 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Summary of Notifiable Diseases --- United States, 2004
Prepared by
PrefaceThe Summary of Notifiable Diseases --- United States, 2004 contains the official statistics, in tabular and graphic form, for the reported occurrence of nationally notifiable infectious diseases in the United States for 2004. Unless otherwise noted, the data are final totals for 2004 reported as of December 2, 2005. These statistics are collected and compiled from reports sent by state health departments to the National Notifiable Diseases Surveillance System (NNDSS), which is operated by CDC in collaboration with the Council of State and Territorial Epidemiologists (CSTE). The Summary is available at http://www.cdc.gov/mmwr/summary.html. This site also includes publications from previous years. The Highlights section presents noteworthy epidemiologic and prevention information for 2004 for selected diseases and additional information to aid in the interpretation of surveillance and disease-trend data. Part 1 contains tables showing incidence data for the nationally notifiable diseases during 2004.* The tables provide the number of cases reported to CDC for 2004 nationwide as well as the distribution of cases by geographic location and the patient's demographic characteristics (age, sex, race, and ethnicity). Part 2 contains graphs and maps that depict summary data for certain notifiable diseases described in tabular form in Part 1. The Selected Reading section presents general and disease-specific references for notifiable infectious diseases. These references provide additional information on surveillance and epidemiologic concerns, diagnostic concerns, and disease-control activities. Comments and suggestions from readers are welcome. To increase the usefulness of future editions, comments about the current report and descriptions about how information is or could be used are invited. Comments should be sent to Public Health Surveillance Team --- NNDSS, National Center for Public Health Informatics at soib@cdc.gov. * Because no cases of anthrax; diphtheria; influenza-associated pediatric mortality; paralytic poliomyelitis; rubella, congenital syndrome; severe acute respiratory syndrome--associated coronavirus (SARS-CoV) disease; smallpox; vancomycin-intermediate Staphylococcus aureus; western equine encephalitis; or yellow fever were reported in the United States during 2004, these diseases do not appear in the tables in Part 1. For certain other nationally notifiable diseases, incidence data were reported to CDC but are not included in the tables or graphs of this Summary. Data on chronic hepatitis B and hepatitis C virus infection (past or present) are undergoing data quality review. Data on ehrlichiosis attributable to other or unspecified agents are being withheld from publication pending the outcome of discussions about the reclassification of certain Ehrlichia species, which will probably affect how data in this category are reported. Data on human immunodeficiency virus (HIV) infections are not included because HIV infection (not acquired immunodeficiency syndrome [AIDS]) reporting has been implemented on different dates and by using different methods than for AIDS case reporting; however, these data are summarized in the Highlights section. BackgroundThe infectious diseases designated as notifiable at the national level during 2004 are listed on page 3. A notifiable disease is one for which regular, frequent, and timely information regarding individual cases is considered necessary for the prevention and control of the disease. A brief history of the reporting of nationally notifiable infectious diseases in the United States is available at http://www.cdc.gov/epo/dphsi/nndsshis.htm. In 1961, CDC assumed responsibility for the collection and publication of data on nationally notifiable diseases. NNDSS is neither a single surveillance system nor a method of reporting. Certain NNDSS data are reported to CDC through separate surveillance information systems and through different reporting mechanisms; however, these data are aggregated and compiled for publication purposes. The list of nationally notifiable diseases is revised periodically. A disease might be added to the list as a new pathogen emerges, or a disease might be deleted as its incidence declines. Public health officials at state health departments and CDC collaborate in determining which diseases should be nationally notifiable. CSTE, with input from CDC, makes recommendations annually for additions and deletions. Although disease reporting is mandated by legislation or regulation at the state and local levels, state reporting to CDC is voluntary. Thus, the list of diseases considered notifiable varies slightly by state. Current and historic national public health surveillance case definitions used for classifying and enumerating cases consistently across reporting jurisdictions are available at http://www.cdc.gov/epo/dphsi/nndsshis.htm. All states report conditions that were designated as internationally quarantinable and notifiable (i.e., cholera, plague, and yellow fever) in compliance with the International Health Regulations (IHR) issued by the World Health Organization (WHO). In May 2005, the World Health Assembly adopted revised IHR. The current IHR will be replaced by the 2005 IHR when it becomes official on June 15, 2007, unless an earlier implementation date is adopted. The 2005 IHR revision stipulates that smallpox, poliomyelitis caused by wild-type poliovirus, human influenza caused by a new subtype, and SARS-CoV are directly reportable to WHO. In addition, the 2005 IHR includes an open-ended algorithm to determine which other conditions or events require mandatory reporting to WHO because they might constitute a public health emergency of international concern. Conditions that use the algorithm to determine notifiability include, but are not limited to, cholera, pneumonic plague, yellow fever, and West Nile fever (1). 1. World Health Organization. Third report of Committee A. Annex 2. Available at http://www.who.int/gb/ebwha/pdf_files/WHA58/A58_55-en.pdf.
Infectious Diseases Designated as Notifiable at the National Level During 2004
|
| Acquired immunodeficiency syndrome (AIDS) | Listeriosis |
| Anthrax | Lyme disease |
| Botulism | Malaria |
| Brucellosis | Measles |
| Chancroid | Meningococcal disease |
| Chlamydia trachomatis, genital infection | Mumps |
| Cholera | Pertussis |
| Coccidioidomycosis | Plague |
| Cryptosporidiosis | Poliomyelitis, paralytic |
| Cyclosporiasis | Psittacosis |
| Diphtheria | Q fever |
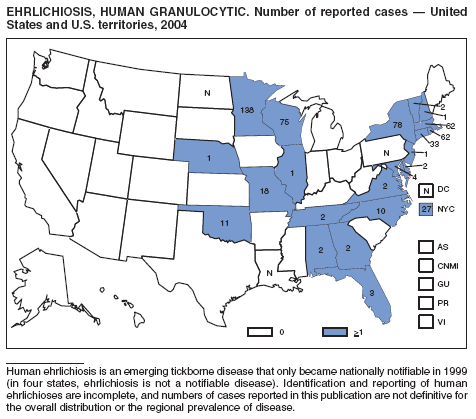
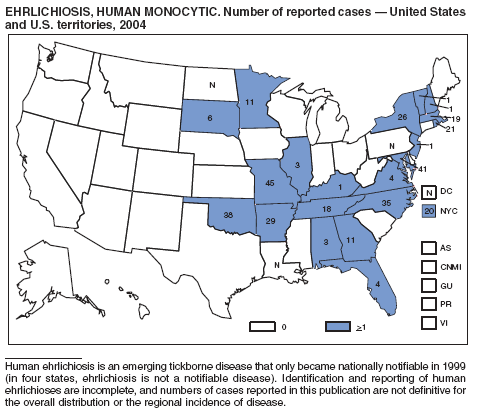
| Ehrlichiosis | Rabies |
Human granulocytic |
Animal |
Human monocytic |
Human |
Human, other or unspecified agent |
Rocky Mountain spotted fever |
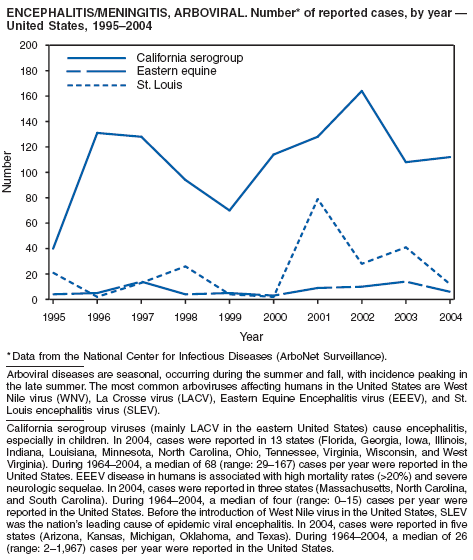
| Encephalitis/meningitis, arboviral | Rubella |
California serogroup |
Rubella, congenital syndrome |
Eastern equine |
Salmonellosis |
Powassan |
Severe acute respiratory syndrome--associated coronavirus (SARS-CoV) disease |
St. Louis |
Shigellosis |
Western equine |
Smallpox* |
West Nile |
Streptococcal disease, invasive, group A |
| Enterohemorrhagic Escherichia coli (EHEC) | Streptococcal toxic-shock syndrome |
|
Streptococcus pneumoniae, invasive disease |
|
|
|
|
| Giardiasis | Syphilis |
| Gonorrhea | Syphilis, congenital |
| Haemophilus influenzae, invasive disease | Tetanus |
| Hansen disease (leprosy) | Toxic-shock syndrome (other than streptococcal) |
| Hantavirus pulmonary syndrome | Trichinellosis† |
| Hemolytic uremic syndrome, postdiarrheal | Tuberculosis |
| Hepatitis A, viral, acute | Tularemia |
| Hepatitis B, viral, acute | Typhoid fever Vancomycin-intermediate Staphylococcus aureus |
| Hepatitis B, viral, chronic | infection (VISA)§ |
| Hepatitis B, perinatal infection | Vancomycin-resistant Staphylococcus aureus infection (VRSA)§ |
| Hepatitis C, acute | Varicella |
| Hepatitis C, virus infection (past or present) | Varicella deaths |
| Human immunodeficiency virus (HIV) infection | Yellow fever |
|
|
|
|
| Influenza-associated pediatric mortality* | |
| Legionellosis | |
* New for 2004, as of October 4, 2004.
† Formerly referred to as trichinosis.
§ New for 2004, as of January 1, 2004.
Provisional data concerning the reported occurrence of nationally notifiable infectious diseases are published weekly in the MMWR. After each reporting year, staff in state health departments finalize reports of cases for that year with local or county health departments and reconcile the data with reports previously sent to CDC throughout the year. These data are compiled in final form in the Summary.
Notifiable disease reports are the authoritative and archival counts of cases. They are approved by the appropriate chief epidemiologist from each submitting state or territory before being published in the Summary. Data published in MMWR Surveillance Summaries or other surveillance reports produced by CDC programs might not agree exactly with data reported in the annual Summary because of differences in the timing of reports, the source of the data, or surveillance methodology.
Data in the Summary were derived primarily from reports transmitted to CDC's National Center for Public Health Informatics from health departments in the 50 states, five territories, New York City, and the District of Columbia. More information regarding infectious notifiable diseases, including case definitions, is available at http://www.cdc.gov/epo/dphsi/phs.htm. Policies for reporting notifiable disease cases can vary by disease or reporting jurisdiction. The case-status categories used to determine which cases reported to NNDSS are published, by disease or condition, are listed in the "print criteria" column of the 2006 NNDSS event code list (available at http://www.cdc.gov/epo/dphsi/phs/files/NNDSSeventcodelistJanuary2006.pdf.
Final data for certain diseases are derived from the surveillance records of the following CDC programs. Requests for further information regarding these data should be directed to the appropriate program.
National Center for Health Statistics (NCHS)
Office of Vital and Health Statistics Systems (deaths from selected notifiable diseases).
National Center for Infectious Diseases (NCID)
Division of Bacterial and Mycotic Diseases (toxic-shock syndrome; streptococcal disease, invasive, group A;
and streptococcal toxic-shock syndrome).
Division of Vector-Borne Infectious Diseases (ArboNET
surveillance data regarding arboviral encephalitis/meningitis).
Division of Viral and Rickettsial Diseases (animal rabies, hantavirus pulmonary syndrome,
influenza-associated pediatric mortality, and SARS-CoV).
National Center for HIV, STD, and TB Prevention (NCHSTP) "
Division of HIV/AIDS Prevention --- Surveillance and Epidemiology (acquired immunodeficiency syndrome
[AIDS] and human immunodeficiency virus [HIV]
infection).
Division of STD Prevention (chancroid, chlamydia,
gonorrhea, and syphilis).
Division of TB Elimination (tuberculosis).
National Immunization Program (NIP)
Epidemiology and Surveillance Division (poliomyelitis and varicella deaths).
Population estimates for the states are derived from NCHS estimates of the July 1, 2000--July 1, 2003, U.S. resident population from the Vintage 2003 postcensal series by year, county, age, sex, race, and Hispanic origin, prepared under a collaborative arrangement with the U.S. Census Bureau (available at http://www.cdc.gov/nchs/about/major/dvs/popbridge/popbridge.htm). Population numbers for territories are 2003 estimates from the U.S. Census Bureau International Data Base Data Access--Display Mode (available at http://www.census.gov/main/ipc/www/idbprint.html). The choice of population denominators for incidence reported in the MMWR is based on 1) the availability of census population data at the time of preparation for publication and 2) the desire for consistent use of the same population data to compute incidence reported by different CDC programs. Incidence in the Summary is calculated as the number of reported cases for each disease or condition divided by either the U.S. resident population for the specified demographic population or the total U.S. residential population, multiplied by 100,000. When a nationally notifiable disease is associated with a specific age restriction, the same age restriction is applied to the population in the denominator of the incidence calculation. In addition, population data from states in which the disease or condition was not notifiable or was not available were excluded from incidence calculations. Unless otherwise stated, disease totals for the United States do not include data for American Samoa, Guam, Puerto Rico, the U.S. Virgin Islands, or the Commonwealth of the Northern Mariana Islands.
Incidence data in the Summary are presented by the date of report to CDC as determined by the MMWR week and year assigned by the state or territorial health department. Data are reported by the state in which the patient resided at the time of diagnosis. For certain nationally notifiable infectious diseases, surveillance data are reported independently to different CDC programs. Thus, surveillance data reported by other CDC programs might vary from data reported in the Summary because of differences in 1) the date used to aggregate data (e.g., date of report or date of disease occurrence), 2) the timing of reports, 3) the source of the data, 4) surveillance case definitions, and 5) policies regarding case jurisdiction (i.e., which state should report the case to CDC).
The data reported in the Summary are useful for analyzing disease trends and determining relative disease burdens. However, these data must be interpreted in light of reporting practices. Disease reporting is likely incomplete, and completeness might vary depending on the disease. The degree of completeness of data reporting might be influenced by the diagnostic facilities available; control measures in effect; public awareness of a specific disease; and the interests, resources, and priorities of state and local officials responsible for disease control and public health surveillance. Finally, factors such as changes in methods for public health surveillance, introduction of new diagnostic tests, or discovery of new disease entities can cause changes in disease reporting that are independent of the true incidence of disease.
