Prescription Pain Reliever (Opioid) Supplement and Call-Back Survey
CDC developed a five-point strategy aimed to prevent opioid-related overdoses and associated harms. To support this strategy, the Division of Reproductive Health undertook activities to better understand opioid use and risks among pregnant and postpartum women, maternal overdose deaths, and related jurisdiction needs. One component built upon existing surveillance infrastructure using the Pregnancy Risk Assessment Monitoring System (PRAMS) and other maternal-infant health surveys. CDC funded 2 new PRAMS-related opioid surveillance activities in 2019:
- PRAMS Opioid Supplement to collect information on prescription opioid pain reliever use during pregnancy.
- The supplement was implemented in 32 PRAMS sites and 2 non-PRAMS sites (total of 34 jurisdictions).
- PRAMS Opioid Call-back Survey to learn about prescription opioid pain reliever use and treatment postpartum, as well as infant health from PRAMS survey respondents 9-10 months after a live birth. The Call-back survey was implemented in 7 PRAMS sites with high rates of opioid-related overdoses.
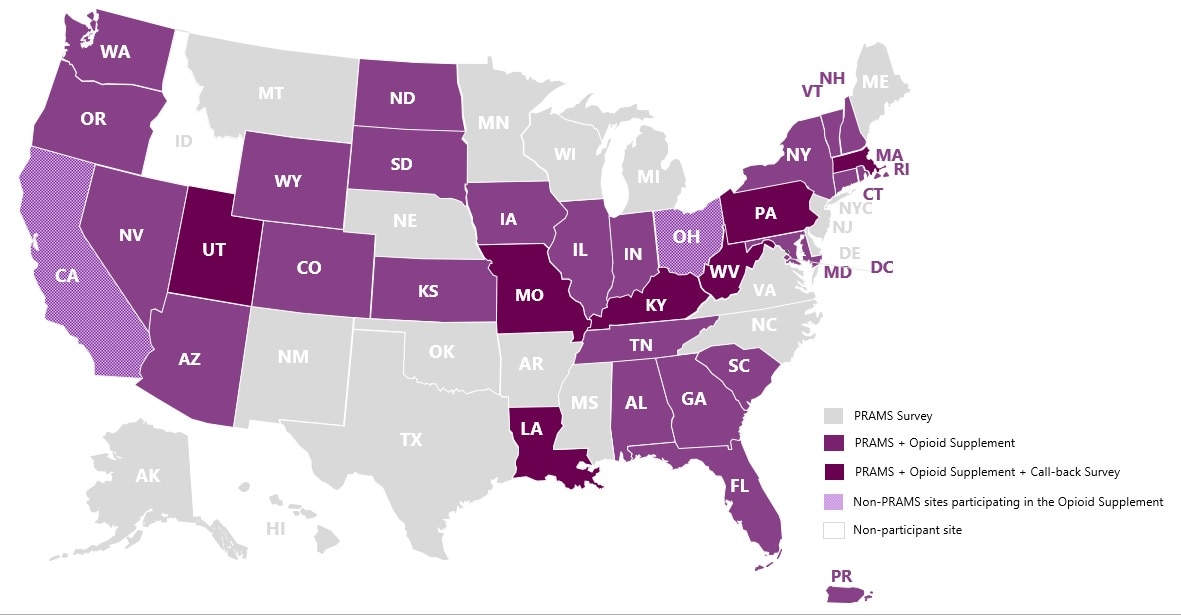
Jurisdictions Participating in the Pregnancy Risk Assessment Monitoring System (PRAMS), Opioid Supplement and Opioid Call-back Survey, 2019.
A total of 32 PRAMS jurisdictions received funding to implement the Opioid Supplement. Two additional sites with maternal-infant health surveys, California (Maternal and Infant Health Assessmentexternal icon) and Ohio (Ohio Pregnancy Assessment Surveyexternal icon), were funded through a partnership with the CDC Foundation to implement the Opioid Supplement.
Overview and Methods
Jurisdictions included the supplemental questions at the end of their PRAMS or maternal-infant surveys with no other changes to typical data collection procedures.
Questionnaire
The Opioid Supplement included a total of 10 questions, taking approximately 5-10 minutes to complete, and collected data on maternal knowledge, behaviors and experiences related to prescription opioid use during pregnancy.
Copies of the Opioid Supplement can be found in the PRAMS Supplements web page.
Overview and Methods
Seven states with a high rate of opioid-related overdose deaths participating in the Opioid Supplement implemented the Opioid Call-back Survey from October 2019 to April 2020. Each site designed a sampling plan to oversample women in areas of the state with populations at increased risk of opioid use. Identification of these areas, typically counties, was completed by each jurisdiction using available data sources and indicators such as reported overdose hospitalizations, overdose deaths, and/or neonatal abstinence syndrome incidence.
Women were contacted to complete the Opioid Call-Back Survey if they completed the regular PRAMS survey and did not decline participating in the call-back. Women were contacted when the infant was 9 months old and were eligible to complete the survey until their infant was 10 months old.
Questionnaire
The Opioid Call-back Survey had a total of 58 questions, took approximately 35 minutes to complete, and assesses opioid misuse and access to medication-assisted therapy, satisfaction with care received, postpartum care received, infant health and development, and receipt of social services and supports.
Copies of the Opioid Call-Back Survey can be accessed below:
- Opioid Call-back English Survey
- Opioid Call-back Spanish Survey
Ko JY, D’Angelo DV, Haight SC, et al. Vital Signs: Prescription Opioid Pain Reliever Use During Pregnancy — 34 U.S. Jurisdictions, 2019external icon. MMWR Morb Mortal Wkly Rep 2020;69:897–903.
Data from the opioid supplement or call-back survey will be available with the release of the 2019 PRAMS data. Please follow instructions to submit a PRAMS proposal found on the PRAMS for Researchers web page.