field survey conducted in endemic areas in China showed that hedgehogs were widely distributed, had heavy tick infestations, and had high SFTSV seroprevalence and RNA prevalence. After experimental infection of Erinaceus amurensis and Atelerix albiventris hedgehogs with SFTSV, we detected robust but transitory viremias that lasted for 9–11 days. We completed the SFTSV transmission cycle between hedgehogs and nymph and adult H. longicornis ticks under laboratory conditions with 100% efficiency. Furthermore, naive H. longicornis ticks could be infected by SFTSV-positive ticks co-feeding on naive hedgehogs; we confirmed transstadial transmission of SFTSV. Our study suggests that the hedgehogs are a notable wildlife amplifying host of SFTSV in China.
Severe fever with thrombocytopenia syndrome (SFTS) is caused by SFTS virus (SFTSV), a new tickborne bandavirus identified in China in 2009 (1), and subsequently in South Korea in 2013 (2), Japan in 2014 (3), Vietnam in 2019 (4), and Myanmar and Pakistan in 2020 (5,6). The symptoms of SFTS include fever, thrombocytopenia, leukocytopenia, and gastrointestinal disorders; case-fatality rate is 2%–30% (1,7,8). The earliest cases in China were reported in the Dabie mountain range, which is located at the intersection of Henan, Hubei, and Anhui Provinces in central China. Shandong, Liaoning, and Zhejiang provinces are the other main hot spots for SFTS in China (9). Within Zhejiang Province, Daishan County, an archipelago of islands located in the East China Sea, is one of the most SFTS-endemic areas (10). The main industries in Daishan County are fishing and tourism. Agriculture is relatively unimportant; 4,000 sheep and 150 cattle were reported on the islands in 2019, as provided by the Department of Agriculture in Daishan County. As of 2020, SFTS cases have been reported in most other provinces of China (9,11,12).
The Asian long-horned tick, Haemaphysalis longicornis, is a primary vector for SFTSV and the dominant human-biting tick in SFTSV-endemic areas (13,14). H. longicornis ticks have both bisexual and parthenogenetic populations; parthenogenetic populations are widely distributed in China and strongly correlated with the distribution of SFTS cases (15). H. longicornis ticks go through a 3-stage life cycle: larva, nymph, and adult. Extensive reports suggest that H. longicornis ticks are the reservoir of SFTSV (16–18); however, transstadial transmission efficiencies of SFTSV varied under laboratory conditions. We compared results from Zhuang et al. (16) and Hu et al. (19): transmission rate from egg pools to larvae pools was 80% in Zhuang and 100% in Hu; from larval pools to nymph pools, 92% in Zhuang and 100% in Hu; and from nymph pools to adults, 40% in Zhuang and 50% in Hu. The corresponding SFTSV prevalence was extremely low, 0.2%–2.2%, in different developmental stages of host-seeking H. longicornis ticks collected from vegetation (17,18,20). These findings suggest that ticks alone are not sufficient to maintain a reservoir of SFTSV in the natural environment, and additional amplifying hosts are required.
Antibodies to SFTSV and viral RNA have been detected in a wide range of domestic animals, including goats, cattle, dogs, and pigs and wild animals such as shrews, rodents, weasels, and hedgehogs. The highest seroprevalence was found in sheep (69.5%), followed by cattle (60.4%), dogs (37.9%) and chickens (47.4%) (21–23). Given that most of the SFTS patients are farmers, who have frequent contacts with many of these susceptible domestic and wild animals, understanding the epidemiology of SFTSV is difficult and complex.
Hedgehogs belong to the family Erinaceinae, which are widely distributed in Europe, Asia, and Africa (24) and are invasive species in Japan and New Zealand (25,26). The Amur hedgehog, Erinaceus amurensis, is closely related to the European hedgehog, E. europaeus, and is common in northern and central China. The African pygmy hedgehog, Atelerix albiventris, native to central and eastern Africa, has been introduced into many countries as pets, including China (25,26). Both the Amur hedgehog and the African pygmy hedgehog can become heavily infested by all kinds of ticks and are known to carry many zoonotic diseases, such as tick-borne encephalitis virus, Bhanja virus, and Tahyna virus (27–29). Hedgehogs are poikilothermal animals and hibernate during winter. During hibernation, their metabolism and immune system are suppressed (30), which has led to the suspicion that hibernating hedgehogs contribute to the long-term persistence of these viruses (31). A few previous studies have reported that SFTSV antibodies and RNA were detected in Amur hedgehogs in Shandong and Jiangsu Province. However, the prevalence of SFTV infection appeared low compared with that in other animals, such as goats, sheep, and cattle (14,32).