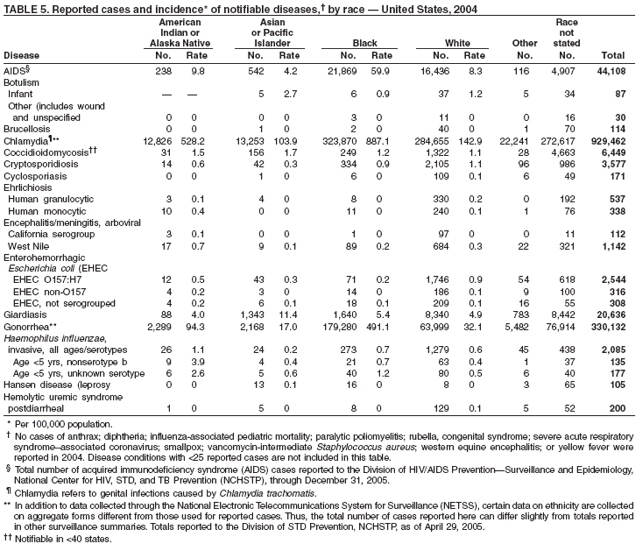
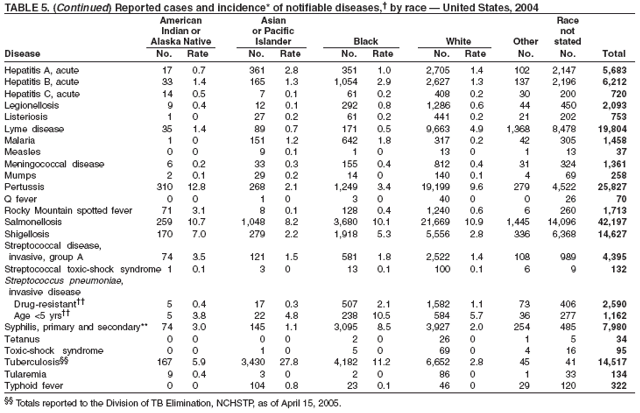
Public health surveillance data are published for selected racial and ethnic populations because these variables can be risk markers for certain notifiable diseases. Race and ethnicity data also can be used to highlight populations for focused prevention efforts. However, caution must be used when drawing conclusions from reported race and ethnicity data. Different racial/ethnic populations might have different patterns of access to health care, potentially resulting in data that are not representative of actual disease incidence among specific racial/ethnic populations. Surveillance data reported to NNDSS are in either individual case-specific form or summary form (i.e., aggregated data for a group of cases). Summary data often lack demographic information (e.g., race); therefore, the demographic-specific rates presented in the Summary might be underestimated.
In addition, not all race and ethnicity data are collected uniformly for all diseases. For example, certain disease programs collect data on race and ethnicity by using one or two variables, based on the 1977 standards for collecting such data issued by the Office of Management and the Budget (OMB). However, beginning in 2003, certain CDC programs, including the tuberculosis program, implemented OMB's 1997 revised standards for collecting such data; these programs collect data on multiple races per person by using multiple race variables. Additionally, although the recommended standard for classifying a person's race or ethnicity is based on self-reporting, this procedure might not always be followed.
Before 1990, data were reported to CDC as cumulative counts rather than individual case reports. In 1990, states began electronically capturing and reporting individual case reports (without personal identifiers) to CDC by using the National Electronic Telecommunication System for Surveillance (NETSS). In 2001, CDC launched the National Electronic Disease Surveillance System (NEDSS), now a component of the Public Health Information Network, to promote the use of data and information system standards that advance the development of efficient, integrated, and interoperable surveillance information systems at the local, state, and federal levels. CDC has developed the NEDSS Base System (NBS), a public health surveillance information system that can be used by states that do not wish to develop their own NEDSS-based systems. The objective of NEDSS is to improve the accuracy, completeness, and timeliness of disease reporting at the local, state, and national level. In 2003, CDC encouraged states using NBS to move from NETSS to NEDSS reporting standards. In 2004, six states used NEDSS to transmit nationally notifiable infectious diseases to CDC; as of March 2005, a total of 10 states used NEDSS to transmit these data to CDC. More information concerning NEDSS is available at http://www.cdc.gov/NEDSS.
A major feature of NBS is the ability to capture data already in electronic form (e.g., electronic laboratory results, which are needed for case confirmation) rather than enter these data manually as in NETSS.
Below are summary highlights for certain national notifiable diseases. Highlights are intended to assist in the interpretation of major occurrences that affect disease incidence or surveillance trends (e.g., outbreaks, vaccine licensure, or policy changes).
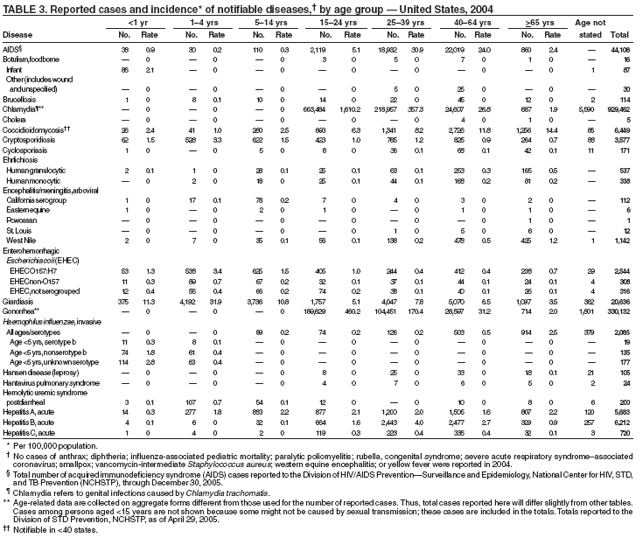
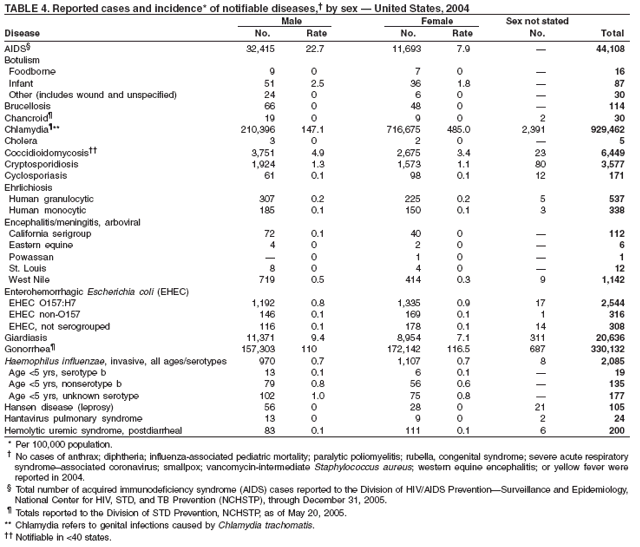
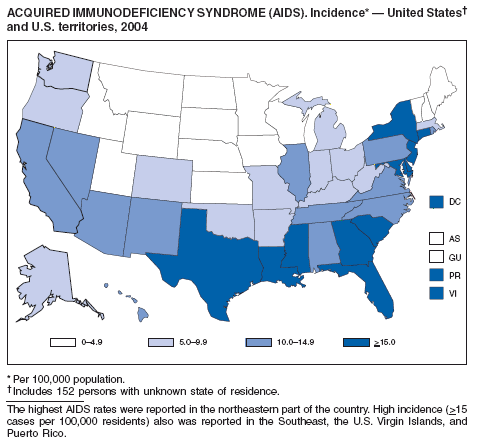
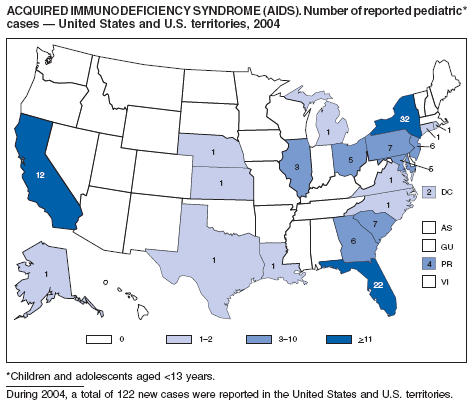
Since 1981, confidential name-based AIDS surveillance has been the cornerstone of national, state, and local efforts to monitor the scope and impact of the HIV epidemic. The data have multiple uses, including developing policy to help prevent and control AIDS. However, because of the introduction of therapies that effectively slow the progression of the infection, AIDS data no longer adequately represent the populations affected by the epidemic. By providing a window into the epidemic at an earlier stage, HIV data, combined with AIDS data, better represent the overall impact. As of the end of 2004, a total of 43 areas (38 states, Puerto Rico, and four U.S. territories) had implemented confidential name-based HIV reporting. These 43 areas have integrated name-based HIV surveillance into their AIDS surveillance systems, whereas other jurisdictions have used other methods for reporting cases of HIV infection. Under no configuration are names or other personal identifying information collected at the national level.
During 1998--1999, declines in AIDS rates began to level. This trend followed a period of sharp declines in reported cases after 1996, when highly effective antiretroviral therapies were introduced. At the end of 2004, an estimated 415,193 persons were living with AIDS. After a substantial decrease in the number of deaths among persons with AIDS during the late 1990s, the rate of decrease flattened through 2004. The number of deaths among persons with AIDS decreased 66% during 1995--2000. During 2001--2003, the number of deaths reported remained stable; however, in 2004, the number of deaths decreased 10%, compared with the number reported in 2001.
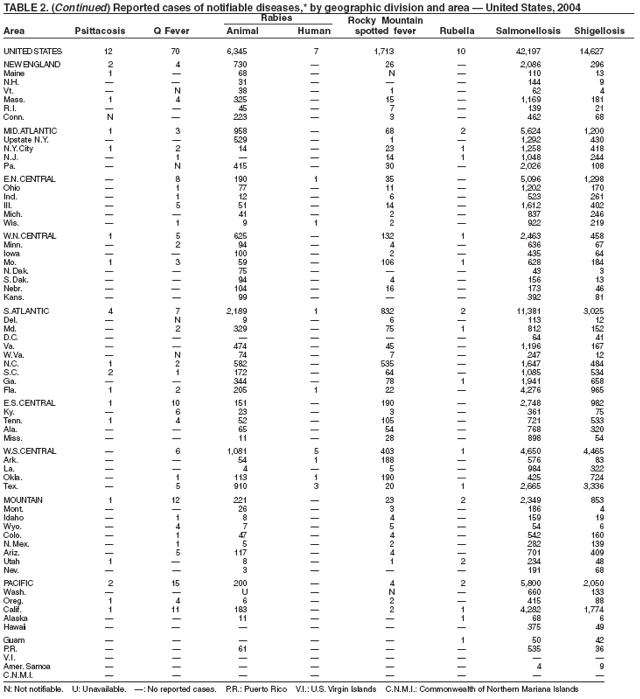
No cases of anthrax were reported in the United States in 2004. One incident was reported in which laboratory workers were inadvertently exposed to viable spores during the conduct of research on inactivated vegetative organisms. Exposed laboratorians were given postexposure antibiotic prophylaxis. No adverse health effects were reported among the laboratorians (1). This incident highlights the importance of appropriate biosafety procedures and adequate sterility testing when working with inactivated Bacillus anthracis or other select agents. Naturally occurring anthrax epizootics are commonly reported in the United States; two occurred in 2004, affecting livestock in South Dakota and livestock and game animals in Texas. No human cases resulted.
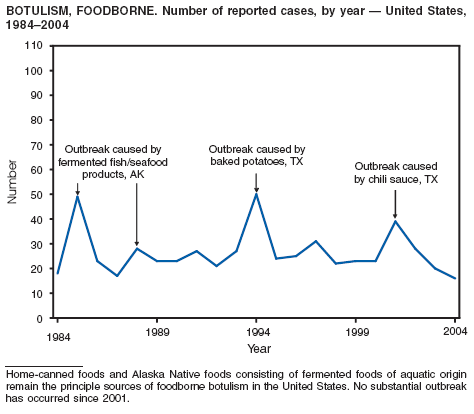
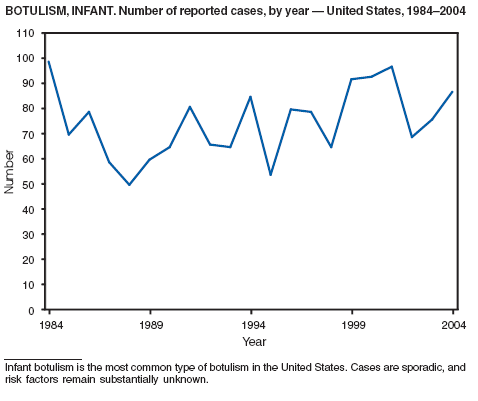
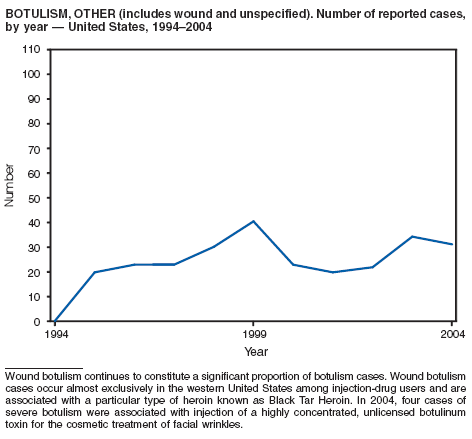
Botulism is a severe paralytic illness caused by the toxins of Clostridium botulinum. Exposure to toxin can occur by ingestion (foodborne botulism), in situ production from C. botulinum colonization of a wound (wound botulism) or the gastrointestinal tract (infant botulism), or by injection of pharmacologic preparations of toxin (treatment-associated botulism) (1). In 2004, cases were attributed to foodborne botulism, wound botulism, infant botulism, and treatment-associated botulism resulting from injection of an unlicensed concentrated preparation of toxin (2).
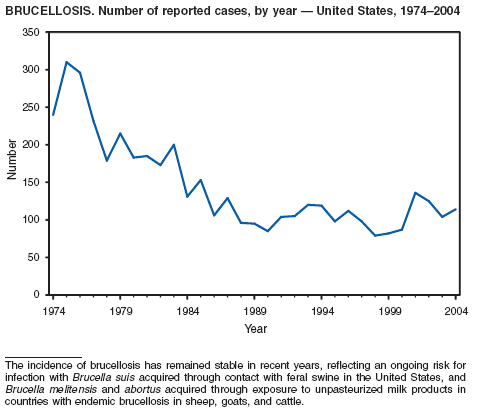
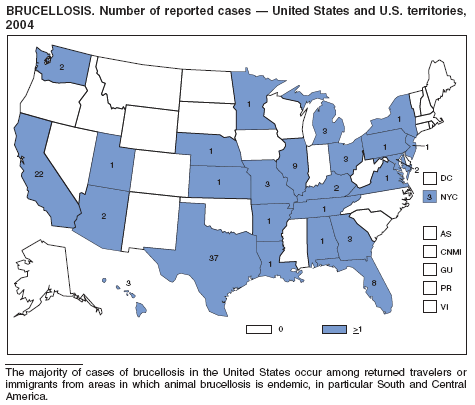
In 2004, five cattle herds in three states were reported by the U.S. Department of Agriculture to be affected by brucellosis. No human cases of brucellosis were associated with the affected herds. One affected state was able to maintain its brucellosis-free designation, and 48 states remain designated free of cattle brucellosis by the U. S. Department of Agriculture (1). Brucella abortus remains enzootic in elk and bison in the greater Yellowstone National Park area, and Brucella suis is enzootic in feral swine in the southeast. Hunters exposed to these animals might be at increased risk for infection. The majority of human cases in the United States occur among returned travelers or immigrants from countries with endemic brucellosis and are associated with consumption of unpasteurized milk or soft cheeses. Pathogenic Brucella species are considered category B biologic threat agents because of a high potential for aerosol transmission. For the same reason, biosafety level 3 practices, containment and equipment are recommended for laboratory manipulations of isolates.
