Volume 24, Number 7—July 2018
Perspective
Integrated Serologic Surveillance of Population Immunity and Disease Transmission
Figure 2
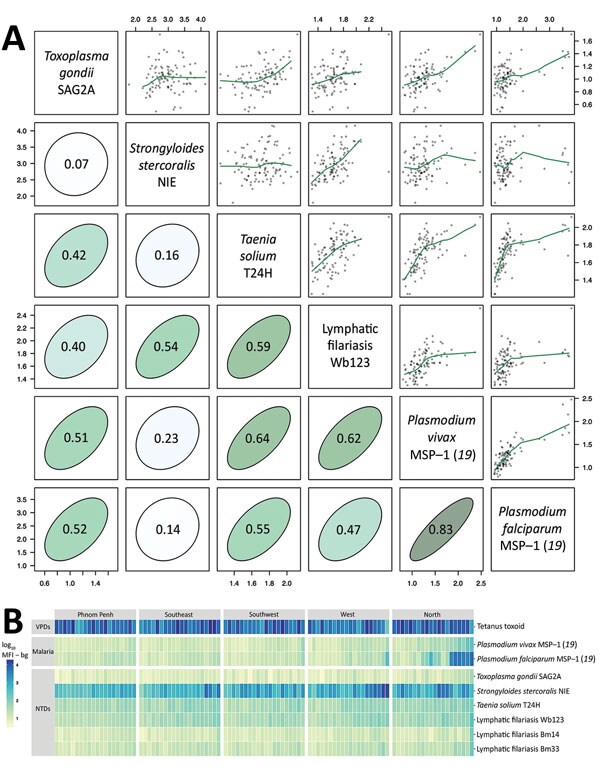
Figure 2. Antibody response to tetanus toxoid and causative agents of malaria and NTDs measured by multiplex bead assay among 2,150 women, Cambodia, 2012. Specimens were measured by using the Luminex platform (Luminex Corporation, Austin, TX, USA) (25). A) Relationship between pairs of antibodies measured by mean antibody response (log10 MFI – bg) in each of the 100 sampling clusters. Scatter plots include nonparametric locally weighted regression fits trimmed to reduce edge effects. Correlation ellipses depict the strength of the association on the basis of the Pearson correlation (r estimates). Both axes indicate mean antibody response. B) Heatmap of mean antibody response to tetanus toxoid and pathogens that cause malaria and NTDs in 100 sampling clusters stratified by region and then sorted by mean antibody response. Data set and computational notebook are available through the Open Science Framework (https://osf.io/2kr8b). MFI – bg, mean fluorescence intensity minus background; MSP, merozoite surface protein; NTDs, neglected tropical diseases; SAG2A, surface antigen 2A; VPDs, vaccine-preventable diseases.
References
- Dowell SF, Blazes D, Desmond-Hellmann S. Four steps to precision public health. Nature. 2016;540:189–91. DOIGoogle Scholar
- World Health Organization. Global vaccine action plan 2011-2020. Geneva: The Organization; 2013 [cited 2017 Nov 22]. http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/
- World Health Organization. Global technical strategy for malaria 2016–2030. Geneva: The Organization; 2015 Nov 4 [cited 2017 Nov 22]. https://market.android.com/details?id=book-LV40DgAAQBAJ
- World Health Organization. Global health sector strategy on HIV 2016–2021. Report no. WHO/HIV/2016.05. Geneva: The Organization; 2016 [cited 2017 Nov 22]. http://apps.who.int/iris/bitstream/handle/10665/246178/WHO-HIV-2016.05-eng.pdf
- Cutts FT, Izurieta HS, Rhoda DA. Measuring coverage in MNCH: design, implementation, and interpretation challenges associated with tracking vaccination coverage using household surveys. PLoS Med. 2013;10:e1001404. DOIPubMedGoogle Scholar
- MacNeil A, Lee C-W, Dietz V. Issues and considerations in the use of serologic biomarkers for classifying vaccination history in household surveys. Vaccine. 2014;32:4893–900. DOIPubMedGoogle Scholar
- Cutts FT, Hanson M. Seroepidemiology: an underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop Med Int Health. 2016;21:1086–98. DOIPubMedGoogle Scholar
- Drakeley C, Cook J. Chapter 5. Potential contribution of sero-epidemiological analysis for monitoring malaria control and elimination: historical and current perspectives. Adv Parasitol. 2009;69:299–352. DOIPubMedGoogle Scholar
- Simonsen J, Strid MA, Mølbak K, Krogfelt KA, Linneberg A, Teunis P. Sero-epidemiology as a tool to study the incidence of Salmonella infections in humans. Epidemiol Infect. 2008;136:895–902. DOIPubMedGoogle Scholar
- Teunis PFM, Falkenhorst G, Ang CW, Strid MA, De Valk H, Sadkowska-Todys M, et al. Campylobacter seroconversion rates in selected countries in the European Union. Epidemiol Infect. 2013;141:2051–7. DOIPubMedGoogle Scholar
- Exum NG, Pisanic N, Granger DA, Schwab KJ, Detrick B, Kosek M, et al. Use of pathogen-specific antibody biomarkers to estimate waterborne infections in population-based settings. Curr Environ Health Rep. 2016;3:322–34. DOIPubMedGoogle Scholar
- Moss DM, Priest JW, Hamlin K, Derado G, Herbein J, Petri WA Jr, et al. Longitudinal evaluation of enteric protozoa in Haitian children by stool exam and multiplex serologic assay. Am J Trop Med Hyg. 2014;90:653–60. DOIPubMedGoogle Scholar