In China, the density of large wild animals is extremely low, especially in East China, where SFTS is endemic. Instead, the most abundant wildlife in these areas are rodents and insectivores (33). However, the potential role of rodents in the transmission of SFTSV was refuted when it was shown that immunocompetent rodents cannot develop SFTSV viremia after artificial inoculation (34). In contrast, hedgehogs are the only small wild animals that consistently show high SFTSV seroprevalence, high density, and high H. longicornis tick infestation in the SFTS-endemic areas (32,35), which has led us to speculate that hedgehogs might play an important role in the natural circulation of SFTSV in China. We conducted all animal studies in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People’s Republic of China. The Committee on the Ethics of Animal Experiments of the Institute of Zoology, Chinese Academy of Sciences, approved the protocols for animal studies (approval no. IOZ20180058).
Field Survey of Hedgehogs in SFTS-Endemic Areas
To confirm the role of hedgehogs as potential wild amplifying hosts for SFTSV, we first performed an animal survey in Daishan County in 2019 (Figure 1, panel A). Daishan County is the worst-affected area for SFTS in Zhejiang Province (10); during 2011–2019, Daishan Center for Disease Control and Prevention reported 133 SFTS cases on 3 Daishan County islands—Daishan Island, Qushan Island, and Changtu Island—but none on Xiushan Island, even though Xiushan Island has a similar landscape, vegetation, and population density as the other major islands (Figure 1, panel B).
For the survey, we set small mammal traps and caught 33 animals on Daishan Island and 75 on Xiushan Island. On Daishan Island, 9/33 (28%) of the captured small mammals were E. amurensis Amur hedgehogs, 6/33 (18%) were Rattus norvegicus brown rats, 12/33 (36%) were Sorex araneus common shrews, and 6/33 (18%) were Apodemus agrarius striped field mice. On Xiushan Island, we caught no hedgehogs; 36/75 (48%) of the small mammals caught were R. norvegicus rats, 33/75 (44%) were S. araneus shrews, and 6/75 (8%) were R. losea lesser rice field rats (Figure 1, panel C). Antibody testing showed that 3/9 (33%) of E. amurensis hedgehogs from Daishan Island were positive for SFTSV (Table 1). Hedgehogs are abundant in the 2 villages in Daishan Island; we estimated population density as >80 animals per square kilometer based on the results of the trapping study (Table 2). In addition, the 9 trapped hedgehogs were all heavily infected by ticks, with an average of 145 ticks per hedgehog, including H. longicornis ticks (Table 3).
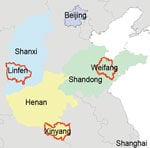
Additional E. amurensis hedgehog serum samples were collected from trapping studies conducted in other SFTS-endemic areas, including Weifang City of Shandong Province, Linfen City of Shanxi Province, and Xinyang City of Henan Province. SFTSV antibodies were detected in 9/35 (25.7%) of hedgehogs from Weifang City, of which 11.1% tested positive for SFTSV RNA; 2/6 (33.3%) from Linfen City, of which 50% tested positive; and 2/8 (25%) from Xinyang City, of which no hedgehogs tested positive. Of the hedgehogs from Weifang, 11.1% were infected by ticks positive for SFTSV RNA, as were 12.5% of those from Linfen (Table 4; Figure 2). We believe these results strongly support our hypothesis that hedgehogs play an important role in the natural circulation of SFTSV. After collecting samples, we conducted several experiments to determine the role of the hedgehogs in SFTSV transmission (Appendix, https://wwwnc.cdc.gov/EID/article/28/12/22-0668-App1.pdf).
Susceptibility of Hedgehogs to Experimental Infection with SFTSV
We inoculated 4 male and 4 female E. amurensis hedgehogs 6–12 months old with 4 × 106 FFU of SFTSV by intraperitoneal route. We observed viremia of ≈9 days in all animals and peak titers of 3.1 log10 RNA copies/μL at days 3–6, suggesting viral multiplication. Two E. amurensis hedgehogs showed a mild weight loss of <25% by day 9 (Figure 3, panels A, B).
