Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Influenza Vaccination Coverage Among Children Aged 6 Months--18 Years --- Eight Immunization Information System Sentinel Sites, United States, 2008--09 Influenza Season
Vaccination is the most effective way to prevent influenza-related morbidity and mortality (1). Annual influenza vaccination was first recommended for children aged 6--23 months in 2004 and for children aged 24--59 months in 2006 (2,3). In August 2008, the Advisory Committee on Immunization Practices (ACIP) expanded its recommendations to include all children aged 5--18 years, beginning with the 2008--09 influenza season (1). Among children aged 6 months--8 years, previously unvaccinated children and children who received only 1 vaccine dose for the first time in the preceding influenza season are recommended to receive 2 influenza vaccine doses (1). Children aged 9--18 years are recommended to receive 1 vaccine dose. To update previous estimates (4) by assessing influenza vaccination coverage among children aged 6 months--18 years during the 2008--09 season, CDC averaged data from the eight immunization information system (IIS) sentinel sites. The results indicated that average (unweighted) vaccination coverage with ≥1 influenza vaccine doses decreased with increasing age from 47.8% for children aged 6--23 months to 9.1% for those aged 13--18 years. Among sites, average coverage with ≥1 doses among children aged 6--23 months increased from 40.8% during the 2007--08 influenza season to 47.8% during the 2008--09 season; however, coverage levels remained suboptimal. Vaccination against both seasonal influenza and 2009 pandemic influenza A (H1N1) are recommended for children in 2009 (5); these findings highlight the need to identify opportunities for and barriers to influenza vaccination of children.
IIS sentinel sites* are useful data sources to assess influenza vaccination coverage because data 1) reflect the most recent influenza season, 2) are provider-verified, 3) can track vaccination patterns throughout the entire August-March influenza season, and 4) can assess coverage among children and adolescents. For the 2008--2012 sentinel site project period, CDC is supporting eight IIS sites that meet the following criteria: 1) >85% of child vaccine provider sites are enrolled in the IIS, 2) >85% of children aged <19 years who resided in the sentinel site region with ≥2 vaccinations are recorded in the IIS, and 3) >70% of doses are reported to the IIS ≤30 days of vaccination. The six sentinel site areas in Arizona, Colorado, Michigan, Minnesota, Oregon, and Wisconsin consist of contiguous counties, postal codes, or census tracts; the other two sentinel sites consist of the entire state of North Dakota and all of New York City. As of March 31, 2009, 5,236,894 children aged 6 months--18 years were enrolled in the sentinel sites (range: 32,917 in Colorado to 2,303,355 in New York City).
To reflect ACIP recommendations for the 2008--09 influenza season (1), full vaccination for children aged 6 months--8 years was defined as 1) receipt of 2 vaccine doses separated by at least 4 weeks in the current season among previously unvaccinated children and children who received 1 dose for the first time during August 1, 2007--March 31, 2008, or 2) receipt of 1 vaccine dose in the current season among all other children. Children aged 9--18 years were considered fully vaccinated with receipt of 1 vaccine dose. Vaccination coverage was calculated for children aged 6--23 months, 2--4 years, 5--10 years, 11--12 years, and 13--18 years who resided in each sentinel site area during the 2008--09 influenza season. Analyses included only children who were in the specified age categories during the entire influenza season to ensure that all children evaluated had the same opportunity for vaccination. Vaccination coverage at each sentinel site was calculated by dividing the number of children vaccinated by the total number of children in each specified age group. The unweighted average for the eight sites (i.e., average site-specific coverage) was calculated by summing the percentages of children vaccinated at each site and dividing by the total number of sites (eight). To determine weekly vaccination patterns, the number of influenza vaccine doses administered each week to children aged 6 months--18 years during the 2008--09 influenza season was determined at each of the eight sites and converted into a percentage of all doses administered during the entire season; those eight percentages were then averaged.
During the 2008--09 influenza season, among children aged 6--23 months, average site coverage for the eight sites with ≥1 vaccine doses was 47.8% (range: 34.3%--60.1%); average full vaccination site coverage was 28.9% (range: 19.8%--39.7%) (Table). Among children aged 2--4 years, average site coverage with ≥1 vaccine doses was 27.8% (range: 17.3%--38.1%); average full vaccination site coverage was 21.8% (range: 12.6%--32.3%). Among children aged 5--10 years, average site coverage with ≥1 vaccine doses was 16.3% (range: 9.4%--23.7%); average full vaccination site coverage was 12.0% (range: 6.2%--19.7%). For children aged 11--12 years and 13--18 years, the average site coverage for ≥1 vaccine dose (making them fully vaccinated) was 12.7% (range: 6.6%--18.0%) and 9.1% (range: 4.8%--14.5%), respectively.