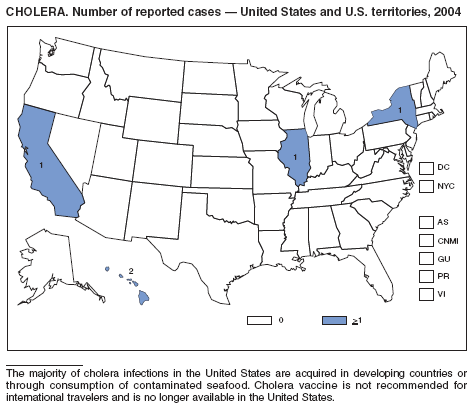
In 2004, five laboratory-confirmed cases of cholera, all caused by toxigenic Vibrio cholerae O1, were reported to CDC. Four (80%) infections were acquired outside the United States, and one (20%) infection, believed to have been acquired through imported seafood, occurred in Hawaii. No patients died. Although the annual average incidence of cholera during 1995--2000 was 10.2 cases per 100,000 population, during 2001--2004, average annual incidence was three cases per 100,000 population (1). This general decrease might reflect a trend towards fewer cases of cholera in Latin America and worldwide, as reported by the World Health Organization (2).
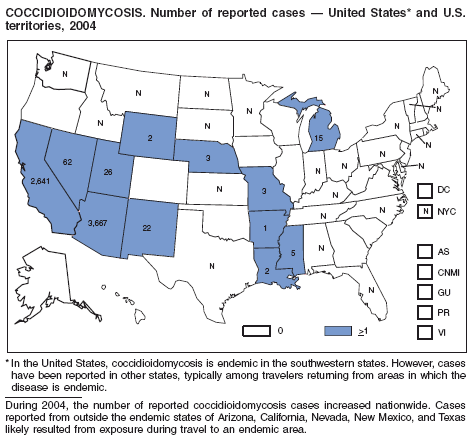
During 2003--2004, the number of reported cases of coccidioidomycosis increased 32% (1). The majority of these cases occurred in California and Arizona. Increases are probably attributable to recent changes in land use, demographics, and climate in endemic areas, although certain cases might be attributable to increased physician awareness and testing. New efforts are needed to determine the amount of undertesting by health-care providers so as to accurately define the burden of disease.
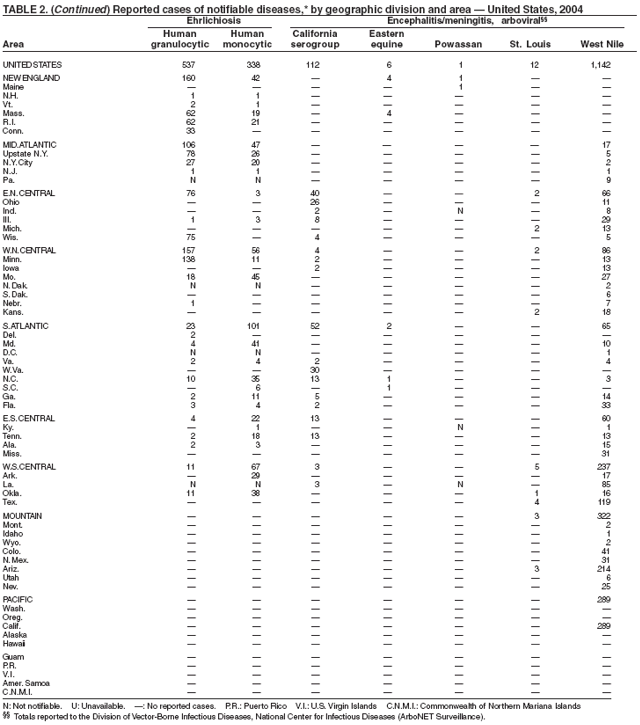
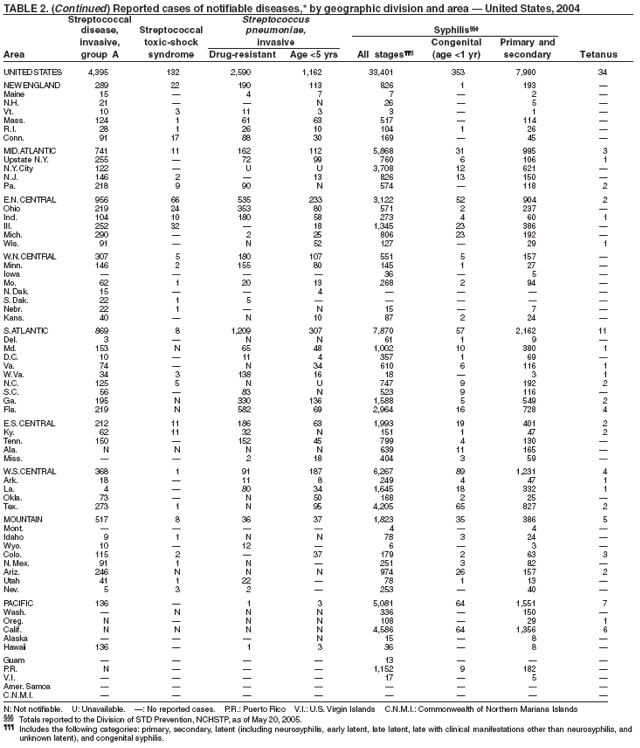
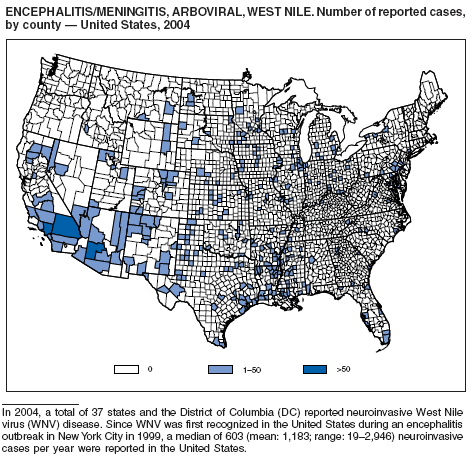
During 2004, for the sixth consecutive year, epidemic and epizootic West Nile virus (WNV) activity occurred in the United States (1), particularly in California, Arizona, and Colorado. A high incidence of neuroinvasive WNV disease continued to occur in the Midwest and Great Plains states. Other states experienced perennial reemergence in areas of previous activity, and continued geographic expansion into counties of Western states.
In 2004, a total of 40 states and the District of Columbia reported cases of WNV disease among humans. Of these cases, 45% were West Nile neuroinvasive disease (WNND), 54% were uncomplicated fever, and 5% were clinically unspecified. Of the WNND cases, 8% were fatal. Four states (Arizona, California, Colorado, and Texas) accounted for 64% of all reported human cases. Illness onset dates were April 23--December 30; December 30 is the latest date on which onset of human WNV disease was reported in the United States. The epidemic peak occurred during the week ending August 16. A total of 224 presumptively WNV-viremic blood donors were identified through nationwide blood screening, 125 (56%) of whom were from Arizona, California, and Colorado.
In addition, 47 states reported WNV-infected dead birds; and 38 states reported WNV-infected horses and other WNV-infected animals. Culex mosquitoes accounted for 94% of reported WNV-positive pools. Cx. tarsalis was the most commonly reported WNV-infected mosquito species and was considered a major epizootic and epidemic vector in states west of the Mississippi river.
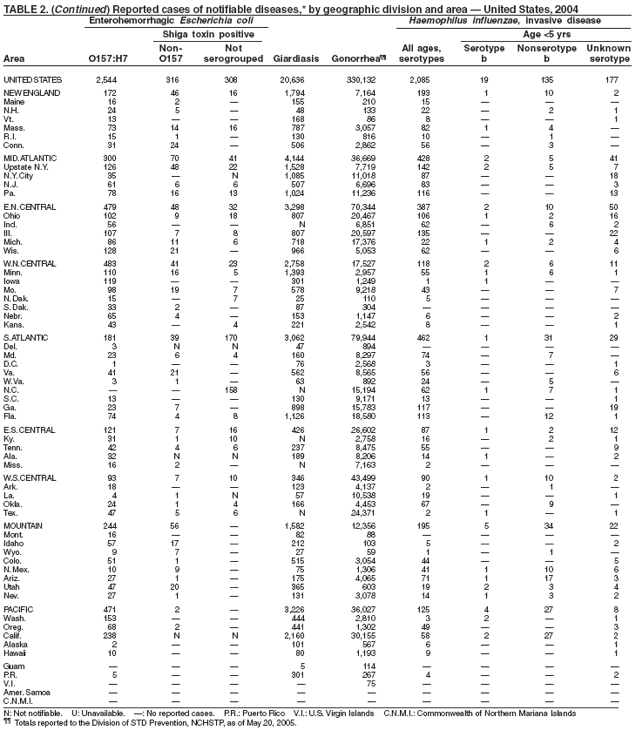
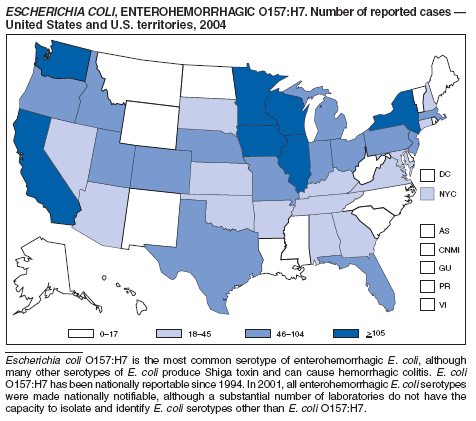
Escherichia coli O157:H7 has been nationally notifiable since 1994 (1). In 2000, the Council for State and Territorial Epidemiologists passed a resolution in which all Shiga-toxin producing E. coli were made nationally notifiable under the name enterohemorrhagic E. coli (EHEC); national surveillance for EHEC began in 2001. Surveillance categories for EHEC include 1) EHEC O157:H7; 2) EHEC, serogroup non-O157; and 3) EHEC, not serogrouped. During 1994--1999, reported infections with the most well-known pathogen in this group, E. coli O157:H7, increased annually, to a peak of 4,744 cases in 1999. This increase was attributable in part to the increasing ability of laboratories to identify this pathogen. During 2003--2004, incidence reported by active surveillance in FoodNet decreased substantially and progressively compared with 1996--1998; incidence of E. coli O157 infections reported by FoodNet sites in 2004 declined below the 2010 national target of one case per 100,000 persons (2).
During 2004, cases of enterohemorrhagic E. coli were reported from 50 states, the District of Columbia, and Puerto Rico. Of these, 80% were classified as EHEC O157:H7; 10% as EHEC, serogroup non-O157; and 10% as EHEC, not serogrouped.
Diagnosis solely on the basis of detection of Shiga toxin does not protect the public's health. Characterizing E. coli isolates by serotype and pulsed-field gel electrophoresis (PFGE) patterns is critical to detect, investigate, and halt outbreaks. Therefore, broth culture media or specimens in which Shiga toxin is detected should be cultured for E. coli or submitted to state public health laboratories for E. coli isolation, and isolates should be confirmed and characterized at state public health laboratories.
Healthy cattle, which harbor the organism as part of the bowel flora, are the main animal reservoir for E. coli O157:H7 and other Shiga-toxin producing E. coli. The majority of reported outbreaks are caused by contaminated food or water. The substantial decline in cases during 2003--2004 coincided with industry and regulatory control activities and with a decrease in the contamination of ground beef (3). Direct transmission from animals and their environments to humans in settings such as petting zoos and through the pet trade remains a growing public health concern (4), and prevention recommendations have been developed and disseminated (5).
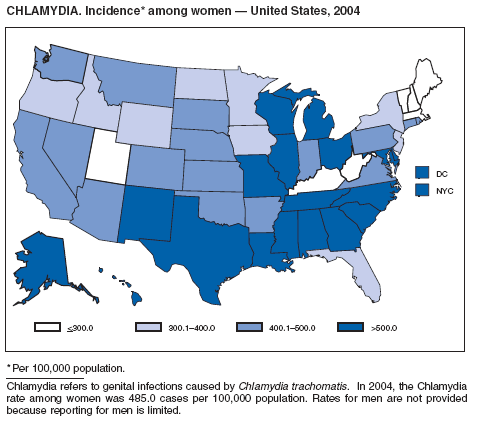
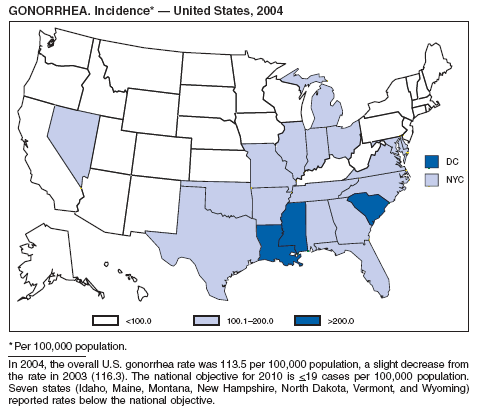
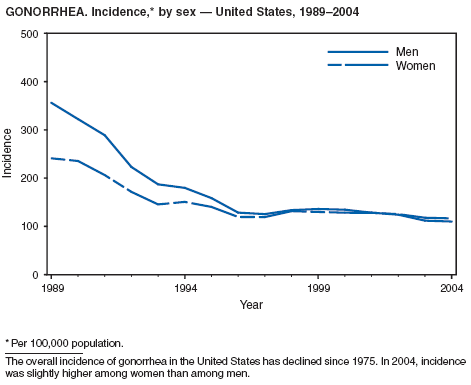
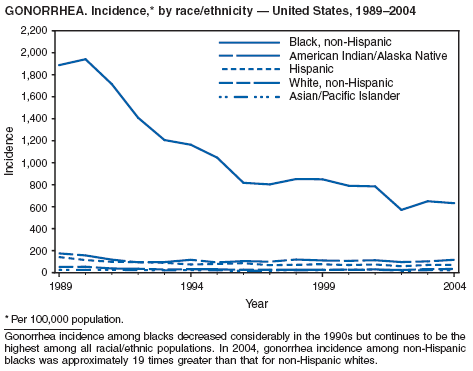
In 2004, the gonorrhea rate (113.5 cases per 100,000 population) was the lowest ever reported in the United States (1). Although the gonorrhea rate among women (116.5) remained slightly higher than that among men (110.0) for the third straight year, rates for both men and women have been decreasing since 2000. Decreases have been recorded predominantly among black men and women; rates among non-Hispanic white men and women and among all Hispanics have increased slightly since 2000. However, the rate per 100,000 population for blacks remains 19 times higher than that for whites, with the highest rate being among persons aged 15--24 years (2,079.8) and persons aged 20--24 years (2,487.2).
