Volume 30, Number 5—May 2024
Research Letter
Deforestation and Bovine Rabies Outbreaks in Costa Rica, 1985–2020
Abstract
In Latin America, rabies virus has persisted in a cycle between Desmodus rotundus vampire bats and cattle, potentially enhanced by deforestation. We modeled bovine rabies virus outbreaks in Costa Rica relative to land-use indicators and found spatial-temporal relationships among rabies virus outbreaks with deforestation as a predictor.
Costa Rica has benefited from effective vaccination campaigns to eliminate canine rabies virus infections. Still, the virus has endured, spread by vampire bats (Desmodus rotundus) to cattle, with rare but documented transfer from bats to humans (1,2). To determine how anthropogenic disturbance affects rabies virus incidence and risk in this system, we investigated the relationship between land-use change and documented bovine rabies virus outbreaks in Costa Rica during 1985–2020.
Since 1985, the National Animal Health Service of Costa Rica (SENASA) has conducted rabies virus surveillance on domestic animals, confirming outbreaks of >1 cases by using fluorescent antibody testing (3). We mapped bovine rabies outbreaks during 1985–2020 reported by SENASA with neighboring land-use data by using QGIS 3.16.2 (QGIS, https://qgis.org). Ten outbreaks from the initial SENASA report (n = 119) were removed because of inaccurate location data, leaving 109 outbreaks for our study.
To evaluate outbreak probability and distribution, we used kernel density estimations with the QGIS default bandwidth to create spatial probability estimations on the basis of known outbreaks. We used a kernel function that smoothed and interpolated probabilities across the study area. We used a kernel radius of 10 km, the maximum vampire bat foraging range, limiting interpolation to the determined area (4). We applied a Kulldorff retrospective space-time scan with an elliptical spatial scan by using SaTScan version 9.7 (SaTScan, https://www.satscan.org) to detect the number of outbreak locations in space and time (5).
We applied logistic regression by using a generalized linear mixed model R-package (https://cran.r-project.org/web/packages/lme4/lme4.pdf) to evaluate the effects of land-use factors on bovine rabies virus outbreak locations compared with random control locations (n = 119). We set the district as a random effect to account for spatial effects. We set the number of control points to match the true number of SENASA-reported outbreaks. We created control locations by using the random points function in QGIS and by using the 2005 and 2017 agricultural land-use data to bind nonoutbreak samples to areas that could house cattle. We matched controls temporally to outbreaks on the basis of the proportion of outbreaks before and after 2006.
For each outbreak and control location, we used district-level human population density and cattle population density as explanatory variables. We used the distance to and area of forest cover from each outbreak and control location within a 10-km buffer of the location. For outbreaks and control events up to 2014, we calculated forest cover by using the 2014 aerial photograph from the Atlas of Costa Rica (http://www.kyriosoft.com/atlas). For outbreaks after 2014, we used a 2018 aerial photograph from the National Territorial Information System (https://www.snitcr.go.cr). We used human population data from 2011 for all detections up to 2011. After 2011, we used a human population density estimate based on national growth trends (6). We used a similar approach for cattle density data based on a dataset from 2014 (7).
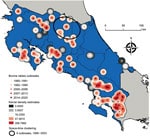
Outbreaks occurring in the northern provinces of Alajuela and Heredia clustered on the basis on their statistically significant closeness in both location and time of occurrence (6 outbreaks during 1999–2003; log likelihood ratio 7.52; p = 0.035) (Figure). The increased number of outbreaks in southern Puntarenas Province may be because of repeated emergence given the lack of space-time clustering (Figure).
We found a positive association between the distance to forested areas and bovine rabies virus outbreaks (generalized linear mixed model estimate 4.33 × 10−4, SE 3.32 × 10−1; Z-value 1.95; p = 0.05) (Table). Each 1-km increase in distance from forested areas increased the probability of an outbreak by 4%. This finding aligns with our understanding of D. rotundus bat feeding preferences and rabies virus transmission risk. Decreased forested roosting site proximity appears to increase D. rotundus bat feeding behavior on cattle (8). Human and cattle densities were not associated with bovine rabies outbreaks (Table). Because human population data were unavailable until 2011 and cattle population data unavailable until 2014, the effect of those population densities may be skewed because agricultural intensification in Costa Rica has undergone major changes during the study period (9).