We inoculated groups of 5 male and 5 female A. albiventris hedgehogs 6–12 months of age with 4 × 106 FFU of SFTSV by intraperitoneal (Figure 3, panels C, D) and subcutaneous (Figure 3, panels E, F) routes. We observed viremia of 9–11 days in all 10 animals; peak titers were 3.2 log10 RNA copies/μL at days 3–7 for the intraperitoneal route and 3.1 log10 RNA copies/μL at days 6–8 for the subcutaneous route (Figure 3, panels D, F). Most animals showed mild weight loss of <20% (Figure 3, panels C, E). Those results suggest that E. amurensis and A. albiventris hedgehogs could develop similar viremias independent of inoculation routes, without substantially compromising their overall health. However, E. amurensis hedgehogs are shy and prone to dying during transport from their stress response. Thus, we performed most of the following experiments with A. albiventris hedgehogs, of which we had a stable supply through the local pet store.
SFTSV Viremia during Hibernation
We inoculated 4 A. albiventris hedgehogs with 4 × 106 FFU of SFTSV and kept them at 4°C to trigger hibernation. Two of the hedgehogs came out of hibernation at day 15 with viremias of 2.7 and 3.3 log10 RNA copies/μL; the other 2 hedgehogs continued in hibernation until day 30 and had viremias of 3.0 and 3.7 log10 RNA copies/μL. All the viremias measured in these hibernating hedgehogs were comparable to the peak virus titers previously measured in the nonhibernating hedgehogs (Figure 4). However, the duration of viremia in these 4 hibernating hedgehogs was much longer than that recorded in the nonhibernating hedgehogs, suggesting that hibernation could potentially extend the course of SFTSV viremia in hedgehogs and contribute to the overwintering of SFTSV in the field.
SFTSV-Induced Pathology
To assess the pathologic changes in hedgehogs resulting from SFTSV infection, we intraperitoneally inoculated 6 A. albiventris hedgehogs with 4 × 106 FFU of SFTSV. We euthanized 2 animals at 3 days, 6 days, and 2 months after infection and collected their organs for viral RNA evaluation and hematoxylin and eosin (H&E) staining. We detected a robust viremia on days 3 and 6 but none at 2 months after infection. We observed the highest level of viral RNA in the spleen, followed by the blood; the lowest level was in the heart (Figure 5). H&E-stained slides from the spleen showed hemorrhagic necrosis and lymphopenia at days 3 and 6. We assessed the severity of the lesions as +++ on day 3 and ++++ on day 6, but the lesions had largely recovered by 2 months, with a severity score of ++ (Appendix Figure 1). These results further confirmed that hedgehogs show a high tolerance to SFTSV without obvious long-term or permanent pathologic changes.
Transmission of SFTSV between H. longicornis Ticks and Hedgehogs
We used laboratory-adapted H. longicornis ticks and A. albiventris hedgehogs to model the natural transmission of SFTSV hypothesized to occur in the wild. We fed naive H. longicornis nymphs on hedgehogs infected by intraperitoneal inoculation with 4 × 106 FFU of SFTSV at day 0. We detected viremia of 3.8 log10 RNA copies/μL in hedgehogs at day 5; fully engorged nymphs dropped off between days 4 and 8. The engorged nymphs molted after 2–3 weeks, and the adult ticks tested 100% positive for SFTSV at a level of 7.2 log10 RNA copies/mg tick.
Two to 3 weeks after they molted into adults, we fed the SFTSV-carrying ticks on 3 naive hedgehogs, 8 ticks per animal. We monitored weight and viremia for 12 days and observed a slow weight loss of <25% by day 12; the viremia peaked on days 8–10 at 4.1 log10 copies/μL. After peaking, the viremia decreased slowly until the 3 hedgehogs were euthanized on day 12 (Figure 6, panels A, B). We collected the fully engorged ticks on days 7–10 and then tested them. All 24 ticks were still positive for SFTSV RNA (Figure 6, panel C). We believe that these data strongly suggest that SFTSV can be efficiently transmitted between hedgehogs and H. longicornis ticks and that transstadial transmission occurs within H. longicornis ticks.