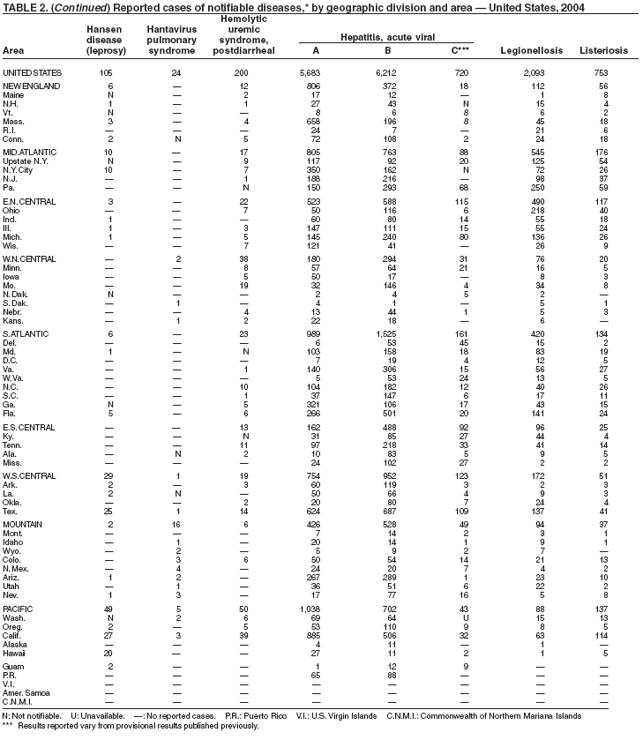
The number of reported cases of Hansen disease (HD) in the United States peaked at 361 in 1985 and has declined since 1988. HD outpatient clinics operated under the guidance and direction of the U.S. Department of Health and Human Services, Health Resources and Services Administration exist in Boston, Massachusetts; Chicago, Illinois; Maricopa County, Arizona; Los Angeles, Martinez, and San Diego, California; Miami, Florida; San Juan, Puerto Rico; Seattle, Washington; New York City, New York; and Dallas, Houston, San Antonio, and Harlingen, Texas. Services provided to HD patients include diagnosis, treatment, follow-up of patients and contacts, monitoring disability, disability prevention, education, maintenance of the referral system of HD health-care services, and maintenance of the HD registry and database. More information is available at http://bphc.hrsa.gov/nhdp/default.htm.
Hepatitis A vaccine is recommended for persons at increased risk for hepatitis A (e.g., international travelers, men who have sex with men [MSM], injection drug users [IDUs], and non-IDUs) and also for children in states and counties that historically have had consistently elevated rates of hepatitis A.
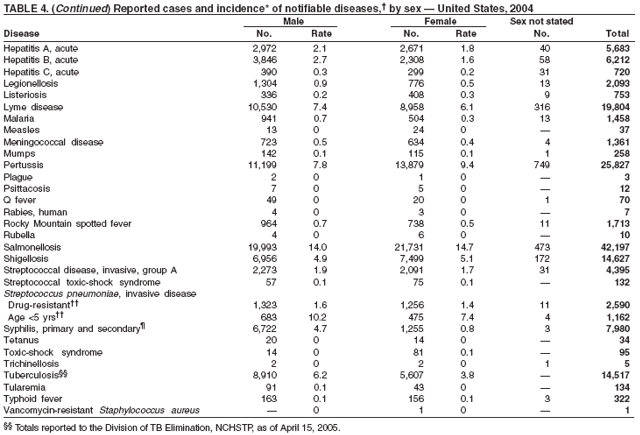
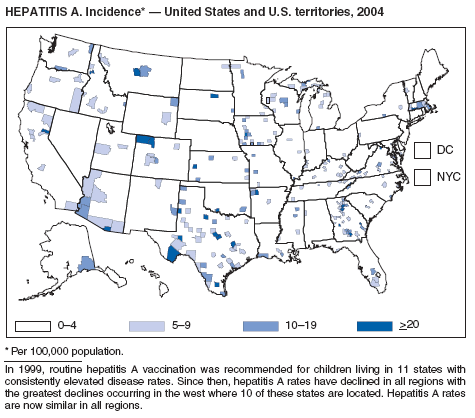
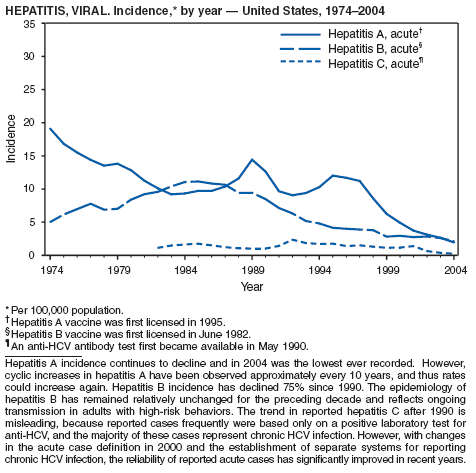
Since routine childhood vaccination was recommended in 1999 in states where hepatitis A rates were consistently elevated, the overall hepatitis A rate has declined dramatically. In 2004, the rate (1.9 per 100,000 population) was the lowest yet recorded, with 5,683 cases reported. Declines have been greater among age groups and regions where routine vaccination of children is recommended, likely reflecting the result of the current vaccination strategy. To maintain and further reduce the current low rates, the strategy was expanded in October 2005 to include routine vaccination nationwide of children aged 12--23 months (3).
Although rates among children have declined among all races and ethnicities, the decline among Hispanic children has been less than that among non-Hispanics. The highest rates among children are now among non-Hispanics in states not covered by recommendations for routine childhood hepatitis A vaccination.
The decline in rates among children, particularly those in vaccinating states, has resulted in a substantial shift in the epidemiologic profile of this disease in the United States. Rates in the western states, which historically have been higher than in other regions, are now similar to rates in the rest of the country, and rates among adults are higher than those among children. In addition, the pattern of reported risk factors has shifted, with an increasing proportion of cases occurring among adults in persons at high risk, including MSM and illegal drug users. In addition, as transmission of hepatitis A virus (HAV) has declined within the United States, the proportion of cases attributed to travel to countries in which hepatitis A is endemic has increased for all age groups and is now the most frequently reported risk factor among persons with hepatitis A virus aged <15 years.
During 1990--2004, the number of acute hepatitis B cases reported annually declined 68% (1). This steady decline has coincided with the implementation of a national strategy to achieve the elimination of hepatitis B. The primary elements of this strategy are 1) screening of all pregnant women for hepatitis B virus (HBV) infection with the provision of postexposure prophylaxis to infants born to infected women; 2) routine vaccination of all infants and children aged <19 years; and 3) vaccination of others at increased risk for hepatitis B (e.g., health-care workers, men who have sex with men [MSM], injection-drug users [IDUs], and household and sex contacts of persons with chronic HBV infection).
In 2004, the rate among children aged <13 years, the cohort born since routine infant vaccination was implemented, was 0.07 per 100,000 population, representing a 94% decline for that age group since 1990. By race and ethnicity, the highest rates among children continue to be recorded among Asian/Pacific Islanders (API), followed by blacks, American Indians/Alaska Natives, and whites. Since 1990, however, the disparity between the population with the highest (API) and the lowest (whites) incidence has been reduced >90%. Evaluation of verified cases reported during 2001--2002 indicated that eight (42%) of the 19 cases that were followed up occurred among children born outside the United States; six (75%) of these eight children were international adoptees (2). Since 1990, rates among adolescents aged 14--18 years have also declined approximately 94%, but the 2004 rate (0.4 per 100,000 population) remains substantially higher than the rate for children aged <13 years.
During 1990--1999, rates among adults declined 63% but have since remained approximately unchanged. Among adults, a high proportion of cases occur among persons in identified risk groups (i.e. IDUs, MSM, and persons with multiple sex partners) indicating a need to strengthen efforts to reach these populations with vaccine.
By December 2003, all 50 states and the District of Columbia had implemented HIV surveillance systems, including both name-based and nonname-based systems. Since 2000, a total of 35 areas (33 states, Guam, and the U.S. Virgin Islands) have had laws or regulations requiring name-based confidential reporting for adults/adolescents with confirmed HIV infection, in addition to reporting of persons with AIDS. In 2002, CDC initiated a system to monitor HIV incidence; in 2003, CDC expanded this system and also initiated a national HIV behavioral surveillance system. CDC will assess the implementation and effectiveness of prevention activities through multiple monitoring systems, including use of new performance indicators for state and local health departments and community-based organizations (1).
At the end of 2004, a total of 209,937 adults and adolescents in the 35 areas were living with HIV infection (not AIDS). Estimated prevalence of HIV infection (not AIDS) in this group was 136.7 per 100,000 population (2). In these areas, 2004 was the first year in which mature HIV surveillance data (i.e., available since at least 2000) could be used to allow for stabilization of data collection and for adjustment of the data in order to monitor trends. Data from additional areas will be included in analyses when >4 years of case reports have accrued.
In the 35 areas (33 states, Guam, and the U.S. Virgin Islands) that have had laws or regulations since 2000 requiring confidential name-based reporting for children aged <13 years with confirmed HIV infection, an estimated 2,636 children were living with HIV infection (not AIDS) at the end of 2004. Estimated prevalence of HIV infection (not AIDS) in this group was 7.9 per 100,000 population (1).
A substantial number of pediatric influenza-associated deaths were reported to CDC during the 2003--04 influenza season (CDC, unpublished data, 2004). In October 2004, CDC added influenza-associated pediatric mortality to the list of conditions voluntarily reportable to the National Notifiable Diseases Surveillance System (NNDSS) (1); at the same time, the Council of State and Territorial Epidemiologists (CSTE) and CDC worked together to draft recommendations for national reporting of pediatric deaths with laboratory confirmation of influenza, and these recommendations were approved at the 2004 CSTE annual meeting (2). Reporting for this condition began in week 40 (week ending October 9, 2004) of the 2004--05 influenza season. The cumulative year-to-date provisional incidence is published each week in the MMWR Table I for low-incidence nationally notifiable infectious diseases.
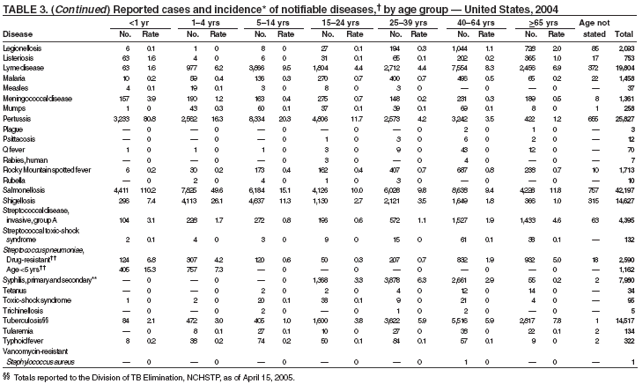
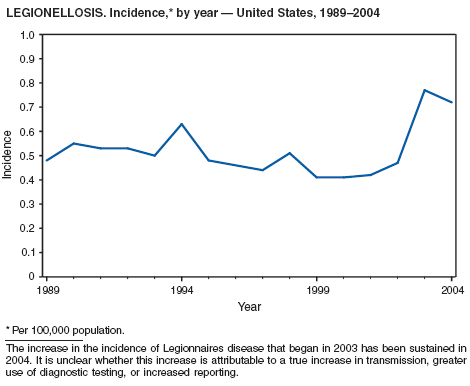
Legionellosis includes two distinct clinical entities: Legionnaires disease (which is characterized by fever, respiratory symptoms, and pneumonia) and Pontiac fever (which is characterized by fever and headache but no evidence of pneumonia). In 2004, the number of cases of legionellosis reported to CDC by state health departments remained above the baseline established before 2003, a year in which an increase in the number of cases was temporally related to excessive rainfall (1). Potential explanations for persistently high case counts include a real increase in the incidence of disease, increased diagnostic testing ordered by clinicians, increased reporting to state health departments, or a combination of all three.
In 2004, an increased number of cases of Legionnaires disease and Pontiac fever associated with overnight travel to hotels or aboard cruise ships were reported (2--4). Approximately 20% of all cases of Legionnaires disease are associated with recent travel; the majority of these cases are thought to be associated with potable water systems in hotels or whirlpool spas in hotels or on board cruise ships. Legionnaires disease is often treated empirically with broad-spectrum antibiotics that are effective against multiple etiologies of community-acquired pneumonia (5), and <10% of all cases are reported to state health departments. The incubation period of Legionnaires disease is 2--10 days, although well-documented cases have occurred >10 days after exposure (6). Because travelers often return home before developing symptoms, clinicians or state health departments are unlikely to be aware of more than one case resulting from a particular travel-related exposure, which complicates the detection of outbreaks.
To address the problem of travel-associated legionellosis, state health departments, the Council of State and Territorial Epidemiologists (CSTE), and CDC are collaborating to improve reporting of travel-associated legionellosis. In 2005, CSTE adopted a position statement aimed at setting goals for timelier reporting of such cases and establishing more current diagnostic criteria (7). Information on preventing travel-associated legionellosis has been published (8).
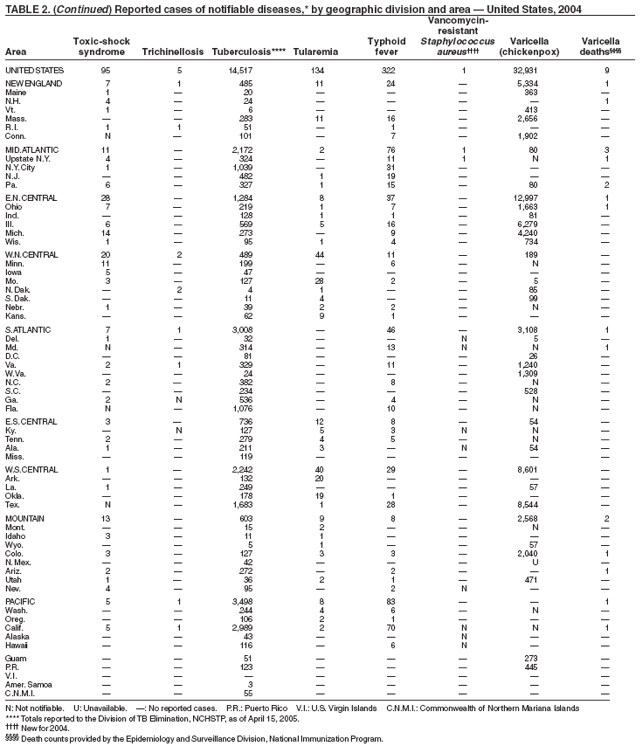
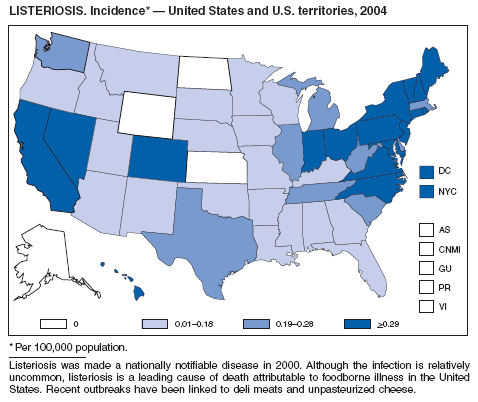
Listeriosis is a severe but uncommon infection caused by Listeria monocytogenes; it was made a nationally notifiable disease in 2000. Listeriosis is primarily foodborne and occurs most frequently among persons who are older, pregnant, or immunocompromised. During 2004, cases of listeriosis were reported from 47 states and the District of Columbia.