All eight sentinel sites reported vaccination coverage for children aged 6 months--4 years for both the 2007--08 and 2008--09 influenza seasons. Average site coverage for ≥1 influenza vaccine doses among children aged 6--23 months increased 17.2%, from 40.8% during the 2007--08 influenza season to 47.8% during the 2008--09 season, and increased 25.2%, from 22.2% to 27.8% for children aged 2--4 years (4) (Figure 1). Average full vaccination site coverage among children aged 6--23 months increased 30.8%, from 22.1% during the 2007--08 influenza season to 28.9% during the 2008--09 season and 32.1%, from 16.5% to 21.8% for children aged 2--4 years. Increases in coverage with ≥1 influenza vaccine doses and full vaccination coverage were observed for children aged 6 months--4 years at each sentinel site.
The weekly pattern of influenza vaccination was similar for all age groups except for children aged 6--23 months. To highlight this difference, data for children aged 2--18 years were consolidated for comparison with children aged 6--23 months. The average weekly percentages of influenza vaccinations increased steadily during September 21--October 25, 2008, and then began to decline among children aged 2--18 years (Figure 2). The percentage of vaccinations of children aged 6--23 months remained steady until November 22, 2008, when they began to decline. However, a greater percentage of vaccinations of children aged 6--23 months occurred during December--March than among children aged 2--18 years. The declines in both age groups began months before influenza activity peaked in the United States in February 2009 (6).
Reported by: R Potter, DVM, Michigan Dept of Community Health. LJ Pabst, MPH, Immunization Svcs Div; AE Fiore, MD, Influenza Div, National Center for Immunization and Respiratory Diseases, CDC.
Editorial Note:
These data describe vaccination coverage among children aged 6 months--18 years during the 2008--09 influenza season, including all children aged 5--18 years who were not included in ACIP recommendations for influenza vaccination until guidance published in August 2008 (1). Vaccination coverage increased from the 2007--08 season to the 2008--09 season among children aged 6 months--4 years at all eight sentinel sites. Increases have been observed previously at some, but not all, sentinel sites and have not been consistent from season to season (7). Monitoring influenza vaccination coverage among children aged 6 months--4 years remains important because younger children are at increased risk for influenza-related hospitalizations (1).
During the 2008--09 influenza season, a greater proportion of children aged 6--23 months were vaccinated late in the season compared with children in all other age groups. Although reasons for this are not clear, children aged 6--23 months likely have more visits to health-care providers, resulting in more opportunities for influenza vaccination; the later vaccinations also might reflect children in this age group returning for a second influenza vaccine dose.
The 2008--09 influenza season was the first for which influenza vaccination coverage at IIS sentinel sites was assessed among children aged 5--18 years, a group newly recommended for vaccination by ACIP. Coverage was low at all sites in this group, suggesting that vaccine providers had not incorporated annual influenza vaccination into routine preventive measures for healthy children aged 5--18 years. IIS sites might underascertain influenza vaccination of older children and adolescents because vaccinations administered at pharmacies, urgent-care clinics, school vaccination clinics, and other sites might be less likely reported to IIS than those administered at health-care provider offices. School vaccination campaigns have been used in past influenza seasons to increase the number of children receiving vaccine and reduce influenza-related illness (8,9). Immunization programs should work with vaccination providers in traditional and complementary settings to ensure that all administered doses are entered into the IIS.
The findings in this report are subject to at least two limitations. First, although IIS sentinel sites have >85% vaccination provider site participation, not all provider sites in all sentinel sites are enrolled in IIS. The lack of information on vaccinations administered by nonenrolled providers might have resulted in underestimates of vaccination coverage. However, during the 2007--08 influenza season, IIS-based coverage was consistent with coverage calculated by the National Immunization Survey for children aged 6--23 months (4,10), suggesting that IIS data are complete at least for children in that age group. Second, these results might not be generalizable to the entire U.S. population and should be viewed as representative of their specific geographic areas only.
Development of a second vaccine recommended for the 2009--10 influenza season, the influenza A (H1N1) 2009 monovalent vaccine, poses a challenge for vaccination providers, particularly with regard to younger children, who might require 2 doses of seasonal influenza vaccine and 2 doses of influenza A (H1N1) 2009 monovalent vaccine to be fully protected. School vaccination clinics and other vaccination sites outside of health-care provider offices might become increasingly important to maximizing opportunities for older children and adolescents to receive influenza vaccine. State and local immunization programs should identify opportunities in traditional and other settings to administer influenza vaccinations to children and adolescents and should work with vaccination providers to ensure that the doses administered are reported to their IIS. Monitoring IIS data will continue to be an important means of providing rapid assessment of progress toward increasing influenza vaccination coverage for seasonal influenza and 2009 pandemic influenza A (H1N1).
Acknowledgments
The findings in this report are based, in part, on contributions provided by staff members at the eight IIS sentinel sites.