Hedgehogs as Amplifying Hosts for SFTSV
SFTSV can be transmitted both transovarially and transstadially in H. longicornis ticks; however, a decreased efficiency has been observed during passaging (16). Thus, an amplifying host will be necessary to improve the transmission efficiency. To determine if hedgehogs can serve as amplifying hosts, we prepared SFTSV-positive adult H. longicornis ticks as described above with 100% efficiency. Next, we fed 5 of the SFTSV-carrying adult H. longicornis ticks together with 14–16 naive nymphs and 3–4 naive adult ticks on each of 3 naive A. albiventris hedgehogs. We collected the fully engorged ticks at 7–10 days after bite and tested them for viral RNA levels. The viral load in the engorged nymphs was 2.5 log10 RNA copies/mg tick and in previously naive adults 2.7 log10 RNA copies/mg tick (Figure 7, panels A, B). After the nymphs molted, the adult ticks tested 100% positive for SFTSV, with a level of 6.9 log10 RNA copies/mg tick (Figure 7, panel C). Thus, these results suggest that hedgehogs could be acting as an amplifying host for SFTSV.
Viremia in the vertebrate host is important for the arbovirus to transmit from host to vector. Previous epidemiologic surveys and experimental infections have revealed that many wild and domesticated animals are susceptible to SFTSV infection (21). However, these studies had similar findings that most vertebrate animals were subclinically infected with SFTSV, with limited viremia (36). For example, 80% of goats developed a viremia after subcutaneous inoculation with 107 PFU of SFTSV, which lasted for <24 hours (37). Similarly, beagle dogs intramuscularly inoculated with 2.51 × 107 50% tissue culture infectious dose of SFTSV did not have a detectable viremia until day 3 (38). Furthermore, the efficient transmission of SFTSV between tick vectors and these potential wild animal hosts has not been proven. In this study, we consistently detected robust viremias of ≈103 RNA copies/μL in both native E. amurensis and exotic A. albiventris hedgehogs after intraperitoneal or subcutaneous inoculation with 4 × 106 FFU of SFTSV at 100% efficiency; viremia lasted for 9–11 days and provided the basis for the effective transmission of SFTSV from host to tick. Moreover, hedgehogs were highly tolerant to SFTSV infection; they experienced slight weight loss and pathology that recovered after the clearance of virus.
H. longicornis ticks overwinter mostly as nymphs, but with an SFTSV-positive rate of 4% as measured by pool (39). Thus, we speculate that their role in overwintering of disease may be limited. Hedgehogs are involved in the overwintering of many pathogens during hibernation (31,40), which could include SFTSV. Our results suggest that the SFTSV viremia can be extended from 9 days when not hibernating to >1 month during hibernation, and with viremias no less than those seen in nonhibernating hedgehogs.
To meet the requirement for hedgehogs to be considered as maintenance hosts for SFTSV, the transmission cycle between vector and host needs to be established. Using laboratory-adapted H. longicornis ticks and A. albiventris hedgehogs, this study showed efficient infection transmission from nymph or adult ticks to hedgehogs, efficient infection transmission from hedgehogs to nymph or adult ticks, and transstadial infection transmission from nymph to adult tick. It is important to note that these results were observed in 100% of tested subjects. Naive nymph and adult H. longicornis ticks cofeeding with SFTSV-infected adult ticks on naive hedgehogs were also 100% infected. Our results show that hedgehogs fulfill the requirements to be considered competent amplifying hosts for SFTSV. Other animals or birds could also maintain the natural circulation of SFTSV; for example, experimentally inoculated spotted doves (Streptopelia chinensis) can develop SFTSV viremia (41). However, transmission between H. longicornis ticks and spotted doves is not proven.
To conclude that hedgehogs are major amplifying hosts of SFTSV in the real world, further studies should investigate abundance, tick association, geographic distribution in areas of transmission, and field exposure. Our initial survey in SFTSV-endemic Daishan Island and nonendemic Xiushan Island reveals that the existence of hedgehogs was related to SFTSV transmission. The epidemiologic surveys we conducted in 4 SFTSV-endemic provinces consistently showed high SFTSV seroprevalence and that the population density of hedgehogs in SFTSV-endemic areas can be >60 animals/km2. Hedgehogs are heavily infested by tick species including H. longicornis; we observed a density of 145 ticks per animal on Daishan Island. Hedgehogs are widely distributed across farms and rural communities, which contain the humans most likely to be bitten by H. longicornis ticks carrying SFTSV (32,35). Furthermore, hedgehogs share the same environment as domestic animals such as dogs, goats, and cows, which are also natural hosts for H. longicornis ticks and show high seroprevalence for SFTS. Thus, it is possible that humans and domestic animals are similarly infected by ticks that had previously fed on SFTSV-positive hedgehogs at an earlier stage in their life cycle. As previously stated, SFTSV-endemic areas in China have few large wild animals; the most common animals are rodents and insectivores (33). Tests on rodents have shown that they are not capable of maintaining SFTSV infection (34). Our results show that of the mammals present in rural China, hedgehogs meet all the requirements to be major wildlife amplifying hosts for SFTSV.