Molecular subtyping of L. monocytogenes isolates and sharing of that information through PulseNet has enhanced the ability of public health officials to detect and investigate outbreaks of listeriosis. Recent outbreaks have been linked to ready-to-eat meat (1) and unpasteurized fresh cheese (2). During 2004, incidence of listeriosis as reported to FoodNet active surveillance was 0.27 cases per 100,000 population, representing a decrease of 40% compared with 1996--1998 (3).
In January 2001, the Food and Drug Administration (FDA), the U.S. Department of Agriculture (USDA), and CDC released a national Listeria Action Plan to help guide control efforts by industry, regulators, and public health officials (4), and in November 2003, FDA and CDC updated their components of the plan (5). Also in 2003, USDA issued new regulations aimed at further reducing L. monocytogenes contamination of ready-to-eat meat and poultry products (6). All clinical isolates should be submitted to state public health laboratories for pulsed-field gel electrophoresis (PFGE) pattern determination, and all persons with listeriosis should be interviewed by a public health specialist or health-care provider using a standard form (available from CDC at telephone 404-639-2206).
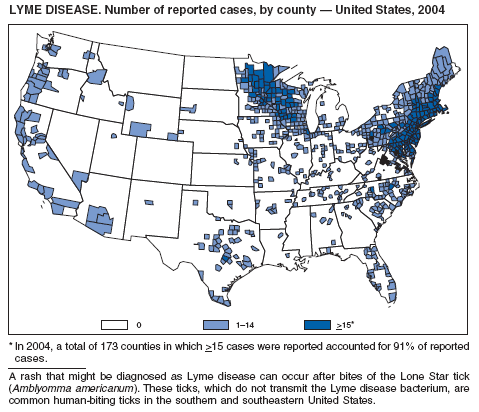
The number of reported Lyme disease cases decreased for the second consecutive year, with 17% fewer cases reported in 2004 than in 2002. However, much of this decrease can be attributed to modifications of the surveillance systems or reporting mechanisms in two high-incidence states.
The risk for Lyme disease and other tickborne illnesses can be reduced by avoiding tick-infested areas, using insect repellant containing N,N-diethyl-m-toluamide (DEET), and checking daily for attached ticks. Persons can also reduce their risk for peridomestic tick exposure through landscape modifications, correctly timed applications of pesticide, and use of commercial bait boxes (1,2).
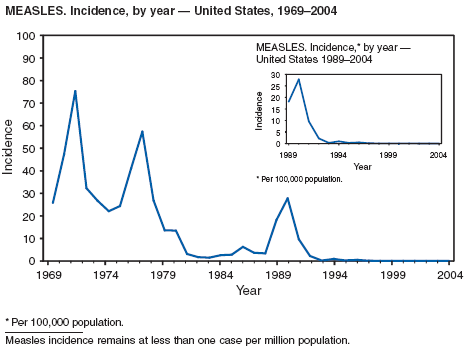
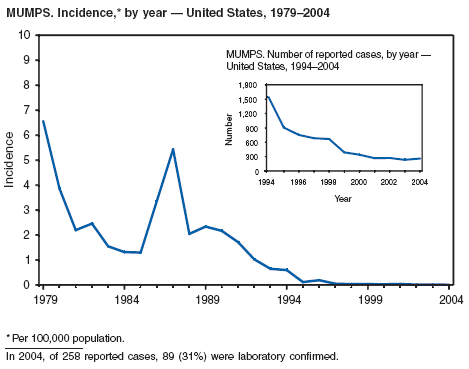
During 2004, the number of confirmed cases of measles reported in the United States was a record low. Cases occurred in 13 states; 27 cases were internationally imported and resulted in six secondary cases. For four cases, the sources are classified as unknown because no link to importation could be detected. The majority of infected persons were aged <5 years. Two outbreaks occurred, both from imported sources. In one outbreak that involved nine persons, measles occurred among nine adopted children from China; a secondary case occurred in an unvaccinated U.S. resident. In a second outbreak that involved three persons, an unvaccinated U.S. resident aged 19 years with a nonmedical exemption returned to the United States from India while infectious (1,2). Two secondary cases resulted, including one in an airline passenger who was seated directly beside the index patient. Measles can be prevented by adhering to recommendations for vaccination, including guidelines for travelers (3,4).
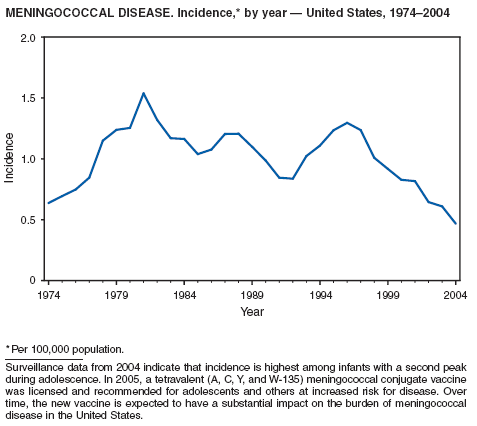
Neisseria meningitidis is a leading cause of bacterial meningitis and sepsis in the United States. During 2004, the number of invasive meningococcal disease cases reported to CDC decreased 22%, compared with the number reported in 2003. The case-fatality ratio (10%--14%) remains high, and 11%--19% of survivors have serious health sequelae. Rates of meningococcal disease are highest among infants, with a second peak at age 18 years (CDC, unpublished data, 2004).
A new tetravalent (A, C, Y, W-135) meningococcal conjugate vaccine ([MCV4] MenactraTM; manufactured by Sanofi Pasteur, Swiftwater, Pennsylvania) was licensed in January 2005 for persons aged 11--55 years. CDC's Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination with MCV4 of young adolescents aged 11--12 years, adolescents at high school entry if not vaccinated previously, college freshmen living in dormitories, and other populations at increased risk (1). The new conjugate vaccine should become a key addition to meningococcal disease prevention strategies.
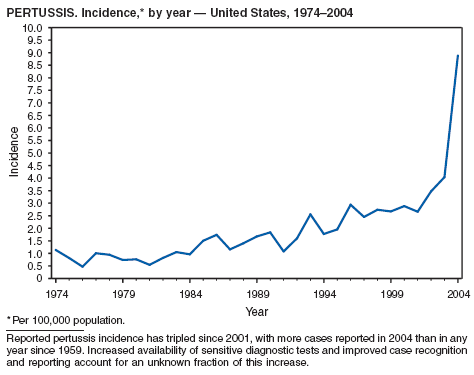
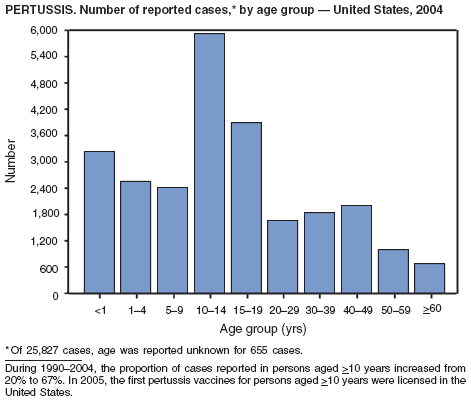
In 2004, incidence of reported pertussis increased for the third year in a row, to 8.9 cases per 100,000 population, more than twice the rate reported in 2003. The number of cases was the highest reported since 1959. Of the cases for which age was reported, 10% occurred among infants aged <6 months who were too young to have received the first 3 of the 5 doses of diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine recommended by age 6 years. This age group had the highest reported rate (136.5 per 100,000 population). Among older infants aged 6--11 months, the rate was 31.8 per 100,000. Among older children and adults, rates were 16.9 among children aged 1--4 years, 12.6 among children aged 5--9 years, 23.9 among children and adolescents aged 10--19 years, and 3.5 among adults aged >20 years.
Pertussis continues to cause morbidity in the United States despite high coverage levels for childhood pertussis vaccine. During 1994--2004, the reported pertussis rate per 100,000 population increased from 1.8 to 8.9. How much of this increase reflects greater recognition and better reporting of cases is unclear (1,2). Although infants have the highest morbidity associated with pertussis, adolescents and adults now account for the majority (67%) of reported cases. They become susceptible to disease when vaccine-induced immunity wanes, approximately 5--10 years after pertussis vaccination (2).
Two tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine, adsorbed (Tdap) products were licensed by the Food and Drug Administration in 2005 as single-dose booster vaccines to provide protection against tetanus, diphtheria, and pertussis. CDC's Advisory Committee on Immunization Practices (ACIP) recommends the routine use of Tdap vaccines among adolescents aged 11--18 years in place of tetanus and diphtheria toxoids (Td) vaccines (3). ACIP also has made a provisional recommendation that adults aged 19--64 years receive a single dose of Tdap to replace the next dose (4). The primary objective of administering the adolescent pertussis booster is to protect adolescents and adults against pertussis. Strategies for use of Tdap in adults are under review.
The number of human plague cases reported in 2004 remained low, compared with an annual average of 13 cases reported during 1980--1999. All cases were acquired from either infected fleas or contact with infected rabbits in Colorado or Wyoming. One case was fatal. In addition to a decreased number of human cases, limited epizootic activity has been reported in the southwestern United States, which is likely secondary to hot summer and dry winter conditions experienced during recent years. Continued surveillance is critical because of the potential use of plague as a bioterrorist agent.
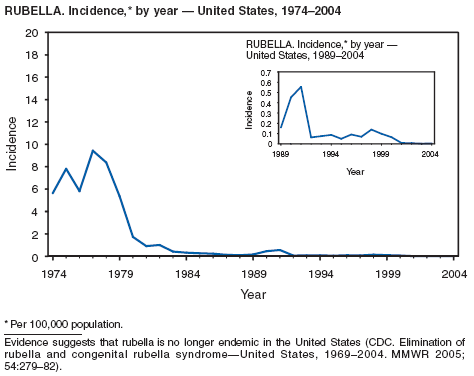
One of the national health goals for 2010 is to eliminate rubella and CRS in the United States (objective 14-1) (1). On October 29, 2004, a nine-person independent panel unanimously agreed that rubella is no longer endemic in the United States (2).
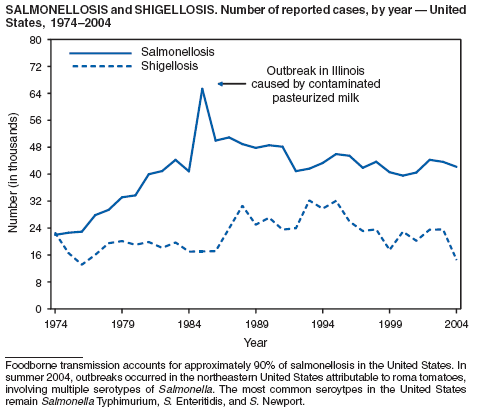
During 2004, as in previous years, the majority (64%) of reported cases of salmonellosis occurred during July--October. Salmonella isolates are reported by serotype through the Public Health Laboratory Information System. Since 1993, the two most frequently reported isolates have been S. enterica serotype Typhimurium and S. enterica serotype Enteritidis (1). During 2004, overall incidence of Salmonella in FoodNet active surveillance sites was 14.5 cases per 100,000 population, representing a decrease of 8% compared with 1996--1998 (2). This decrease was attributable largely to a 41% decrease in incidence of S. enterica serotype Typhimurium. Incidence of S. Enteritidis remained unchanged, and incidence of S. Newport and S. Javiana increased 41% and 167%, respectively (2). A substantial proportion of S. enterica serotype Typhimurium and S. enterica serotype Newport isolates are resistant to multiple drugs; national surveillance of S. enterica serotype Typhimurium strains conducted in 2002 indicated that 40% were resistant to one or more drugs and that 34% had a five-drug resistance pattern characteristic of a single phage type, DT104 (3). During 1998--2002, the proportion of five-drug--resistant strains of S. enterica serotype Newport increased substantially, from 1 in 1998 to 22% in 2002 (3,4).
The approximately 14,000 cases of shigellosis reported to CDC in 2004 represent a substantial decrease from the reported annual totals since 1978, which have uniformly exceeded 17,000 cases. Shigella sonnei infections continue to account for >75% of shigellosis in the United States (1). Prolonged, multistate outbreaks of S. sonnei infections transmitted in child care centers, where maintenance of good hygienic conditions requires special care account for much of the problem (2,3). Shigellae also can be transmitted through contaminated foods, sexual contact, and water used for drinking or recreational purposes (1). A new serotype of Shigella boydii has been reported in the United States and Canada (4).
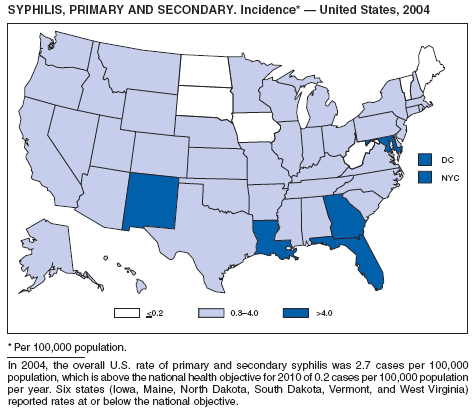
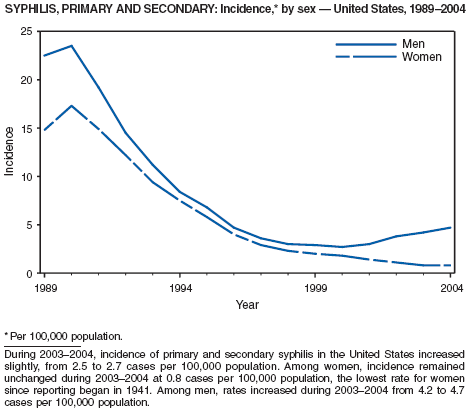
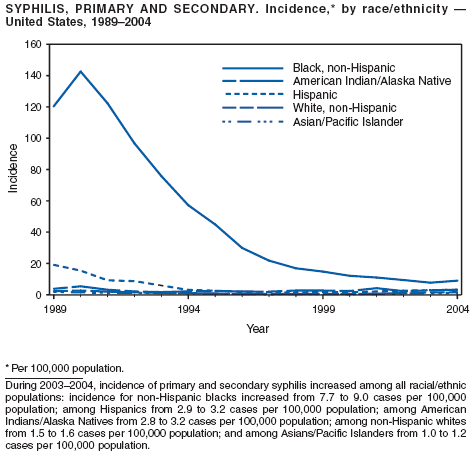
In 2004, rates of primary and secondary syphilis increased for the fourth consecutive year to the highest level (2.7 cases per 100,000 population) reported in the United States since 1997 (1). These increases occurred only among men; however, for the first time in >10 years, the rate of primary and secondary syphilis among women did not decrease but remained essentially unchanged from 2003. Rates increased among both black and white men. CDC is collaborating with partners from throughout the United States to revise the Syphilis Elimination Plan for 2005--2010 (2).
