CDC Specimen Test Order and Reporting (CSTOR)
The CDC Specimen Test Order and Reporting (CSTOR) Web Portal is the central online resource for CDC Infectious Disease (ID) laboratory partners, including the Association of Public Health Laboratories (APHL)external icon, to submit specimens and access test results and reports.
CSTOR Web Portal allows infectious disease specimen submitters to:
- Request and obtain approval to send samples to CDC for testing
- Submit specimen data and create shipping paperwork online
- Validate specimen data and provide submitter support to correct errors and complete missing information
- View and download test results and reports easily and securely online
Streamlining the Specimen Submission Lifecycle
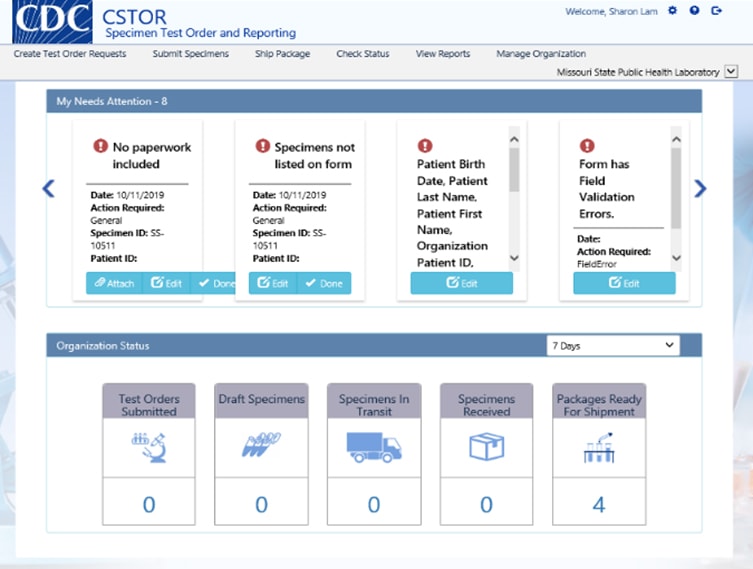
There are six modules to help submit, track, and access specimen information and test results. These include:
- Request Test Order – Manage test requests to CDC; CSTOR is updated with the latest Test Order Directory
- Submit Specimens – Submit specimen data via a web form or import a batch of specimens using any Excel file
- Ship Package – Create manifests and packing slips for specimen shipments
- Check Status – Track the status of test order requests, specimen submissions, and packages
- View Reports – View and download reports securely, and access test result data in a variety of formats
- Manage Organization – Administrators may add and remove users in their organization and edit data
An alternative to CDC Specimen Submission Form 50.34, CSTOR removes manual processes and tracks the status of each submitted specimen. Connected to CDC’s Enterprise Laboratory Information Management System (ELIMS), CSTOR accelerates specimen receipt, reduces processing time, and expedites delays.
Saves time and minimizes manual processes
- Import specimens to and export results from a LIMS in bulk
- Additional access for laboratorians and epidemiologists
Improves data quality and standardization
- Validate data as CSTOR guides you to fix errors before submission
- Easily complete submission form with auto-populated submitter fields
Centralizes all CDC submission information
- Receive alerts in one place instead of receiving multiple emails
- Electronically track test order requests, approvals, and rejections
Provides secure data access to the entire laboratory
- Securely access protected health information, including all eReports, without individual decryption
If specimen submitting organizations are interested in using CSTOR, please contact the CSTOR Team.