SFTSV may also spread to other countries with competent hosts and vectors. E. europaeus hedgehogs were introduced to New Zealand by human intervention (25,26). The summer density of hedgehogs in 3 studies in New Zealand was estimated at 250–800 hedgehogs/km2 (42–44). In addition, H. longicornis ticks are common in New Zealand and are all parthenogenetic (Appendix Figure 2) (45). New Zealand is also on the East Asian–Australian flyway, so it could be considered to have a high risk for SFTSV disease incursion through SFTSV–positive H. longicornis ticks infested in migratory birds (46).
In conclusion, our data strongly support our initial hypothesis that hedgehogs can maintain the natural circulation of SFTSV in rural areas. The high density and wide distribution, the high-level susceptibility and tolerance of hedgehogs to SFTSV, the heavy H. longicornis tick infestation rates, and the ability to amplify the infection level of feeding ticks are all compelling evidence that hedgehogs are a likely wildlife amplifying host of SFTSV.
Dr. Zhao is a PhD student at Institute of Zoology, Chinese Academy of Sciences, China. Her primary research interest is interaction of vectors and vector-borne pathogens.
Acknowledgments
We thank Rachel Summers for constructing the New Zealand hedgehog distribution map.
This research was funded by the State Key Research Development Program of China (2019YFC12005004, 2019YFC12005001), National Key R&D Program of China (2021YFC2300903), the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDPB16), the key program of Chinese Academy of Sciences (CAS) (KJZD-SW-L11), Natural Science Foundation of Zhejiang Province (NO. LY21H100002), and the Open Research Fund Program of State Key Laboratory of Integrated Pest Management (IPM2109).
References
- Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–32. DOIPubMedGoogle Scholar
- Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, et al. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis. 2014;20:1358–61. DOIPubMedGoogle Scholar
- Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis. 2014;209:816–27. DOIPubMedGoogle Scholar
- Tran XC, Yun Y, Van An L, Kim SH, Thao NTP, Man PKC, et al. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg Infect Dis. 2019;25:1029–31. DOIPubMedGoogle Scholar
- Zohaib A, Zhang J, Saqib M, Athar MA, Hussain MH, Chen J, et al.; Sajjad-Ur-Rahman. Sajjad-Ur-Rahman. Serologic evidence of severe fever with thrombocytopenia syndrome virus and related viruses in Pakistan. Emerg Infect Dis. 2020;26:1513–6. DOIPubMedGoogle Scholar
- Win AM, Nguyen YTH, Kim Y, Ha NY, Kang JG, Kim H, et al. Genotypic heterogeneity of Orientia tsutsugamushi in scrub typhus patients and thrombocytopenia syndrome co-infection, Myanmar. Emerg Infect Dis. 2020;26:1878–81. DOIPubMedGoogle Scholar
- Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14:763–72. DOIPubMedGoogle Scholar
- Liu S, Chai C, Wang C, Amer S, Lv H, He H, et al. Systematic review of severe fever with thrombocytopenia syndrome: virology, epidemiology, and clinical characteristics. Rev Med Virol. 2014;24:90–102. DOIPubMedGoogle Scholar
- Sun J, Lu L, Wu H, Yang J, Liu K, Liu Q. Spatiotemporal patterns of severe fever with thrombocytopenia syndrome in China, 2011-2016. Ticks Tick Borne Dis. 2018;9:927–33. DOIPubMedGoogle Scholar
- Fu Y, Li S, Zhang Z, Man S, Li X, Zhang W, et al. Phylogeographic analysis of severe fever with thrombocytopenia syndrome virus from Zhoushan Islands, China: implication for transmission across the ocean. Sci Rep. 2016;6:19563. DOIPubMedGoogle Scholar
- Lin TL, Ou SC, Maeda K, Shimoda H, Chan JP, Tu WC, et al. The first discovery of severe fever with thrombocytopenia syndrome virus in Taiwan. Emerg Microbes Infect. 2020;9:148–51. DOIPubMedGoogle Scholar
- Zhu L, Yin F, Moming A, Zhang J, Wang B, Gao L, et al. First case of laboratory-confirmed severe fever with thrombocytopenia syndrome disease revealed the risk of SFTSV infection in Xinjiang, China. Emerg Microbes Infect. 2019;8:1122–5. DOIPubMedGoogle Scholar