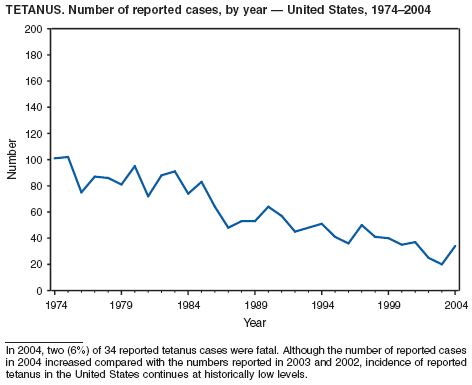
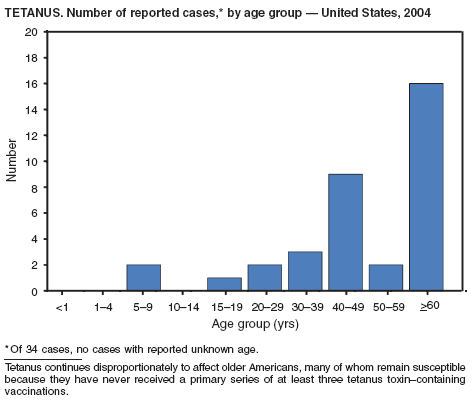
Two fatal cases of tetanus were reported in 2004: a woman aged 85 years with a history of a single tetanus toxoid (TT) booster 50 years previously and a diabetic woman aged 78 years with no documented tetanus vaccinations. The majority (76%) of tetanus cases occurred among persons aged >40 years; 47% occurred among persons aged >60 years. No neonatal cases were reported.
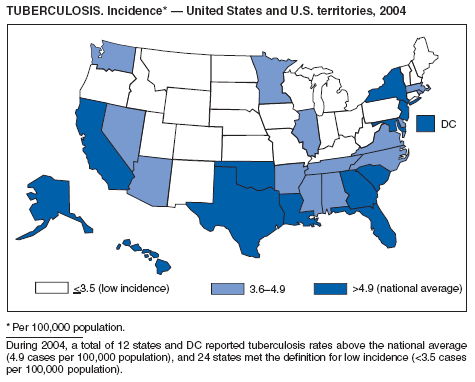
During 2003--2004, the tuberculosis (TB) rate per 100,000 population declined 3.2%, from 5.1 to 4.9 cases (1). This rate remains higher than the national objective of 3.5 cases per 100,000 population set for 2000 (2).
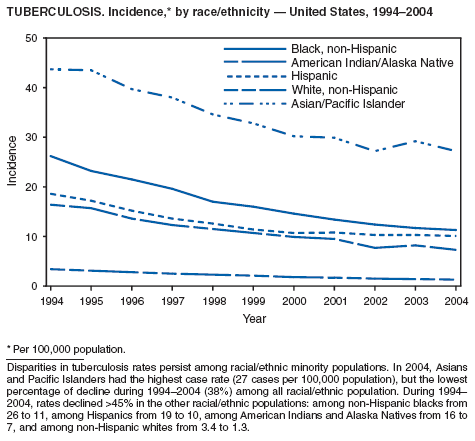
In 2004, disparities in TB rates persisted among members of racial and ethnic minority populations. In descending order, the highest rates per 100,000 population were reported among Asians (27.6), Native Hawaiians or Other Pacific Islanders (16.3), non-Hispanic blacks (11.3), Hispanics (10.1]), American Indians or Alaska Natives (7.3), and non-Hispanic whites (1.3). In 2004, for the first time, Hispanics (29%) exceeded blacks (28%) as the racial or ethnic population with the largest percentage of all TB cases (1).
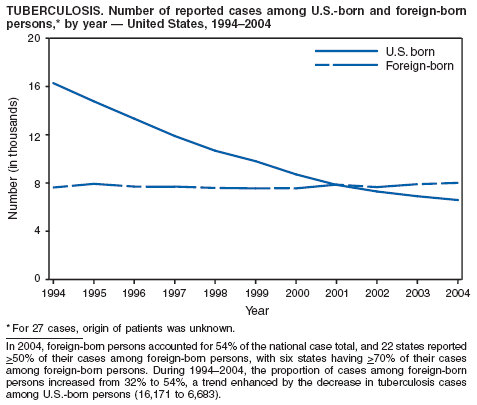
In 2004, foreign-born persons accounted for 54% of the national case total, and 22 states reported >50% of their cases among foreign-born persons (1). The percentage of foreign-born persons among all persons with TB has risen steadily since 1993, when foreign-born persons accounted for 29% (1) of the national case total, and five states reported >50% of their cases among foreign-born persons (3). Although the TB rate among foreign-born persons decreased during 1993--2004 (from 34.0 to 22.8 per 100,000 population), the decrease among U.S.-born persons has been greater (from 7.4 to 2.6). In 2004, the case rate was 8.8 times greater among foreign-born persons than among U.S.-born persons; since 1993, this rate ratio has increased steadily.
CDC is collaborating with public health partners in implementing TB control initiatives for recent international arrivals and residents along the border between the United States and Mexico and in strengthening TB programs in countries with a high incidence of TB disease (4). CDC has updated its comprehensive national action plan to reflect the realignment of its priorities with the 2000 Institute of Medicine report on TB (5). The updated plan ensures that priority prevention activities are undertaken with optimal collaboration and coordination among national and international public health partners (6).
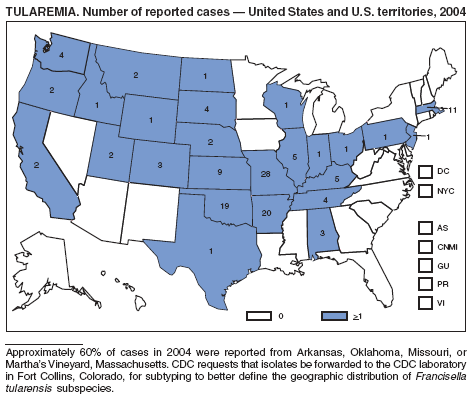
The number of cases reported in 2004 remained stable compared with the annual average reported during 1990--2000 (1). The majority of cases resulted from typical environmental exposures including arthropod bites or contact with infected mammals. Noteworthy were cases of laboratory-acquired pneumonic tularemia among researchers working with cultures contaminated with virulent Francisella tularensis and a case associated with a pet hamster bite (2). CDC requests that F. tularensis isolates be submitted to the CDC laboratory in Fort Collins, Colorado, for subtyping and PFGE testing.
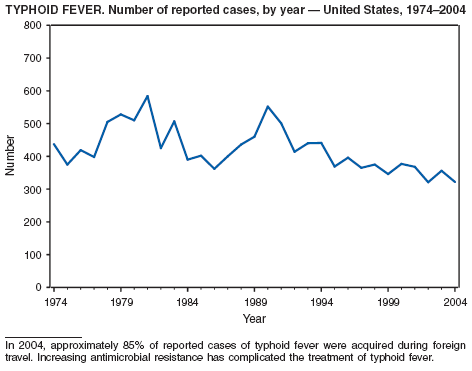
In 2004, the number of cases of typhoid fever in the United States reported to CDC was the second lowest total for any year since 1972. Despite recommendations that travelers to countries in which typhoid fever is endemic should be vaccinated with either of two effective vaccines available in the United States, approximately three fourths of all cases occur among persons who report international travel during the preceding month. Persons visiting friends and relatives in south Asia appear to be at particular risk, even during short visits (1). Salmonella Typhi strains with decreased susceptibility to ciprofloxacin are increasingly frequent in that region and might require treatment with alternative antimicrobial agents (2). Typhoid fever outbreaks in the United States usually are limited in size but can cause substantial morbidity; they are most often foodborne and warrant thorough investigation (3). A sexually transmitted outbreak of typhoid fever has been reported (4).
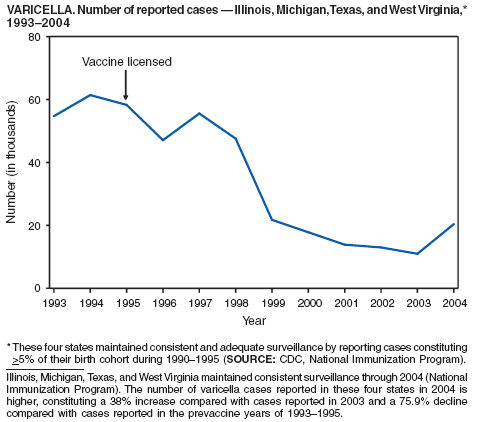
In 2004, nine varicella deaths were reported to CDC from eight states. Ages of the deceased varied (range: 14 months--79 years). Five deaths occurred among children aged 14 months--10 years, and four occurred among adults aged 22--79 years.
In 1999, the Council of State and Territorial Epidemiologists (CSTE) recommended that varicella deaths be reported to CDC to monitor the impact of routine varicella vaccination on varicella-related mortality (1). However, reporting of varicella deaths is incomplete, limiting the usefulness of mortality data in assessing the impact of the varicella vaccination program. CDC encourages states to report varicella deaths so risk factors for varicella-related mortality can be identified and the percentage of deaths that would have been directly preventable by following current recommendations for vaccination can be determined. In 2002, as an adjunct to mortality surveillance, varicella infection was again designated a nationally notifiable condition. CSTE has recommended that states implement statewide individual case reporting by 2005. The objectives of varicella morbidity surveillance at state and national levels are to monitor the epidemiology of varicella by age, place, and over time, to monitor the impact of widespread and increasing immunization on the epidemiology of varicella, and to allow prompt implementation of disease control measures (2).
Part 1
Summaries of Notifiable Diseases in the United States, 2004
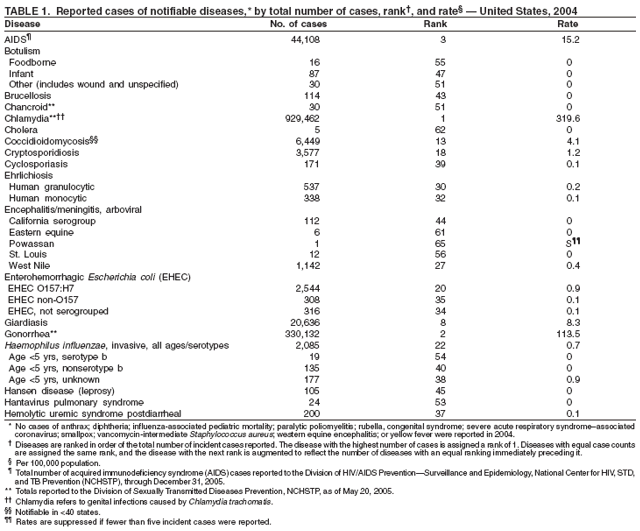
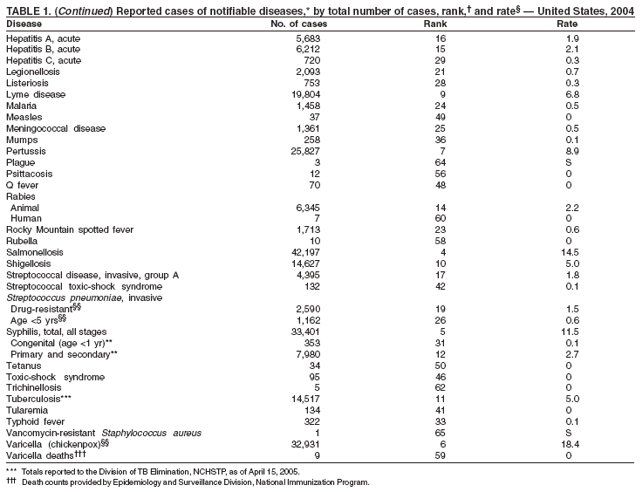

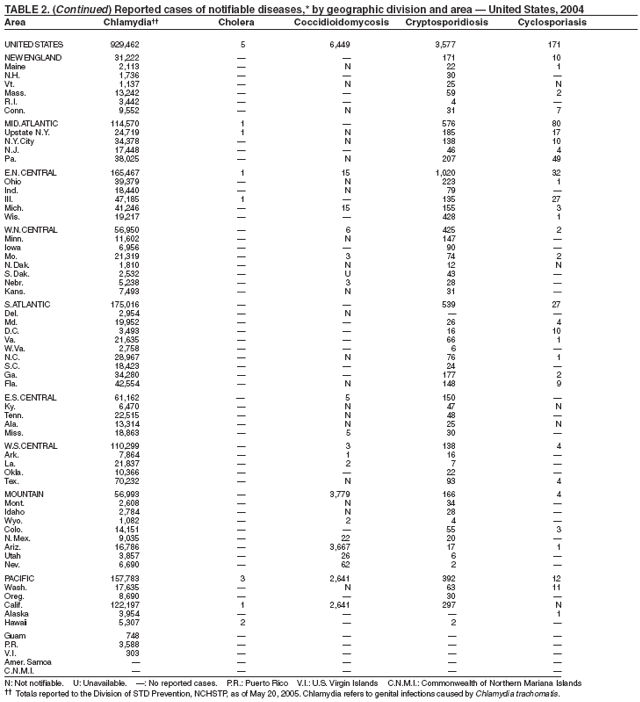

Part 2
Graphs and Maps for Selected Notifiable Diseases in the United States, 2004

General
Bayer R, Fairchild AL. Public health: surveillance and privacy. Science 2000;290:1898--9.
CDC. Racial disparities in nationally notifiable diseases---United States, 2002. MMWR 2005;54:9--11.
CDC. Case definitions for infectious conditions under public health surveillance. MMWR 1997;46(No. RR-10). Additional information available at http://www.cdc.gov/epo/dphsi/casedef/index.htm.
CDC. Framework for program evaluation in public health. MMWR 1999;48(No. RR-11).
CDC. Manual of procedures for the reporting of nationally notifiable diseases to CDC. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC; 1995.
CDC. Manual for the surveillance of vaccine-preventable diseases. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC; 1999. Available at http://www.cdc.gov/nip/publications/surv-manual/.
CDC. National Electronic Disease Surveillance System (NEDSS): a standards-based approach to connect public health and clinical medicine. Journal of Public Health Management and Practice 2001;7:43--50.
CDC. Public Health Information Network (PHIN): overview. Available at http://www.cdc.gov/phin/overview.html.
CDC. Sexually transmitted disease surveillance 1998. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC; 1999.
Chang M-H, Glynn MK, Groseclose SL. Endemic, notifiable bioterrorism-related diseases, United States, 1992--1999. Emerg Infect Dis 2003;9:556--64.
