Selection Criteria for Milwaukee Protocol
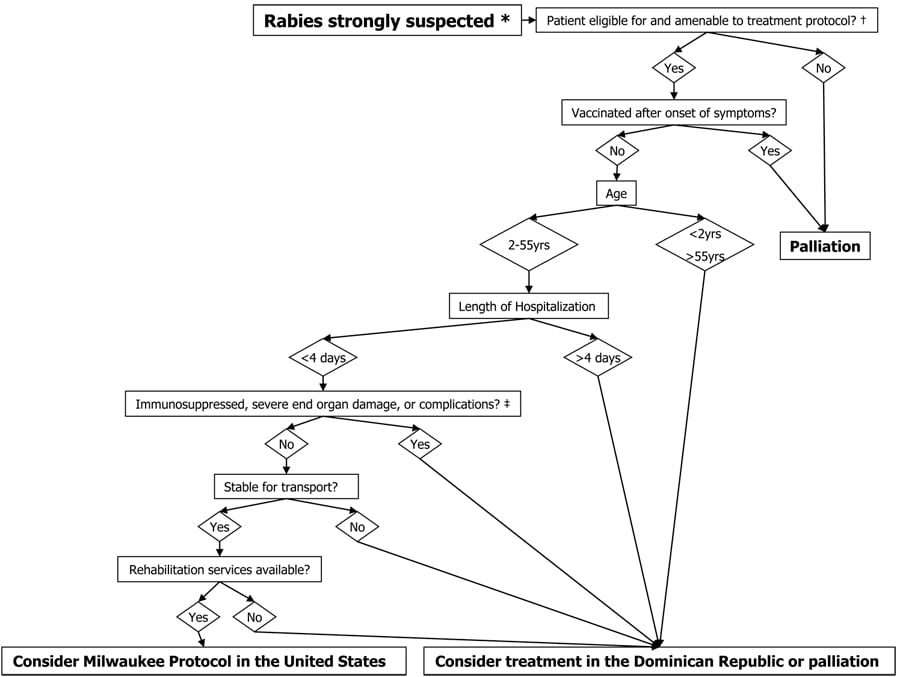
* – Based on compatible clinical presentation including encephalitis and/or myelitis AND a history of animal exposure.
– Patient without allergy to ketamine OR benzodiazepines OR amantadine OR BH4 OR L-arginine OR nimodipine. No contraindication or impracticality of transcranial doppler monitoring AND continuous EEG monitoring. Patient amenable to placement of an invasive device for measurement of intracranial pressure and tissue oxygen content OR brain biopsy under defined circumstances, serial lumbar punctures or phlebotomy to monitor immune response to rabies, repeat skin biopsies of nuchal skin.
– HIV, HTLV-1, pregnancy, coma, cerebral edema, cardiomyopathy, pulmonary edema, renal failure, refractory hypotension, status epilepticus, CSF protein >200mg/dL
§ – Expected survival time >48 hours, normal blood pressure for age with no more than low-dose vasopressor support, AND normal ventilation and oxygenation parameters if sedated and/or intubated.

