Healthy People 2000 Progress Review
Food and Drug Safety
Date: September 1, 1999
Time: 2-4 p.m. EST
This progress review will be broadcast live from the Food and Drug Administration (FDA) Center for Devices and Radiological Health studio. Details on how to view the broadcast are provided on the FDA Web site.
Dr. David Satcher, Assistant Secretary for Health and Surgeon General, will be joined by a panel of experts representing the public and private health sectors. Dr. Linda Suydam, Senior Associate Commissioner, Food and Drug Administration, Mr. Thomas J. Billy, Administrator, Food Safety and Inspection Service, USDA and Dr. Edward Sondik, Director of the National Center for Health Statistics will review the progress of the objectives in the Food and Drug Safety priority area of Healthy People 2000. The participants will engage in a discussion that focuses on:
- Assuring that consumers receive useful information with new prescriptions
- Assuring that health care providers report serious adverse events
- Reducing foodborne illnesses
- Increasing the use of basic food safety techniques when cooking at home
- Improving food safety in restaurants, stores, and institutions
Briefing Book Materials
Data Section
- Priority Area 12: Food and Drug Safety Data Summary Table [PDF – 35 KB]
- Detailed Data Tables [PDF – 242 KB]
Presentations
Drug Safety
Presentation made by:
Edward J. Sondik, Ph.D.
Director, National Center for Health Statistics
Drug Safety (Part 1)
September 1, 1999
Food and Drug Administration (FDA) Center for Devices and Radiological Health studio
I am pleased to report on food and drug safety today and to summarize the status of our progress in reaching the Year 2000 targets established for each of the eight objectives in this priority area. Food and drug safety is truly a priority area, because this topic affects all of us in many aspects of our daily lives. Issues raised in food and drug safety are not limited to certain settings or certain groups but affect the population as a whole. Therefore, it’s critical to establish and develop systems to monitor closely these objectives. The results show that there has been substantial progress in improving both food and drug safety. Let’s look first at drug safety.

The goal in objective 12.5 is to increase the use of linked computer systems in pharmacies to alert us to potential drug interactions or hazardous or ineffective drug combinations. As you can see in this first slide, the use of computers is at a very high level in pharmacies–98 percent in 1995. Unfortunately, while the computer capabilities may be in place in individual pharmacies, we don’t have specific data to assess the extent to which linked systems are in place to inform pharmacists and protect patients.
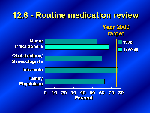
Of course critical to the safe and effective use of medications, is the careful and systematic review of those medications by a patient’s medical team. Objective 12.6 established a target of 75 percent of primary care providers routinely reviewing with their older patients all prescribed and over-the-counter medicines each time a new medication is prescribed. Early in this decade, internists had already met this target but for other health providers only about 50 to 60 percent were conducting this important medication review. Since that time we have new data to show an improvement by nurse practitioners and hope that this encouraging sign indicates that other health providers are doing so as well.
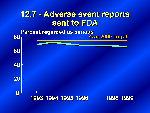
Our next slide shows data from FDA’s MedWatch, a program that monitors adverse reactions to drugs and other medical products. FDA has established detailed criteria for serious adverse events — such as a life-threatening situation, a permanent disability, or death — and has requested that only these serious reactions be reported under the voluntary system. This slide shows the percent of serious adverse events among the reports from health professionals and consumers that comprise the voluntary system — about 8 percent of the total reports to MedWatch. Objective 12.7 aimed at increasing the reporting of serious adverse events to 75 percent of all voluntary reports. Data through May 1999 show that the proportion of serious events has dropped slightly as a total of all reported events and is below the target. We’ll hear more about MedWatch and how to report adverse events later.
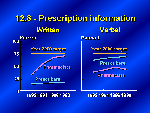
Clearly patients should be active and involved in decisions about their medications, and they need to be well informed to do so. Health care providers are important channels of information for patients, especially about the benefits and possible reactions to prescribed medications. Healthy People 2000 set as a goal that 75 percent of patients would receive useful information, verbally or in writing, from the doctor or the pharmacist. And here there is good news: We see progress for both, particularly among pharmacists. Since 1992 there has been a significant increase in the sharing of written information for prescribed medicines. We can also see that pharmacists by and large rely on written material, and that prescribers, on the other hand, are more likely to talk to their patients about their medications.
This completes the review of the data on the four objectives in Drug Safety. Progress has been made, but there is a lack of current data for some key components. Filling these data gaps with national, reliable studies with comparable data is critical for Healthy People 2010.
Food Safety
Presentation made by:
Edward J. Sondik, Ph.D.
Director, National Center for Health Statistics
Food Safety (Part 2)
September 1, 1999
Food and Drug Administration (FDA) Center for Devices and Radiological Health studio
Perhaps no other year 2000 objectives have the potential to affect so many Americans literally on a day to day basis. Annually, it is estimated that millions of Americans become sick from eating unsafe food and thousands die. We may hear about the most serious outbreaks of foodborne infections through the media. But behind these news reports, there’s a network of reporting systems to capture data on individual cases of illness associated with foodborne pathogens.

Healthy People established the objective to reduce infections caused by Salmonella, Campylobacter jejuni, E.Coli O157:H7 and Listeria moncytogenes, because of the frequency of these food-borne infections and their severity.

I am very pleased to report that each of these four foodborne infections has met the Year 2000 targets. Cases of campylobacter jejuni, down by 50 percent; Salmonella, down by 22 percent; E.Coli O157:H7, down by 75 percent and Listeria monocytogenes down by 29 percent. The magnitude of the problem for each is different but they all show a substantial decline. Truly remarkable progress over the past 10 years. Also noteworthy is that we have a data system capable of monitoring these diseases and documenting progress. From a decade ago when there were many reporting systems operating in this area, yet still data gaps, we now have a coordinated surveillance system, FoodNet. We’ll have more details on FoodNet in following discussions but it is a strong, comprehensive system based on collaborative efforts of State and local health departments and CDC, FDA, and USDA.
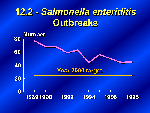
Turning from individual cases to reported outbreaks, Salmonella enteriditis infection outbreaks have declined from 77 outbreaks in 1989 to 45 in 1998. However, objective 12.2 still remains substantially above the year 2000 target of 25 outbreaks.

Consumers have a major responsibility in protecting their health and that of family members. The food preparer in the home can observe the relatively simple practices of washing utensils and cutting boards with soap after contact with raw meat and poultry and refrigerating perishable foods. The data we have show some modest progress in refrigeration and washing cutting boards.
We have other evidence of improvements in consumer food safety practices beyond the ones shown here. Since 1993, the percentage of the population who say they eat raw oysters has dropped from 16 to 12, and the percentage who say they eat foods containing raw eggs has dropped from 52 to 37. The proportion of food preparers who say they wash their hands with soap after handling raw meat has increased from 66 to 76 percent. If we include people who either wash their cutting boards or use a different board after cutting raw food, 79 percent of food preparers reported safe practices in 1998.
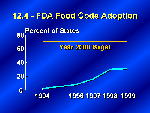
In the retail, restaurant, and institutional side of this equation, FDA established food protection codes which have now been adopted in 16 states just since 1994 when they were first announced. In addition, a very encouraging sign is that another 25 states say that adoption of the code is in process.
Reviewing the data for the drug and food safety objectives shows that progress has been made, particularly in reducing infections caused by key foodborne pathogens. Filling the existing data gaps is a high priority for the future Healthy People 2010 objectives.