Chin JE, ed. Control of communicable diseases manual. 17th ed. Washington, DC: American Public Health Association; 2000.
Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol 2002;155:866--74.
Effler P, Ching-Lee M, Bogard A, Ieong M-C, Nekomoto T, Jernigan D. Statewide system of electronic notifiable disease reporting from clinical laboratories: comparing automated reporting with conventional methods. JAMA 1999;282;1845--50.
Freimuth V, Linnan HW, Potter P. Communicating the threat of emerging infections to the public. Emerg Infect Dis 2000;6:337--47.
Government Accountability Office. Emerging infectious diseases: review of state and federal surveillance efforts. Washington, DC: Government Accountability Office. GAO-04-877; 2004. Available at http://www.gao.gov/new.items/d04877.pdf.
Hopkins RS. Design and operation of state and local infectious disease surveillance systems. J Public Health Management Practice 2005;11:184--90.
Jajosky RA, Groseclose SL. Evaluation of reporting timeliness of public health surveillance systems for infectious diseases. BMC Public Health 2004;4:29.
Koo D, Caldwell B. The role of providers and health plans in infectious disease surveillance. Eff Clin Pract 1999;2:247--52. Available at http://www.acponline.org/journals/ecp/sepoct99/koo.htm.
Koo D, Wetterhall S. History and current status of the National Notifiable Diseases Surveillance System. J Public Health Management Practice 1996;2:4--10.
Lin SS, Kelsey JL. Use of race and ethnicity in epidemiologic research: concepts, methodological issues, and suggestions for research. Epidemiol Rev 2000;22:187--202.
Martin SM, Bean NH. Data management issues for emerging diseases and new tools for managing surveillance and laboratory data. Emerg Infect Dis 1995;1:124--8. Niskar AS, Koo D. Differences in notifiable infectious disease morbidity among adult women---United States, 1992--1994. J Womens Health 1998;7:451--8.
Panackal AA, M'ikanatha NM, Tsui FC, et al. Automatic electronic laboratory-based reporting of notifiable infectious diseases at a large health system. Emerg Infect Dis 2002;8:685--91.
Pinner RW, Koo D, Berkelman RL. Surveillance of infectious diseases. In: Lederberg J, Alexander M, Bloom RB, eds. Encyclopedia of microbiology. 2nd ed. San Diego, CA: Academic Press; 2000;4:506--25.
Pinner RW, Jernigan DB, Sutliff SM. Electronic laboratory-based reporting for public health. Military Medicine 2000;165(suppl 2):20--4.
Roush S, Birkhead G, Koo D, Cobb A, Fleming D. Mandatory reporting of diseases and conditions by health care professionals and laboratories. JAMA 1999;282:164--70.
Silk, BJ, Berkelman RL. A review of strategies for enhancing the completeness of notifiable disease reporting. J Public Health Management Practice 2005;11:191--200.
Teutsch SM, Churchill RE, eds. Principles and practice of public health surveillance. 2nd ed. New York, NY: Oxford University Press; 2000.
Thacker SB, Choi K, Brachman PS. The surveillance of infectious diseases. JAMA 1983;249:1181--5.
AIDS
CDC. HIV/AIDS surveillance report, 2004. Atlanta, GA: US Department of Health and Human Services, CDC, Vol. 16; 2005. Available at: http://www.cdc.gov/hiv/stats/hasrlink.htm.
Nakashima AK, Fleming PL. HIV/AIDS surveillance in the United States, 1981--2001. J Acquir Immune Defic Syndr 2003;32:68--85.
Anthrax
Hugh-Jones M. 1996--97 global anthrax report. J Appl Microbiol 1999;87:189--91.
Inglesby TV, O'Toole T, Henderson DA, et al. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 2002;287:2236--52.
Jernigan DB, Raghunathan PL, Bell BP, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis 2002;8:1019--28.
Martin SW, Tierney BC, Aranas A, et al. An overview of adverse events reported by participants in CDC's anthrax vaccine and antimicrobial availability program. Pharmacoepidemiol Drug Saf 2005;14:393--401.
Sejvar JJ, Tenover FC, Stephens DS. Management of anthrax meningitis. Lancet Infect Dis 2005;5:287--95.
Botulism
Sobel J, Tucker N, MacLaughlin J, Maslanka S. Foodborne botulism in the United States, 1999--2000. Emerg Infect Dis 2004;10:1606--12. Available at http://www.cdc.gov/ncidod/EID/vol10no9/03-0745.htm.
CDC. Botulism in the United States, 1899--1996: handbook for epidemiologists, clinicians and laboratory workers. Atlanta, GA: US Department of Health and Services, CDC; 1998.
Shapiro R, Hatheway C, Swerdlow DL. Botulism in the United States: a clinical and epidemiologic review. Ann Intern Med 1998;129:221--8.
Brucellosis
CDC. Brucellosis: (Brucella melitensis, abortus, suis, and canis). Atlanta, GA: US Department of Health and Human Services, CDC; 2005. Available at http://www.cdc.gov/ncidod/dbmd/diseaseinfo/brucellosis_g.htm.
CDC. Brucellosis case definition. Atlanta, GA: US Department of Health and Human Services, CDC; 2001. Available at http://www.bt.cdc.gov/Agent/Brucellosis/CaseDef.asp.
CDC. Human exposure to Brucella abortus strain RB51---Kansas, 1997. MMWR 1998;47:172--5.
Stevens, MG, Olsen SC, Palmer MV, Cheville NF. US Department of Agriculture, Agricultural Research Service National Animal Disease Center, Iowa State University. Brucella abortus strain RB51: a new brucellosis vaccine for cattle. Compendium 1997;19:766--74.
Yagupsky P, Baron EJ. Laboratory exposures to Brucellae and implications for bioterrorism. Emerg Infect Dis 2005;11:1180--5.
Chomel BB, DeBess EE, Mangiamele DM, et al. Changing trends in the epidemiology of human brucellosis in California from 1973 to 1992: a shift toward foodborne transmission. J Infect Dis 1994;170:1216--23.
Chancroid
DiCarlo RP, Armentor BS, Martin DH. Chancroid epidemiology in New Orleans men. J Infect Dis 1995;172:446--52.
Mertz, KJ, Weiss JB, Webb RM, et al. An investigation of genital ulcers in Jackson, Mississippi, with use of a multiplex polymerase chain reaction assay: high prevalence of chancroid and human immunodeficiency virus infection. J Infect Dis 1998;178:1060--6. Mertz KJ, Trees D, Levine WC, et al. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis 1998;178:1795--8.
Chlamydia trachomatis, Genital Infection
CDC. Sexually transmitted disease surveillance 2002 supplement: Chlamydia Prevalence Monitoring Project, annual report 2002. Atlanta, GA: US Department of Health and Human Services, CDC; 2003. Available at http://www.cdc.gov/std/chlamydia/chlamydia-stats-all-years.htm.
Gaydos CA, Howell MR, Pare B, et al. Chlamydia trachomatis infections in female military recruits. N Engl J Med 1998;339:739--44.
Mertz KJ, McQuillian GM, Levine WC, et al. A pilot study of chlamydial infection in a national household survey. Sex Transm Dis 1998;25:225--8.
Miller WC, Ford CA, Handcock MS, et al. Prevalance of chlamydial and gonococcal infections among young adults in the United States. JAMA 2004;291:2229--36.
Cholera
Steinberg EB, Greene KD, Bopp CA, Cameron DN, Wells JG, Mintz ED. Cholera in the United States, 1995--2000: trends at the end of the millennium. J Infect Dis 2001;184:799--802.
World Health Organization. Cholera, 2004. Wkly Epidemiol Rec 2005;80:261--8.
Mintz ED, Tauxe RV, Levine MM. The global resurgence of cholera. In: Noah ND, O'Mahony M, eds. Communicable disease epidemiology and control. Chichester, UK: John Wiley & Sons; 1998:63--104.
Coccidioidomycosis
Park BJ, Sigel K, Vaz V et al., An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998--2001. J Infect Dis 2005;191:1981--7.
Cryptosporidiosis
Roy SL, DeLong SM, Stenzel SA, et al. Risk factors for sporadic cryptosporidiosis among immunocompetent persons in the United States from 1999 to 2001. J Clin Microbiol 2004;42:2944--51.
CDC. DPDx Diagnostic procedures---stool specimens---detection of parasite antigens. Atlanta, GA: US Department of Health and Human Services, CDC; 2004. Available at http://www.dpd.cdc.gov/DPDx/html/DiagnosticProcedures.htm.
Cyclosporiasis
CDC. Outbreak of cyclosporiasis associated with snow peas---Pennsylvania, 2004. MMWR 2004;53:876--8.
Ho AY, Lopez AS, Eberhard MG, et al. Outbreak of cyclosporiasis associated with imported raspberries, Philadelphia, Pennsylvania, 2000. Emerg Infect Dis 2002;8:783--8.
Herwaldt BL. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis 2000;31:1040--57.
Diphtheria
Dewinter LM, Bernard KA, Romney MG. Human clinical isolates of Corynebacterium diphtheriae and Corynebacterium ulcerans collected in Canada from 1999 to 2003 but not fitting reporting criteria for cases of diphtheria. Clin Microbiol 2005;43:3447--9.
Ehrlichiosis (Human Granulocytic and Human Monocytic)
Demma LJ, Holman RC, McQuiston JH, Krebs JW, Swerdlow DL. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001--2002. Am J Trop Med Hyg 2005;73:400--9.
Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen [Review]. J Clin Microbiol 2003; 16:37--64.
IJdo JW, Meek JI, Cartter ML, et al. The emergence of another tick-borne infection in the 12-town area around Lyme, Connecticut: human granulocytic ehrlichiosis. J Infect Dis 2000;181:1388--93.
McQuiston JH, Paddock CD, Holman RC, Childs JE. The human ehrlichioses in the United States [Review]. Emerg Infect Dis 1999;5:635--42.
Childs JE, Sumner JW, Nicholson WL, Massung RF, Standaert SM, Paddock CD. Outcome of diagnostic tests using samples from patients with culture-proven human monocytic ehrlichiosis: implications for surveillance. J Clin Microbiol 1999;37:2997--3000.
Encephalitis, Arboviral (California Serogroup Viral, Eastern Equine, St. Louis, West Nile, Western Equine)
Campbell GL, Marfin AM, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis 2002;2:519--29.
Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area. N Engl J Med 2001;344:1807--14.
Giardiasis
Stuart JM, Orr HJ, Warburton FG, Jeyakanth S, Pugh C, Morris I, et al. Risk factors for sporadic giardiasis: A case-control study in Southwestern England. Emerg Infect Dis 2003;9:229--33.
CDC. DPDx diagnostic procedures--stool specimens---detection of parasite antigens. Atlanta, GA: US Department of Health and Human Services, CDC; 2004. Available at http://www.dpd.cdc.gov/DPDx/HTML/DiagnosticProcedures.htm.
Gonorrhea
CDC. Sexually transmitted diseases treatment guidelines, 2002. MMWR 2002;51(No. RR-6).
CDC. Sexually transmitted diseases surveillance 2002 supplement: Gonococcal Isolate Surveillance Project (GISP) annual report 2002. Atlanta, GA: US Department of Health and Human Services, CDC; 2003.
Fox KK, del Rio C, Holmes KK, et al. Gonorrhea in the HIV era: a reversal in trends among men who have sex with men. Am J Public Health 2001;91:959--64.
Haemophilus influenzae, Invasive Disease
Fry AM, Lurie P, Gidley M, Schmink S, Lingappa J, Rosenstein NE. Haemophilus influenzae type b (Hib) disease among Amish children in Pennsylvania: reasons for persistent disease. Pediatrics 2001;108:1--6.
Hansen Disease (Leprosy)
Britton WJ, Lockwood NJ. Leprosy. Lancet 2004;363: 1209--19.
Hartzell JD, Zapor M, Peng S, Straight T. Leprosy: a case series and review. South Med J 2004;971252--6.
Hastings R, Ed. Leprosy. 2nd Ed. New York, NY: Churchill Livingstone; 1994.
Joyce MP, Scollard DM. Leprosy (Hansen's disease). In: Rakel RE, Bope ET, Eds. Conn's Current Therapy 2004: Latest approved methods of treatment for the practicing physician. 56th ed. Philadelphia, PA: Saunders; 2004:100--5.
Ooi WW, Moschella SL. Update on leprosy in immigrants in the United States: Status in the Year 2000. Clin Infect Dis 2001;32:930--7.
Bruce S, Schroeder TL, Ellner K, Rubin H, Williams T, Wolf JE Jr. Armadillo exposure and Hansen's disease: an epidemiologic survey in southern Texas. J Am Acad Dermatol 43(2 Pt1):223--8.
Hepatitis A
Armstrong GL, Bell BP. Hepatitis A virus infections in the United States: model-based estimates and implications for childhood immunization. Pediatrics 2002;109:839--45.
Bell BP, Shapiro CN, Alter MJ, et al. The diverse patterns of hepatitis A epidemiology in the United States---implications for vaccination strategies. J Infect Dis 1998;178:1579--84.
Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. JAMA 2005; 294:194--201.
Shapiro CN, Coleman PJ, McQuillan GM, Alter MJ, Margolis HS. Epidemiology of hepatitis A: seroepidemiology and risk groups in the USA. Vaccine 1992;10(suppl 1): S59--62.
Hepatitis B
Armstrong GL, Mast EE, Wojczynski M, Margolis HS. Childhood hepatitis B virus infections in the United States before hepatitis B immunization. Pediatrics 2001;108:1123--8.
Goldstein ST, Alter MJ, Williams IT, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982--1998: implications for vaccination programs. J Infect Dis 2002;185:713--9. McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: The National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health 1999;89:14--8.
Margolis HS, Alter MJ, Hadler SC. Hepatitis B: evolving epidemiology and implications for control [Review]. Semin Liver Dis 1991;11:84--92.
Hepatitis C
Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341:556--62.
Armstrong GA, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31:777--82.
Influenza-Associated Pediatric Mortality
Council of State and Territorial Epidemiologists. Influenza-associated pediatric mortality; 2004. Atlanta, GA: Council of State and Territorial Epidemiologists; 2004. Available at http://www.cste.org/PositionStatementsResolutions2.htm.
Legionellosis
Cowgill KD, Lucas CE, Benson RF, et al. Recurrence of legionnaires disease at a hotel in the United States Virgin Islands over a 20-year period. Clin Infect Dis 2005;40:1205--7.
Fields BS, Benson RF, Besser RE. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev 2002;15:506--26.
European Working Group on Legionella Infections. European guidelines for control and prevention of travel associated Legionnaires' disease. London, UK: United Kingdom Health Protection Agency; 2005.
Joseph CA. Legionnaires' disease in Europe 2000--2002. Epidemiol Infect 2004;132:417--24.
Marston BJ, Lipman HB, Breiman RF. Surveillance for Legionnaires' disease: risk factors for morbidity and mortality. Arch Intern Med 1994;154:2417--22.
