Counterfeit Rabies Vaccine and Rabies Immune Globulin in the Philippines
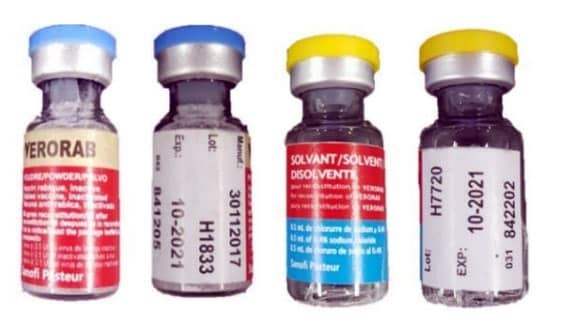
CDC Travelers’ Health posted information for international travelers on August 2, 2019 in response to a World Health Organization (WHO) notice regarding counterfeit rabies vaccine and rabies immune globulin in the Philippines. Three rabies vaccines (Verorab, Speeda, and Rabipur) and one immune globulin (Equirab) have been confirmed by the manufacturers as counterfeit. The Philippines Food and Drug Administration is actively investigating the counterfeit products. More information about the involved products is available in the WHO Medical Product Alert No 8/2019external icon.
Rabies is a deadly virus that can infect people if they are bitten or scratched by a rabid animal, but rabies is 100% preventable with post-exposure prophylaxis (PEP) that includes rabies vaccine and rabies immune globulin. Counterfeit vaccine or immune globulin might not be effective in preventing rabies.
If you received rabies vaccine or rabies immune globulin in the Philippines, contact your healthcare provider or state or local health department immediately to determine if you need revaccination.
While traveling abroad, do not approach or touch cats, dogs, or other animals. If you are bitten or scratched by an animal, wash all wounds thoroughly with soap and running water, and seek medical attention immediately. The US embassy in your destination country (http://www.usembassy.gov/external icon) can help you locate medical services.
Travelers visiting the Philippines may be advised to seek PEP in a neighboring country with a reliable supply or return to the United States regardless of whether or not you already received rabies vaccine or immune globulin (antirabies serum) in the Philippines. Consider buying a travel insurance policy that will allow you to return to the United States early to get medical care, if needed. If you choose to receive treatment in the Philippines, be vigilant about what you receive, request a copy of package information (such as brand name, batch or lot number, and expiration dates) for all doses received, and speak with the healthcare provider to ensure only certified products are used.
Further investigation and advanced laboratory testing is ongoing, but contents, efficacy, and safety of the counterfeit products are unknown. Please report any adverse events from the counterfeit vaccine to a healthcare provider and the Vaccine Adverse Events Reporting Systemexternal icon (for vaccines) or MedWatchexternal icon (for immune globulin).
To learn more about rabies please visit the CDC rabies website or contact your state or local health department.