- Goodhew EB, Priest JW, Moss DM, Zhong G, Munoz B, Mkocha H, et al. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis. 2012;6:e1873. DOIPubMedGoogle Scholar
- Hamlin KL, Moss DM, Priest JW, Roberts J, Kubofcik J, Gass K, et al. Longitudinal monitoring of the development of antifilarial antibodies and acquisition of Wuchereria bancrofti in a highly endemic area of Haiti. PLoS Negl Trop Dis. 2012;6:e1941. DOIPubMedGoogle Scholar
- Lammie PJ, Moss DM, Brook Goodhew E, Hamlin K, Krolewiecki A, West SK, et al. Development of a new platform for neglected tropical disease surveillance. Int J Parasitol. 2012;42:797–800. DOIPubMedGoogle Scholar
- Poirier MJP, Moss DM, Feeser KR, Streit TG, Chang G-JJ, Whitney M, et al. Measuring Haitian children’s exposure to chikungunya, dengue and malaria. Bull World Health Organ. 2016;94:817–825A. DOIPubMedGoogle Scholar
- Feeser KR, Cama V, Priest JW, Thiele EA, Wiegand RE, Lakwo T, et al. Characterizing reactivity to Onchocerca volvulus antigens in multiplex bead assays. Am J Trop Med Hyg. 2017;97:666–72. DOIPubMedGoogle Scholar
- Curtis KA, Kennedy MS, Charurat M, Nasidi A, Delaney K, Spira TJ, et al. Development and characterization of a bead-based, multiplex assay for estimation of recent HIV type 1 infection. AIDS Res Hum Retroviruses. 2012;28:188–97. DOIPubMedGoogle Scholar
- Metcalf CJE, Farrar J, Cutts FT, Basta NE, Graham AL, Lessler J, et al. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet. 2016;388:728–30. DOIPubMedGoogle Scholar
- Hens N, Shkedy Z, Aerts M, Damme CFPV, Beutels P. Modeling infectious disease parameters based on serological and social contact data: a modern statistical perspective. New York: Springer-Verlag New York; 2012.
- Solomon AW, Engels D, Bailey RL, Blake IM, Brooker S, Chen J-X, et al. A diagnostics platform for the integrated mapping, monitoring, and surveillance of neglected tropical diseases: rationale and target product profiles. PLoS Negl Trop Dis. 2012;6:e1746. DOIPubMedGoogle Scholar
- van Gageldonk PGM, van Schaijk FG, van der Klis FR, Berbers GAM. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods. 2008;335:79–89. DOIPubMedGoogle Scholar
- Scobie HM, Mao B, Buth S, Wannemuehler KA, Sørensen C, Kannarath C, et al. Tetanus immunity among women aged 15 to 39 years in Cambodia: a national population-based serosurvey, 2012. Clin Vaccine Immunol. 2016;23:546–54. DOIPubMedGoogle Scholar
- Arnold BF, van der Laan MJ, Hubbard AE, Steel C, Kubofcik J, Hamlin KL, et al. Measuring changes in transmission of neglected tropical diseases, malaria, and enteric pathogens from quantitative antibody levels. PLoS Negl Trop Dis. 2017;11:e0005616. DOIPubMedGoogle Scholar
- Priest JW, Jenks MH, Moss DM, Mao B, Buth S, Wannemuehler K, et al. Integration of multiplex bead assays for parasitic diseases into a national, population-based serosurvey of women 15-39 years of age in Cambodia. PLoS Negl Trop Dis. 2016;10:e0004699. DOIPubMedGoogle Scholar
- Frenk J. The global health system: strengthening national health systems as the next step for global progress. PLoS Med. 2010;7:e1000089. DOIPubMedGoogle Scholar
- Sturrock HJW, Bennett AF, Midekisa A, Gosling RD, Gething PW, Greenhouse B. Mapping malaria risk in low transmission settings: challenges and opportunities. Trends Parasitol. 2016;32:635–45. DOIPubMedGoogle Scholar
- Zhou X-N, Bergquist R, Tanner M. Elimination of tropical disease through surveillance and response. Infect Dis Poverty. 2013;2:1. DOIPubMedGoogle Scholar
- United Nations. Sustainable development goals [cited 2017 Feb 14]. http://www.un.org/sustainabledevelopment
- Woolhouse ME, Hagan P. Seeking the ghost of worms past. Nat Med. 1999;5:1225–7. DOIPubMedGoogle Scholar
- Hotez PJ, Alvarado M, Basáñez M-G, Bolliger I, Bourne R, Boussinesq M, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e2865. DOIPubMedGoogle Scholar