Lyme Disease
Stafford KC III. Tick management handbook: an integrated guide for homeowners, pest control operators, and public health officials for the prevention of tick-associated disease. New Haven, CT: Connecticut Agricultural Experiment Station; 2004. Available at http://www.cdc.gov/ncidod/dvbid/lyme/resources/handbook.pdf.
Hayes EB and Piesman J. How can we prevent Lyme disease? N Engl J Med 2003;348:2424--30.
Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 200518:484--509.
Medical Letter. Treatment of Lyme disease. Med Lett Drugs Ther 2005;47:41--3.
CDC. Caution regarding testing for Lyme disease. MMWR 2005;54:125.
Malaria
CDC. Transmission of malaria in resort areas---Dominican Republic, 2004. MMWR 2005;53:1195--8.
CDC. Congenital malaria---Nassau County, New York, 2004. MMWR 2005;54:383--4.
Magill, AJ. The prevention of malaria. Primary Care 2002;29:815--42.
Measles
Papania M, Hinman A, Katz S, Orenstein W, McCauley M, eds. Progress toward measles elimination---absence of measles as an endemic disease in the United States. J Infect Dis 2004;189(Suppl 1):S1--257.
Rota PA, Liffick SL, Rota JS, et al. Molecular epidemiology of measles viruses in the United States, 1997--2001. Emerg Infect Dis 2002;8:902--8.
De Serres G, Gay NJ, Farrington CP. Epidemiology of transmissible diseases after elimination. Am J Epidemiol 2000;151:1039--48.
Meningococcal Disease
Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med 2001;344:1378--88.
Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of meningococcal disease in the United States, 1992--1996. J Infect Dis 1999;180:1894--901.
Pertussis
Bisgard KM, Rhodes P, Connelly BL, et al. Pertussis vaccine effectiveness among children 6 to 59 months of age in the United States, 1998--2001. Pediatrics 2005;116:e285--94.
Bisgard KM, Pascual FB, Ehresmann KR, et al. Infant pertussis: who was the source? Pediatr Infect Dis J 2004;23:985--9.
CDC. Pertussis---United States, 2001--2003. MMWR 2005;54:1283--6.
Lee GM, Lebaron C, Murphy TV, Lett S, Schauer S, Lieu TA. Pertussis in adolescents and adults: should we vaccinate? Pediatrics 2005;115:1675--84.
Plague
CDC. Imported plague---New York City, 2002. MMWR 2003;53:725--8.
Enscore RE, Biggerstaff BJ, Brown TL, et al. Modeling relationships between climate and the frequency of human plague cases in the southwestern United States, 1960--1997. Am J Trop Med Hyg 2002;66:186--96.
Inglesby TV, Dennis DT, Henderson DA, et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense [Review]. JAMA 2000;283:2281--90.
Dennis DT, Gage KL, Gratz N, Poland JD, Tikhomirov E. Plague manual: epidemiology, distribution, surveillance and control. Geneva, Switzerland: World Health Organization; 1999.
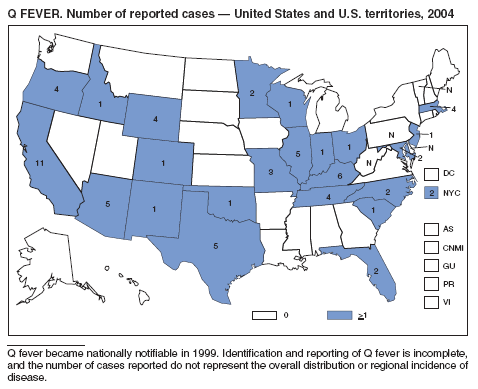
Q Fever
Mcquiston JH, Nargund VN, Miller JD, Priestly R, Shaw EI, Thompson HA. Prevalence of antibodies to Coxiella burnetii among veterinary school dairy herds in the United States, 2003. Vector-Borne and Zoonotic Dis 2005;90--1.
McQuiston JH, Childs JE. Q fever in humans and animals in the United States [Review]. Vector Borne and Zoonotic Dis 2002;179--191.
CDC. Q Fever---California, Georgia, Pennsylvania, and Tennessee, 2000--2001. MMWR 2002;41:924--7.
Raoult D, Tissot-Dupont H, Foucault C, et al. Q fever 1985--1998. Clinical and epidemiologic features of 1,383 infections [Review]. Medicine 2000;79:109--25.
Bernard KW, Parham GL, Winkler WG, Helmick CG. Q fever control measures: recommendations for research facilities using sheep. Infection Control 1982;3:461--5.
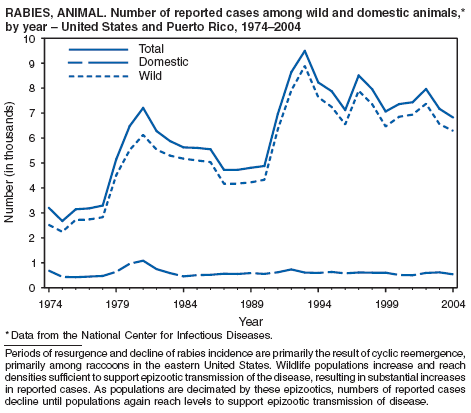
Rabies, Animal and Human
Willoughby RE Jr, Tieves KS, Hoffman GM, et al. Survival after treatment of rabies with induction of coma. N Engl J Med 2005;352:2508--14.
Srinivasan A, Burton EC, Kuehnert MJ, et al. Transmission of rabies virus from an organ donor to four transplant recipients. N Engl J Med 2005;352:1103--11.
Krebs JW, Mandel EJ, Swerdlow DL, Rupprecht CE. Rabies surveillance in the United States during 2003. J Am Vet Med Assoc 2004;227:1912--25.
Noah DL, Drenzek CL, Smith JS, et al. Epidemiology of human rabies in the United States, 1980 to 1996 [Review]. Ann Intern Med 1998;128:922--30.
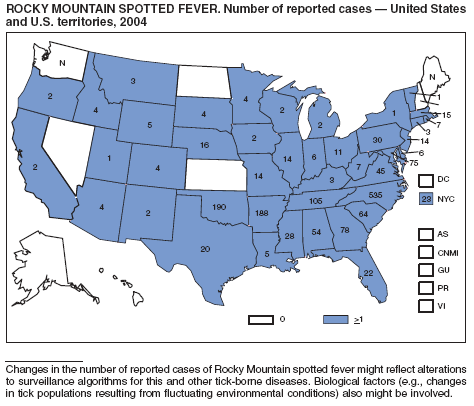
Rocky Mountain Spotted Fever
Demma, LJ, Traeger MS, Nicholson WL, et al. Rocky Mountain spotted fever from an unexpected tick reservoir in Arizona. N Engl J Med 2005;353:587--94.
Treadwell TA, Holman RC, Clarke MA et al. Rocky Mountain spotted fever in the United States, 1993--1996. Am J Trop Med Hyg 2000;63:21--6.
Thorner AR, Walker, DH, Petri WA. Rocky Mountain spotted fever [Review]. Clin Infect Dis 1998;27:1353--60.
Dalton MJ, Clarke MJ, Holman RC, et al. National surveillance for Rocky Mountain spotted fever, 1981--1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg 1995;52:405--13.
Rubella
Danovaro-Holliday MC, Gordon E, Woernle C, et al. Identifying risk factors for rubella susceptibility in a population at risk in the United States. Am J Public Health 2003;93:289--91.
Reef SE, Frey TK, Theall K, et al. The changing epidemiology of rubella in the 1990s: on the verge of elimination and new challenges for control and prevention. JAMA 2002;287;464--72. Reef S, Plotkin S, Cordero J, et al. Preparing for congenital rubella syndrome elimination: summary of the Workshop on Congenital Rubella Elimination in the United States. Clin Infect Dis 2000;31:85--95.
Shigellosis
Gupta A, Polyak CS, Bishop RD, Sobel J, Mintz ED. Laboratory-confirmed shigellosis in the United States, 1989--2002: epidemiologic trends and patterns. Clin Infect Dis 2004;38:1372--7.
Kalluri P, Cummings K, Abbott S, et al. Epidemiological features of a newly described serotype of Shigella boydii. Epidemiol Infect 2004;132:579--83.
Shane A, Crump J, Tucker N, Painter J, Mintz E. Sharing Shigella: risk factors and costs of a multi-community outbreak of shigellosis. Arch Pediatr Adolesc Med 2003;157:601--3.
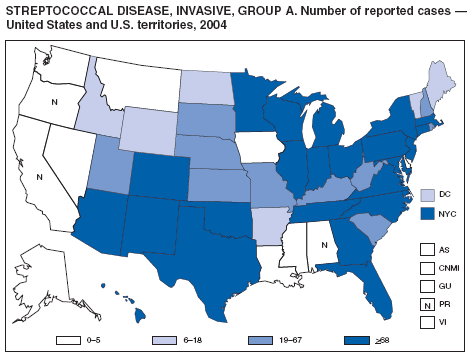
Streptococcal Disease, Invasive, Group A
The Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002;35:950--9.
CDC. Active Bacterial Core Surveillance report. Emerging Infections Program Network. Group A streptococcus, 2003---provisional. Atlanta, GA: US Department of Health and Human Services, CDC; 2005. Available at http://www.cdc.gov/ncidod/dbmd/abcs/survreports/gas04prelim.pdf.
O'Brien KL, Beall B, Barrett NL, et al. Epidemiology of invasive group A streptococcus disease in the United States, 1995--1999. Clin Infect Dis 2002;35:268--76.
Factor SH, Levine OS, Schwartz B, et al. Invasive group A streptococcal disease: risk factors for adults. Emerg Infect Dis 2003;9:970--7.
CDC. Investigating clusters of group A streptococcal disease. Atlanta, GA: US Department of Health and Human Services, CDC; 2005. Available at http://www2.cdc.gov/ncidod/dbmd/abcs/calc/calc_new/new_page.htm.
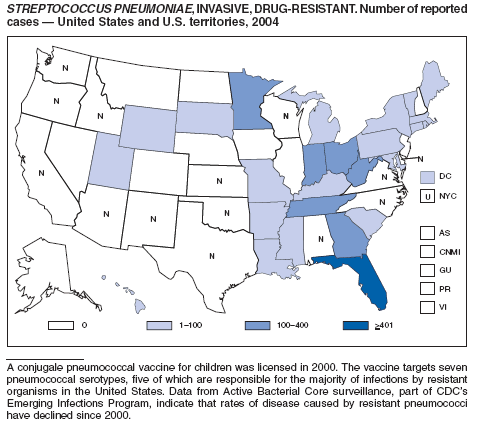
Streptococcus pneumoniae, Invasive, Drug-Resistant
Flannery B, Schrag S, Bennett NM, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections in the United States, 1998--2002. JAMA 2004;291:2197--2203.
Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med 2000;343:1917--24.
Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease following the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003;348:1737--46.
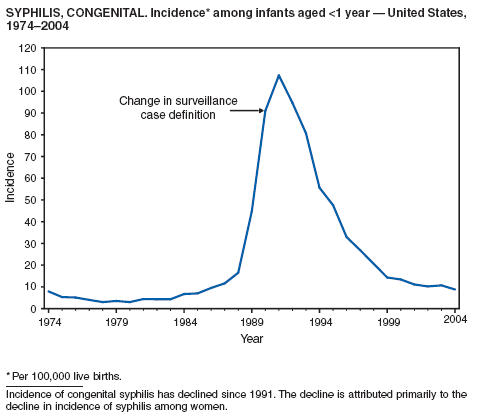
Syphilis, Congenital
CDC. Congenital syphilis---United States, 2002. MMWR 2004;53:716--9.
Syphilis, Primary and Secondary
CDC. The national plan to eliminate syphilis from the United States. Atlanta, GA: US Department of Health and Human Services, CDC; 1999.
CDC. Primary and secondary syphilis---United States, 2002. MMWR 2003;52:1117--20.
CDC. Sexually transmitted disease surveillance supplement 2002: syphilis surveillance report. Atlanta, GA: US Department of Health and Human Services, CDC; 2004.
Tetanus
Pascual FB, McGinley EL, Zanardi LR, Cortese MM, Murphy TV. Tetanus surveillance---United States, 1998--2000. In: Surveillance Summaries, June 20, 2003. MMWR 2003;52(No. SS-3):1--8.
CDC. Tetanus---Puerto Rico, 2002. MMWR 2002;51:613--5.
McQuillan GM, Kruszon-Moran D, Deforest A, Chu SY, Wharton M. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med 2002;136:660--6.
Trichinellosis
CDC. Trichinellosis associated with bear meat---New York and Tennessee, 2003. MMWR 2004;53:606--10.
Moorhead A, Grunenwald PE, Dietz VJ, Schantz PM. Trichinellosis in the United States, 1991--1996: declining but not gone. Am J Trop Med Hyg 1999;60:66--9.
Tuberculosis
CDC. Reported tuberculosis in the United States, 2003. Atlanta, GA: US Department of Health and Human Services, CDC; 2004. Available at http://www.cdc.gov/nchstp/tb.
CDC. Trends in tuberculosis---United States, 2004. MMWR 2005;54:245--9.
Saraiya M, Cookson ST, Tribble P, et al. Tuberculosis screening among foreign-born persons applying for permanent US residence. Am J Public Health 2002;92:826--9.
Talbot EA, Moore M, McCray E, Binkin NJ. Tuberculosis among foreign-born persons in the United States, 1993--1998. JAMA 2000;284:2894--900.
Tularemia
CDC. Outbreak of tularemia among commercially distributed prairie dogs, 2002. MMWR 2002;51:688,699.
CDC. Tularemia---United States, 1990--2000. MMWR 2002;51:182--4.
Dennis DT, Inglesby TV, Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. JAMA 2001;285:2763--73.
Feldman KA, Enscore RE, Lathrop SL, et al. Outbreak of primary pneumonic tularemia on Martha's Vineyard. N Engl J Med 2001:345:1219--26.
Petersen JM, Schriefer ME.Tularemia: emergence/ re-emergence. Vet Res 2005;36:455--67.
Typhoid Fever
Steinberg EB, Bishop RB, Dempsey AF, et al. Typhoid fever in travelers: who should be targeted for prevention? Clin Infect Dis 2004;39:186--91.
Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull WHO 2004;84:346--53.
Olsen SJ, Bleasdale SC, Magnano AR, et al. Outbreaks of typhoid fever in the United States, 1960--1999. Epidemiol Infect 2003;130:13--21.
Reller M, Olsen S, Kressel A. Sexual transmission of typhoid fever: a multi-state outbreak among men who have sex with men. Clin Infect Dis 2003;37:141--4.
Varicella
CDC. Varicella-related deaths---United States, January 2003--June 2004. MMWR 2005;54:272--4.
CDC. Outbreak of varicella among vaccinated children---Michigan, 2003. MMWR 2004;53:389--92.
Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA. 2004;292:704-8.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 5/4/2006
|
|
|||||
|
HOME |
ABOUT MMWR |
MMWR SEARCH |
DOWNLOADS |
RSS
|
CONTACT
|
|||||
|
|
|||||
|