- Yun Y, Heo ST, Kim G, Hewson R, Kim H, Park D, et al. Phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in South Korea and migratory bird routes between China, South Korea, and Japan. Am J Trop Med Hyg. 2015;93:468–74. DOIPubMedGoogle Scholar
- Li Z, Bao C, Hu J, Liu W, Wang X, Zhang L, et al. Ecology of the tick-borne phlebovirus causing severe fever with thrombocytopenia syndrome in an endemic area of China. PLoS Negl Trop Dis. 2016;10:
e0004574 . DOIPubMedGoogle Scholar - Zhang X, Zhao C, Cheng C, Zhang G, Yu T, Lawrence K, et al. Rapid spread of severe fever with thrombocytopenia syndrome virus by parthenogenetic Asian longhorned ticks. Emerg Infect Dis. 2022;28:363–72. DOIPubMedGoogle Scholar
- Zhuang L, Sun Y, Cui XM, Tang F, Hu JG, Wang LY, et al. Transmission of severe fever with thrombocytopenia syndrome virus by Haemaphysalis longicornis ticks, China. Emerg Infect Dis. 2018;24:24. DOIPubMedGoogle Scholar
- Park SW, Song BG, Shin EH, Yun SM, Han MG, Park MY, et al. Prevalence of severe fever with thrombocytopenia syndrome virus in Haemaphysalis longicornis ticks in South Korea. Ticks Tick Borne Dis. 2014;5:975–7. DOIPubMedGoogle Scholar
- Luo LM, Zhao L, Wen HL, Zhang ZT, Liu JW, Fang LZ, et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis. 2015;21:1770–6. DOIPubMedGoogle Scholar
- Hu YY, Zhuang L, Liu K, Sun Y, Dai K, Zhang XA, et al. Role of three tick species in the maintenance and transmission of Severe Fever with Thrombocytopenia Syndrome Virus. PLoS Negl Trop Dis. 2020;14:
e0008368 . DOIPubMedGoogle Scholar - Wang S, Li J, Niu G, Wang X, Ding S, Jiang X, et al. SFTS virus in ticks in an endemic area of China. Am J Trop Med Hyg. 2015;92:684–9. DOIPubMedGoogle Scholar
- Chen C, Li P, Li KF, Wang HL, Dai YX, Cheng X, et al. Animals as amplification hosts in the spread of severe fever with thrombocytopenia syndrome virus: A systematic review and meta-analysis. Int J Infect Dis. 2019;79:77–84. DOIPubMedGoogle Scholar
- Huang XY, Du YH, Wang HF, You AG, Li Y, Su J, et al. Prevalence of severe fever with thrombocytopenia syndrome virus in animals in Henan Province, China. Infect Dis Poverty. 2019;8:56. DOIPubMedGoogle Scholar
- Niu G, Li J, Liang M, Jiang X, Jiang M, Yin H, et al. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis. 2013;19:756–63. DOIPubMedGoogle Scholar
- He K, Chen JH, Gould GC, Yamaguchi N, Ai HS, Wang YX, et al. An estimation of Erinaceidae phylogeny: a combined analysis approach. PLoS One. 2012;7:
e39304 . DOIPubMedGoogle Scholar - Brockie RE. Distribution and abundance of the hedgehog (Erinaceus europaeus) L. in New Zealand, 1869–1973. N Z J Zool. 1975;2:445–62. DOIGoogle Scholar
- Isaac JL. Introduced mammals of the world: their history, distribution and influence. Austral Ecol. 2005;30:237–8. DOIGoogle Scholar
- Jahfari S, Ruyts SC, Frazer-Mendelewska E, Jaarsma R, Verheyen K, Sprong H. Melting pot of tick-borne zoonoses: the European hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles urban and suburban areas. Parasit Vectors. 2017;10:134. DOIPubMedGoogle Scholar
- Dziemian S, Sikora B, Piłacińska B, Michalik J, Zwolak R. Ectoparasite loads in sympatric urban populations of the northern white-breasted and the European hedgehog. Parasitol Res. 2015;114:2317–23. DOIPubMedGoogle Scholar
- Bouma HR, Carey HV, Kroese FG. Hibernation: the immune system at rest? J Leukoc Biol. 2010;88:619–24. DOIPubMedGoogle Scholar
- Simková A. Quantitative study of experimental Tahyna virus infection in hibernating hedgehogs. J Hyg Epidemiol Microbiol Immunol. 1966;10:499–509.PubMedGoogle Scholar
- Sun Y, Liu MM, Luo LM, Zhao L, Wen HL, Zhang ZT, et al. Seroprevalence of severe fever with thrombocytopenia syndrome virus in hedgehog from China. Vector Borne Zoonotic Dis. 2017;17:347–50. DOIPubMedGoogle Scholar
- Jiang Z, Liu S, Wu Y, Jiang X, Zhou K. China’s mammal diversity (2nd edition). 2017;25(8):886–95.
