Fit evaluation of NIOSH Approved N95 filtering facepiece respirators with various skin protectants: a pilot study
July 2023
NIOSH Dataset RD-1070-2023-0
- Bergman-2023-JOEH-Skin-protectants-Accepted-version [PDF – 780 KB]
- Fit with Protectants data dictionary [DOC – 22 KB]
- Fit with Protectants Materials and Methods [DOC – 192 KB]
- Memo-signed [PDF – 245 KB]
- NPPTL-DATASET-Fit of FFRs with Skin Protectants [XLS – 18 KB]
- Overview Fit with Protectants-clean [DOC – 29 KB]
- signatures [PDF – 139 KB]
Materials and Methods
Subjects
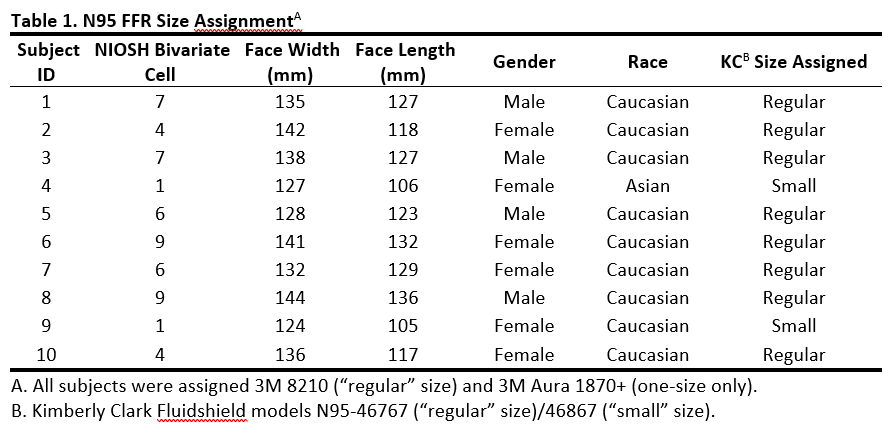
The study was conducted under a protocol approved by the University of Cincinnati’s Institutional Review Board (IRB). A sample of ten subjects (4 males and 6 females), who provided written informed consent, participated in quantitative fit testing of three N95 models with the application of three different skin protectants, as well as a control condition of no skin protectant. Manual caliper measurements of menton-sellion length (face length) and bizygomatic breadth (face width) were taken for all subjects on their first visit (Table 1). These measurements were then used to classify each subject into 1 of 10 cells according to the NIOSH Bivariate Panel, an anthropometric sizing system used to classify test subjects for respirator testing (Zhuang, Bradtmiller, & Shaffer 2007).
Respirators
Three commonly used NIOSH Approved® N95® filtering facepiece respirator models in healthcare were evaluated: Aura 1870+ (3M), 8210/8110S (3M), and Fluidshield (models N95-46767/46867, Kimberly-Clark (KC), Irving, TX, USA). The 3M Aura 1870+ is a tri-fold design (one size only). The other 3M model is cup-shaped and available in “regular” (8210) and “small” (8110S) sizes. The KC is a duckbill design available in two sizes – “regular” (46767) and “small” (46867).
For a subject to be included in the study, they had to initially pass a quantitative fit test under the control condition (no application of a skin protectant) on at least one of the three N95 models. This preliminary fit testing of the control condition also determined each subject’s best-fitting N95 size per model (for those models available in more than one size) to be worn during the evaluation using the skin protectants. Because the 3M Aura 1870+ is available in one-size only, all subjects tested this available size. For the N95 models available in two sizes (3M 8210/8110S and KC 46767/46867), subjects in panel cells 1–5 were initially tested in the “small” model size, and subjects in panel cells 6–10 were tested in the “regular” model size. Three fit tests were performed with the initially selected size. If the subject passed two of three fit tests, the initial size was kept for the remainder of the study. If the subject could only pass one of three tests or failed all three tests in the initial size, then the subject performed three additional fit tests using the alternative size. If the subject passed two or three fit tests with the alternative size, the alternative size was kept for the remainder of the study. If the subject failed all three fit tests in the alternative size, or could only pass one fit test with the alternative size, then the subject was assigned the FFR size (either “small” or “regular”) which achieved the highest single fit factor amongst the two sizes. Following this procedure, all subjects were assigned the 3M 8210 (“regular” size) and only two of the ten subjects were assigned the Kimberly Clark “small” size (Table 1).