- Kroidl I, Saathoff E, Maganga L, Makunde WH, Hoerauf A, Geldmacher C, et al. Effect of Wuchereria bancrofti infection on HIV incidence in southwest Tanzania: a prospective cohort study. Lancet. 2016;388:1912–20. DOIPubMedGoogle Scholar
- Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14:1118–26. DOIPubMedGoogle Scholar
- Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, et al. Helminth infection, fecundity, and age of first pregnancy in women. Science. 2015;350:970–2. DOIPubMedGoogle Scholar
- Moore SM, Azman AS, Zaitchik BF, Mintz ED, Brunkard J, Legros D, et al. El Niño and the shifting geography of cholera in Africa. Proc Natl Acad Sci U S A. 2017;114:4436–41. DOIPubMedGoogle Scholar
- Kovats RS, Bouma MJ, Hajat S, Worrall E, Haines A. El Niño and health. Lancet. 2003;362:1481–9. DOIPubMedGoogle Scholar
- Scobie HM, Patel M, Martin D, Mkocha H, Njenga SM, Odiere MR, et al. Tetanus immunity gaps in children 5–14 years and men >15 years of age revealed by integrated disease serosurveillance in Kenya, Tanzania, and Mozambique. Am J Trop Med Hyg. 2017;96:415–20. DOIPubMedGoogle Scholar
- Mulders MN, Serhan F, Goodson JL, Icenogle J, Johnson BW, Rota PA. Expansion of Surveillance for Vaccine-preventable Diseases: Building on the Global Polio Laboratory Network and the Global Measles and Rubella Laboratory Network Platforms. J Infect Dis. 2017;216(suppl_1):S324–30. DOIPubMedGoogle Scholar
- Corran P, Coleman P, Riley E, Drakeley C. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23:575–82. DOIPubMedGoogle Scholar
- Masson J, Douglass J, Roineau M, Aye KS, Htwe K, Warner J, et al. Concordance between plasma and filter paper sampling techniques for the lymphatic filariasis bm14 antibody ELISA. Trop Med Infect Dis. 2017;2:6. DOIGoogle Scholar
- Formenti F, Buonfrate D, Prandi R, Marquez M, Caicedo C, Rizzi E, et al. Comparison of S. stercoralis serology performed on dried blood spots and on conventional serum samples. Front Microbiol. 2016;7:1778. DOIPubMedGoogle Scholar
- Snijdewind IJM, van Kampen JJA, Fraaij PLA, van der Ende ME, Osterhaus ADME, Gruters RA. Current and future applications of dried blood spots in viral disease management. Antiviral Res. 2012;93:309–21. DOIPubMedGoogle Scholar
- Jacobson JO, Cueto C, Smith JL, Hwang J, Gosling R, Bennett A. Surveillance and response for high-risk populations: what can malaria elimination programmes learn from the experience of HIV? Malar J. 2017;16:33. DOIPubMedGoogle Scholar
- Chipeta MG, Terlouw DJ, Phiri KS, Diggle PJ. Adaptive geostatistical design and analysis for prevalence surveys. Spat Stat. 2016;15:70–84. DOIGoogle Scholar
- Helb DA, Tetteh KKA, Felgner PL, Skinner J, Hubbard A, Arinaitwe E, et al. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc Natl Acad Sci U S A. 2015;112:E4438–47. DOIPubMedGoogle Scholar
- Osgood-Zimmerman A, Millear AI, Stubbs RW, Shields C, Pickering BV, Earl L, et al. Mapping child growth failure in Africa between 2000 and 2015. Nature. 2018;555:41–7. DOIPubMedGoogle Scholar
- Takahashi S, Metcalf CJE, Ferrari MJ, Tatem AJ, Lessler J. The geography of measles vaccination in the African Great Lakes region. Nat Commun. 2017;8:15585. DOIPubMedGoogle Scholar
- Gething PW, Casey DC, Weiss DJ, Bisanzio D, Bhatt S, Cameron E, et al. Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N Engl J Med. 2016;375:2435–45. DOIPubMedGoogle Scholar
- Solomon AW, Pavluck AL, Courtright P, Aboe A, Adamu L, Alemayehu W, et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22:214–25. DOIPubMedGoogle Scholar
- Moyes CL, Temperley WH, Henry AJ, Burgert CR, Hay SI. Providing open access data online to advance malaria research and control. Malar J. 2013;12:161. DOIPubMedGoogle Scholar