- Matsuno K, Orba Y, Maede-White K, Scott D, Feldmann F, Liang M, et al. Animal models of emerging tick-borne phleboviruses: determining target cells in a lethal model of SFTSV infection. Front Microbiol. 2017;8:104. DOIPubMedGoogle Scholar
- Li Z, Hu J, Bao C, Li P, Qi X, Qin Y, et al. Seroprevalence of antibodies against SFTS virus infection in farmers and animals, Jiangsu, China. J Clin Virol. 2014;60:185–9. DOIPubMedGoogle Scholar
- Casel MA, Park SJ, Choi YK. Severe fever with thrombocytopenia syndrome virus: emerging novel phlebovirus and their control strategy. Exp Mol Med. 2021;53:713–22. DOIPubMedGoogle Scholar
- Jiao Y, Qi X, Liu D, Zeng X, Han Y, Guo X, et al. Experimental and natural infections of goats with severe fever with thrombocytopenia syndrome virus: evidence for ticks as viral vector. PLoS Negl Trop Dis. 2015;9:
e0004092 . DOIPubMedGoogle Scholar - Park SC, Park JY, Choi JY, Oh B, Yang MS, Lee SY, et al. Experimental infection of dogs with severe fever with thrombocytopenia syndrome virus: Pathogenicity and potential for intraspecies transmission. Transbound Emerg Dis. 2022;69:3090–6. DOIPubMedGoogle Scholar
- Kim JY, Jung M, Kho JW, Song H, Moon K, Kim YH, et al. Characterization of overwintering sites of Haemaphysalis longicornis (Acari: Ixodidae) and tick infection rate with severe fever with thrombocytopenia syndrome virus from eight provinces in South Korea. Ticks Tick Borne Dis. 2020;11:
101490 . DOIPubMedGoogle Scholar - Nosek J, Grulich I. The relationship between the tick-borne encephalitis virus and the ticks and mammals of the Tribec mountain range. Bull World Health Organ. 1967;36(Suppl):31–47.PubMedGoogle Scholar
- Li Z, Bao C, Hu J, Gao C, Zhang N, Xiang H, et al. Susceptibility of spotted doves (Streptopelia chinensis) to experimental infection with the severe fever with thrombocytopenia syndrome phlebovirus. PLoS Negl Trop Dis. 2019;13:
e0006982 . DOIPubMedGoogle Scholar - Parkes J. Some aspects of the biology of the hedgehog (Erinaceus europaeus L.) in the Manawatu, New Zealand. N Z J Zool. 1975;2:463–72. DOIGoogle Scholar
- Brockie RE. The hedgehog population and invertebrate fauna of the west coast sand dunes. Proceedings of the New Zealand Ecological Society. 1957;5:27–9.
- Campbell PA. The feeding behaviour of the hedgehog (Erinaceus europaeus L.) in pasture land in New Zealand. Proceedings of the New Zealand Ecological Society. 1973;20:35–40.
- Heath A. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N Z Vet J. 2016;64:10–20. DOIPubMedGoogle Scholar
- Zhang X, Zhao C, Cheng C, Zhang G, Yu T, Lawrence K, et al. Rapid spread of severe fever with thrombocytopenia syndrome virus by parthenogenetic Asian longhorned ticks. Emerg Infect Dis. 2022;28:363–72. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: November 15, 2022
Table of Contents – Volume 28, Number 12—December 2022
| EID Search Options |
|---|
|
|
|
|
|
|






Please use the form below to submit correspondence to the authors or contact them at the following address:
Aihua Zheng, Institute of Zoology, Chinese Academy of Sciences, 1 Beichen West Rd, Chaoyang District, Beijing 100101, China
Top