For a subject to have been included in the study, they had to initially pass one quantitative fit test under the control condition (no application of a skin protectant) on at least one of the three N95 models. It is important to acknowledge that the aim of the study was to determine how fit was impacted with skin protectant application, including the hypothetical situation where fit might improve with the application. From this perspective, subjects could have failed all three fit tests for the control condition for as many as two of the three N95 models. This pilot study’s criteria for admission into the study differs from practice in an OSHA-regulated workplace, where a respirator user must pass a fit test to use a specific respirator model.
Blank samples
The three skin protectants evaluated were a bandage (Band-Aid® Flexible Fabric Bandage (3/4” x 3”), Johnson & Johnson, New Brunswick, NJ, USA), a surgical tape (DuraporeTM Surgical Tape, 3M), and a barrier cream (CavilonTM Durable Barrier Cream, product no. 3355, 3M). These skin protectant types were chosen because they are common items found in healthcare settings and are also of three different types (bandage, surgical tape, and barrier cream). Although guidance for two different CavilonTM products are available from 3M, the CavilonTM product (no. 3355) was not found to be recommended for use as a skin protectant to be used with respirators by 3M (3M 2020). At the time of this writing, none of the three skin protectants in this study were recommended for use with the N95 respirators in this study by the FFR manufacturers. Thus, using these specific protectant/N95 respirator combinations in the workplace would not be consistent with each of the NIOSH Approved N95 filtering facepiece respirator tested in this study.
Fit Testing
Three fit tests on the same respirator were performed for each combination of subject, skin protectant, and N95 model; a brand new N95 FFR was used for each test combination. The control condition was tested first for all subjects. The three skin protectant applications were then randomized for testing. Males were instructed to arrive for testing with shaven faces, but all subjects, both male and female, were not instructed to remove any applied facial products or makeup or wash their face prior to testing.
Skin protectants were self-applied by the subjects to the vulnerable areas on their face related to pressure-induced injury: the nose bridge and cheek areas (demonstrated on manikin heads, Figure 1). For the barrier cream, a droplet ~5 mm diameter was squeezed onto a sterile, 100%-cotton round wipe for facial application. The barrier cream was applied prior to performing the first fit test and additional cream was not applied between fit tests. The respirator sealing surface was not wiped between fit tests. After the three fit tests were completed, the subject wiped their face with a clean round wipe. Because the subjects wiped their faces following the third fit test, it was not expected that any residual cream would affect fit test results for the bandage or surgical tape tests (if those tests were randomly selected next).
Users were allowed to don and self-adjust their N95s. Following this adjustment, the test operator verified if the respirator was correctly donned by visually inspecting the placement of the N95 on the face and the placement of the straps on the head and around the neck. A user seal check was performed prior to fit testing. A rest period of two to three minutes was given for subjects between fit tests. Fit testing was performed with a calibrated PortaCount® Respirator Fit Tester (8048, TSI) operating in “N-95 mode”. Daily operational checks of the PortaCount® were performed prior to subject testing. Fit testing was performed in a test chamber using supplemented sodium chloride aerosol.
The OSHA-accepted ambient aerosol condensation nuclei counter protocol was used, which consists of eight sequential exercises: normal breathing, deep breathing, turning head side to side, moving head up and down, talking (reciting the “rainbow passage”), grimacing, bending over (at the waist as if to touch the toes), and normal breathing (Occupational Safety and Health Administration (OSHA), 1998). Passing the quantitative fit test is achieving a FF ≥100 (Occupational Safety and Health Administration (OSHA), 1998). The maximum FF outputted by the PortaCount® in “N-95 mode” is “200+”; where “200+” resulted, 201 was recorded.

Disclaimer
Mention of any company or product does not constitute endorsement by NIOSH or CDC.
References
3M. 2020. Protect your skin while wearing facial personal protective equipment (PPE). Retrieved from https://multimedia.3m.com/mws/media/1830444O/protecting-your-skin-while-wearing-ppe.pdf.
Occupational Safety and Health Administration (OSHA). 1998. Respiratory Protection: Final Rule (Vol. 29 CFR 1910.134). Washington, DC: US Government Printing Office, Office of the Federal Register.
Zhuang Z, Bradtmiller B, Shaffer RE. 2007. New respirator fit test panels representing the current U.S. civilian work force. J Occup Environ Hyg. 4(9): 647-659. doi:10.1080/15459620701497538