Our results show an association between deforestation and bovine rabies virus outbreaks, highlighting the importance of considering negative health effects in risk assessments for forest conversion proposals (10). Our results indicated the southern region of Costa Rica has the highest probability of bovine rabies outbreaks, indicating the need for localized, preventative interventions in the south. On the basis of recent findings, we must caution against bat culling as a response to this threat, because disrupting bat dispersal in unexpected ways may increase the spread of the rabies virus (2). Because rabies virus remains endemic in Latin America, an increased focus on integrating spatial, dietary, and surveillance data for D. rotundus bats is needed to provide additional insights into land-use effects on the persistence and spread of the rabies virus.
Ms. Jones is a recent graduate of the joint Environmental Sciences and Environmental Health Bachelor of Science–Masters of Public Health Program at Emory University, Atlanta, Georgia. Her primary research interests are the application of one health and planetary health approaches to mitigating zoonoses.
Acknowledgment
We thank Mariano Arroyo of the National Animal Health Services of Costa Rica for data access.
References
- Badilla X, Pérez-Herra V, Quirós L, Morice A, Jiménez E, Sáenz E, et al. Human rabies: a reemerging disease in Costa Rica? Emerg Infect Dis. 2003;9:721–3. DOIPubMedGoogle Scholar
- Viana M, Benavides JA, Broos A, Ibañez Loayza D, Niño R, Bone J, et al. Effects of culling vampire bats on the spatial spread and spillover of rabies virus. Sci Adv. 2023;9:
eadd7437 . DOIPubMedGoogle Scholar - Hutter SE, Brugger K, Sancho Vargas VH, González R, Aguilar O, León B, et al. Rabies in Costa Rica: documentation of the surveillance program and the endemic situation from 1985 to 2014. Vector Borne Zoonotic Dis. 2016;16:334–41. DOIPubMedGoogle Scholar
- Rocha F, Ulloa-Stanojlovic FM, Rabaquim VCV, Fadil P, Pompei JC, Brandão PE, et al. Relations between topography, feeding sites, and foraging behavior of the vampire bat, Desmodus rotundus. J Mammal. 2020;101:164–71. DOIGoogle Scholar
- Kulldorff M, Heffernan R, Hartman J, Assunção RM, Mostashari F. Space-time permutation model: a space-time permutation scan statistic for the early detection of disease outbreaks. PLoS Med. 2005;2:216–24. DOIGoogle Scholar
- National Institute of Statistics and Censuses. District population estimates and projections 2000 to 2025. 2014 [cited 2023 Jul 1] https://inec.cr/wwwisis/documentos/INEC/Estimaciones%20y%20Proyecciones/Estimaciones_Proyecciones_Distritales_2000-2025_2014.pdf
- National Institute of Statistics and Censuses. VI National agricultural census: characteristics of farms and producers [in Spanish]. 2015 Jul [cited 2023 Jul 1] https://admin.inec.cr/sites/default/files/media/reagropeccenagro2014-ti-006_6.pdf
- Fleischer R, Jones C, Ledezma-Campos P, Czirják GÁ, Sommer S, Gillespie TR, et al. Gut microbial shifts in vampire bats linked to immunity due to changed diet in human disturbed landscapes. Sci Total Environ. 2024;907:
167815 . DOIPubMedGoogle Scholar - Jadin I, Meyfroidt P, Lambin EF. International trade, and land use intensification and spatial reorganization explain Costa Rica’s forest transition. Environ Res Lett. 2016;11:
035005 . DOIGoogle Scholar - Gillespie TR, Jones KE, Dobson AP, Clennon JA, Pascual M. COVID-Clarity demands unification of health and environmental policy. Glob Change Biol. 2021;27:1319–21. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 30, Number 5—May 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Thomas Gillespie, Emory University, 400 Dowman Dr, Ste E510, Atlanta, GA, 30307, USA
Top