References
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR 2008;57(No. RR-7).
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2004;53(No. RR-6).
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;55(No. RR-10).
- CDC. Influenza vaccination coverage among children aged 6--59 months---eight immunization information system sentinel sites, United States, 2007--08 influenza season. MMWR 2008;57:1043--6.
- CDC. Use of influenza A (H1N1) 2009 monovalent vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-10).
- CDC. Update: Influenza activity---United States, September 28, 2008--April 4, 2009, and composition of the 2009--10 influenza vaccine. MMWR 2009;58:369--74.
- CDC. Influenza vaccination coverage among children aged 6--59 months---six immunization information system sentinel sites, United States, 2006--07 influenza season. MMWR 2007;56:963--5.
- Poehling KA, Talbot HK, Williams JV, et al. Impact of a school-based influenza immunization program on disease burden: comparison of two Tennessee counties. Vaccine 2009;27:2695--700.
- King JC Jr, Cummings GE, Stoddard J, et al. A pilot study of the effectiveness of a school-based influenza vaccination program. Pediatrics;116:e868--73.
- CDC. Influenza vaccination coverage among children aged 6--23 months---United States, 2007--08 influenza season. MMWR 2009;58:1063--6.
* An IIS is a confidential, population-based, computerized data system designed primarily to consolidate vaccination records for all children within a geographic area from multiple vaccination providers. Data are collected from health-care providers, vital records, and billing systems. Additional information regarding IIS sentinel sites is available at http://www.cdc.gov/vaccines/programs/iis/activities/sentinel-sites.htm.
FIGURE 1. Average percentage* of children aged 6--23 months and 2--4 years† who received influenza vaccination, by vaccination status --- Immunization Information System Sentinel Sites, 2007--08 and 2008--09 influenza seasons
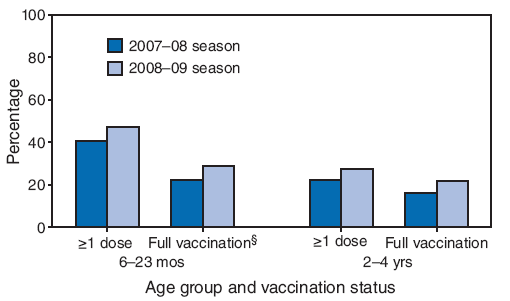
* Unweighted average percentage of children in the two age groups who received vaccination at the eight sentinel sites.
† 2007--08 season: 6--23 months (n = 302,333), 2--4 years (n = 808,711); 2008--09 season: 6--23 months (n = 263,597), 2--4 years (n = 767,422).
§ Full vaccination for children aged 6 months--23 months and 2--4 years was defined as 1) receipt of 2 vaccine doses separated by at least 4 weeks in the current season among vaccine naïve children and children who received 1 dose for the first time during August 1, 2007--March 31, 2008, or 2) receipt of 1 vaccine dose in the current season among all other children.
Alternative Text: The figure above shows the average percentage of children aged 6-23 months and 2-4 years who received influenza vaccination, by vaccination status, according to Immunization Information System sentinel sites for the 2007-08 and 2008-09 influenza seasons. According to the figure, the average coverage for the eight sites with >1 influenza vaccine doses among children aged 6-23 months increased 17.2%, from 40.8% during the 2007-08 influenza season to 47.8% during the 2008-09 season, and increased 25.2%, from 22.2% to 27.8%, for children aged 2-4 years.
FIGURE 2. Average percentage* of all influenza doses administered to children aged 6 months--18 years, by week of administration --- Immunization Information System sentinel sites, 2008--09 influenza season
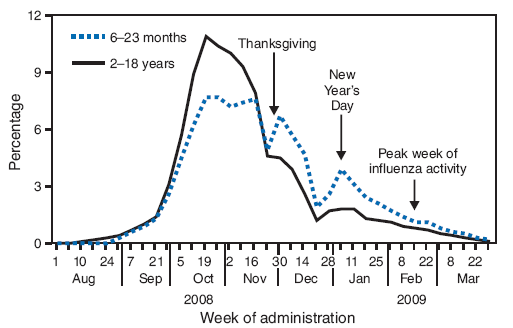
* Unweighted average percentage of doses administered at the eight sentinel sites.
Alternative Text: The figure above shows the average percentage of all influenza doses administered to children aged 6 months-18 years, by week of administration, from the Immunization Information System sentinel sites for the 2008-09 influenza season. According to the figure, the weekly pattern of influenza vaccination was similar for all age groups except for children aged 6-23 months. To highlight this difference, data for children aged 2-18 years were consolidated for comparison with children aged 6-23 months. The average weekly percentages of influenza vaccinations increased steadily during September 21-October 25, 2008, and then began to decline among children aged 2-18 years.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 10/1/2009


